Abstract
Histological data show perivascular recruitment of inflammatory cells in lung inflammation. However, the process of perivascular inflammation is yet-to-be characterized in any systematic manner at cell and molecular levels. Therefore, we investigated impact of genetic background on perivascular inflammation in acute or chronic airway inflammation in different strains of mice. Further, to address molecular mechanisms of perivascular inflammation, we examined immunohistochemical expression of vascular adhesion protein-1 (VAP-1) in chronic airway inflammation. Histological scoring revealed time and strain specific differences in perivascular recruitment of inflammatory cells in chronic and acute airway inflammation (P < 0·05). The data show that A/J strain is significantly more susceptible for perivascular inflammation followed by BALB/c and C57BL/6, while C3H/HeJ strain showed no perivascular accumulation of inflammatory cells. Of the two strains examined for perivascular inflammation in acute airway inflammation, BALB/c showed more accumulation of inflammatory cells compared to C57BL/c. VAP-1 expression occurred in the endothelium of pulmonary arteries but not in alveolar septa or airways in the control as well as challenged mice. In the inflamed lungs from A/J mice, the VAP-1 staining in pulmonary arteries was more intense compared to the other strains. VAP-1 staining was generally observed throughout the pulmonary arterial wall in chronic lung inflammation. These data show that periarterial inflammation is influenced by the genetic background, and may be partially regulated by VAP-1.
Keywords: Perivascular inflammation, VAP-1, lung, immunohistology, airway
Introduction
One of the most consistent histological signs of acute or chronic inflammation in an organ is accumulation of inflammatory cells [1]. Inflammatory cells are recruited to the site of injury through a cascade of interrelated cellular and molecular events. These cellular and molecular events include activation of vascular cells, production of chemoattractants and elaboration of adhesion molecules [2]. These events induce attachment of blood-borne inflammatory cells such as neutrophils and eosinophils to vascular endothelium and eventual transmigration across the vascular barrier. Inflammatory cell adhesion and transmigration in most of the organs occurs in postcapillary venules [3]. However, capillaries are the main sites of inflammatory cell recruitment in the lungs [4]. This alludes to the concept of vascular heterogeneity within and between organs.
Lung diseases such as asthma and sepsis manifest profound recruitment of inflammatory cells [5,6]. Natural and experimental airway inflammation is characterized by significant peribronchial recruitment of eosinophils and lymphocytes [6]. However, we recently presented an extensive review of the literature to highlight perivascular infiltration of inflammatory cells in inflamed lungs [7,8]. Careful perusal of histological data in papers dealing with airway inflammation shows accumulation of inflammatory cells in perivascular spaces [9–16]. Interestingly, such a common histological phenomenon is still to be described in a systematic manner and with molecular tools to define the main players.
There is evidence supporting influence of genetic background on susceptibility for airway inflammation in mice [17–19]. Previously, A/J strain of mice, compared to C3H/HeJ or C57BL/6 J, were shown to be more susceptible to acetylcholine or 5-hydroxytryptamine induced airway hyperresponsiveness [18–20]. Recent data show that not only A/J strains are the most susceptible for OVA-induced chronic airway inflammation followed by BALB/c, C57BL/6 and C3H/HeJ mice, they also showed more eosinophilic inflammation, collagen deposition, and higher levels of IL-4, IL-5 and IL-13 in their lungs. We also reported that BALB/c mice showed more hyper-responsiveness but fewer eosinophils in bronchoalveolar lavage compared to C57BL/6 [18]. Interestingly, eosinophils were observed in peribronchial and peripheral lung areas of sensitized and challenged BALB/c mice but only in peripheral areas in C57BL/6. Although histological perivascular inflammation is commonly observed in various models of airway inflammation, to our knowledge there are no data to show the impact of genetic background on perivascular inflammation. Therefore, we used two distinct models of OVA-induced airway inflammation to determine impact of genetic background on perivascular inflammation.
Recruitment of inflammatory cells is facilitated by a variety of adhesion molecules [21]. Recently, vascular adhesion protein-1 (VAP-1) has been identified as a novel cell adhesion molecule [22,23]. VAP-1 is a 170 kD sialoglycoprotein amine oxidase, which is mostly absent from the vasculature of noninflamed organs except in large and mid-sized pulmonary vessels and liver sinusoids [22–24]. VAP-1 and selectins together promote recruitment of lymphocytes, which is blocked through inhibition of VAP-1-associated monoamine oxidase activity [25]. Furthermore, antibody-mediated direct inhibition of VAP-1 neutralizes granulocyte recruitment in peritonitis [22]. VAP-1 is also expressed in granulocytes in inflamed lungs and may play a role in their adhesion to vascular endothelium [24]. Currently, there is no information on the expression VAP-1 in airway inflammation.
We conducted this study in two distinct models of allergic airway inflammation to examine if perivascular lung inflammation is strain dependent. We also examined the expression of VAP-1 in chronic airway inflammation in four strains of mice. The data presented in this manuscript show influence of genetic background of the mice on the intensity of periarterial recruitment of inflammatory cells and VAP-1 expression.
Materials and methods
Induction of acute airway inflammation
The protocol has been detailed previously [26,27]. Briefly, the mice (BALB/c and C57B/6; n = 6 each) were sensitized with an intraperitoneal injection of 20 µg of OVA (Grade V; Sigma Co., St. Louis, USA) with 2·25 mg alum (AlumImuject; Pierce Rockford, USA) on days 1 and 14. Mice were provoked on day 28, 29 and 30 (BALB/c and C57BL/6) with ultrasonically nebulized 1% OVA and euthanized 48 h after the last provocation. Control mice received the PBS injections on day 0 and 14 followed by intranasal challenge with OVA. The lung tissues were collected and embedded in paraffin.
Induction of chronic airway inflammation
The protocol has been described in detail previously [17]. Briefly, anaesthetized mice (A/J, BALB/c. C57BL/6 and C3H/HeJ strains; N = 6 each) were instilled intranasally with 50 µl of OVA (1 mg/ml) thrice every week for 4 or 8 weeks. The mice were euthanized 24 h after the last challenge and lung tissues were collected and embedded in paraffin.
Histological examination
Sections were prepared from paraffin blocks and stained with haematoxylin-eosin. Because the pattern of perivascular cell accumulation in the acute and chronic airway inflammation was different, we designed separate systems to evaluate. In the acute airway inflammation, the grading system was as follows:
0: no or occasional cells;
1: few loosely arranged cells;
2: many cells in the peripheral parts of the perivascular space;
3: numerous cells in the perivascular space.
In the chronic model, the perivascular inflammation was graded as follows:
0: no inflammation;
1: one to two concentric rows of inflammatory cells;
2: Three or more concentric rows of inflammatory cells;
3: Continuous perivascular and peribronchial cell accumulation (Fig. 1a–d).
Fig. 1.
Histology analyses of H&E stained sections: Lung sections graded as (a) normal, (b) Grade I, (c) Grade II and (d) Grade III. *Lumen of an artery. Original magnification: ×20
Immunohistology for vascular adhesion protein-1
The detailed protocol for VAP-1 staining of lung sections has been described previously [24]. In this study, we stained lung sections with VAP-1 only from the mice subjected to chronic airway inflammation. The reason for this was that lung tissues from acute challenge experiments were all used up in previous analyses. Briefly, lung sections were deparaffinized in xylene and rehydrated in descending concentrations of ethanol. The tissue sections were treated with pepsin (1 mg/ml of 0·1 N HCl) for 45 min to unmask antigens prior to blocking and incubation with primary rat anti-mouse VAP-1 antibody (1 : 25 in 1% BSA in PBS) overnight at 4°C. Following three washings, the sections were incubated for 45 min with rat immunoglobulins (1 : 100 in mouse serum in PBS; DAKO) and rat alkaline phosphatase anti-alkaline phosphatase (1 : 100 in PBS; DAKO). The secondary antibody steps were repeated with incubation times of 15 min. The colour was developed with Fast Blue (Sigma Co) followed by counter staining with haematoxylin. The following controls were included. Staining of adjacent sections with anti-CD3 antibody, omission of primary or both primary and secondary antibodies.
Statistical analyses
The data were analysed by one-way analyses of variance followed by multiple group comparisons with Duncan's test. The differences were deemed to be significant with P < 0·05.
Results
Histopathology
Semi-quantification was performed along a scale of 0–3 based on the extent of perivascular accumulation of inflammatory cells followed by statistical analyses (Fig. 1a–d).
Strain differences in chronic airway inflammation
Irrespective of the strain, lung sections from all the control mice were free of any perivascular and peribronchial inflammation (Figs 2 & 3). Our data showed differences within the strains in the extent of perivascular cell accumulation in OVA-induced chronic airway inflammation (Fig. 2). There was a progressive increase in perivascular inflammation primarily comprised of lymphocytes and eosinophils in A/J strain after 4 and 8 weeks of the challenge compared to the controls. Furthermore, the 8 week A/J mice showed more perivascular inflammation compared to the 4 week group. The BALB/c strain showed more perivascular inflammation at 4 weeks of the challenge compared to the other two BALB/c groups, which did not differ from each other. C57BL/6 mice showed more perivascular cell accumulation after 8 weeks of challenge compared the control and the 4 week groups while there were no differences between the later two groups. Interestingly, none of the C3H/HeJ groups showed any perivascular accumulation of inflammatory cells.
Fig. 2.
Histological scores of perivascular recruitment of inflammatory cells in chronic allergic airway inflammation. Groups bearing superscripts (for example a) were significantly (P < 0·05) different from the groups bearing superscripts other than ‘a’ (for example b or c) while the groups with similar superscripts did not differ significantly.
Fig. 3.
Histological scores of perivascular recruitment of inflammatory cells in acute allergic airway inflammation. Groups bearing superscripts (for example a) were significantly (P < 0·05) different from the groups bearing superscripts other than ‘a’ (for example b) while the groups with similar superscripts did not differ significantly.
After 4 weeks of OVA challenges, A/J and BALB/c strains of mice showed similar degree of perivascular inflammation but more than C57BL/6 and C3H/HeJ strains while C57BL/6 and C3H/HeJ did not differ from each other. A/J mice showed more perivascular recruitment of inflammatory cells compared to all other groups after 8 weeks of the challenges (P < 0·05). At 8 weeks of OVA challenge, C3H/HeJ strain had more perivascular inflammation compared to C57BL/6 and C3H/HeJ.
Strain differences in acute airway inflammation
We also examined lung sections from a model of acute airway inflammation (Fig. 3). C57BL/6 mice showed more perivascular inflammation compared to the strain-specific controls while there were no differences between the control and the treated BALB/c mice. Furthermore, the challenged C57BL/6 mice contained more perivascular inflammatory cells compared to the treated BALB/c.
VAP-1 immunohistology
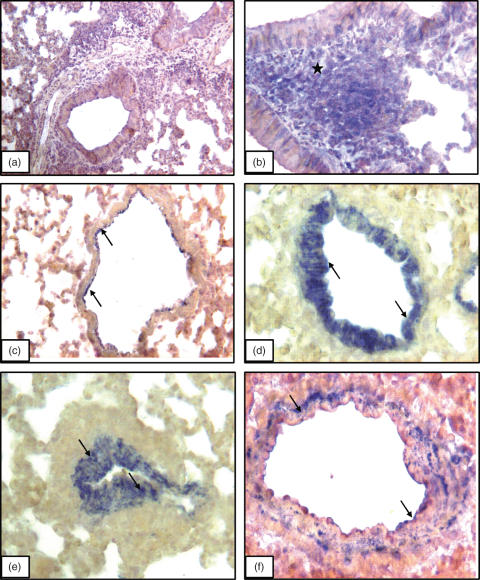
Immunohistology controls comprised of omission of primary antibody (Fig. 4a) and staining with a pan-lymphocyte marker CD3 (Fig. 4b). VAP-1 was localized on the arterial endothelium as well as, in some instances, on basal side of the arterial wall of lung sections from control A/J strain (Fig. 4c) as well as normal mice from other strains (data not shown). Airway epithelium and alveolar septa were negative for VAP-1 (Fig. 4c). A/J strain of mice, compared to BALB/c, C57BL/6 and C3H/HeJ strains, with chronic lung inflammation showed most pronounced increase in VAP-1 staining in pulmonary arteries after 4 weeks (Fig. 4d) and 8 weeks (Fig. 4e) of the challenges. Compared to VAP-1 staining in the normal lungs from A/J strain, VAP-1 antibody stained the whole wall of the arteries in the inflamed lungs (Fig. 4d–e). The staining intensity in the pulmonary arteries of inflamed lungs from BALB/c mice (Fig. 4f), C57BL/6 and C3H/HeJ (data not shown) was much less compared to the A/J strains (Table 1). Occasionally, VAP-1 staining was noticed in peribronchial inflammatory cells as well as basal surface of airway epithelium.
Fig. 4.
VAP-1 immunostaining: Lung section incubated without primary antibody but with secondary antibody (a) show no staining while CD3 antibody stains (asterisks) peribronchial lymphocytes (b). VAP-1 staining (arrows) in lung section from a normal A/J mouse (c) and those challenged for (d) 4 weeks and (e) 8 weeks. (f) Lung section from a BALB/c mouse challenged for 8 weeks and shows VAP-1 staining in the wall of pulmonary artery. Original magnification: a ×10; b, c and e ×20; d and f ×40
Table 1.
Scoring of VAP-1 in staining in lung sections from mice with chronic airway inflammation.
| Pulmonary arteries | Airway epithelium | Alveolar septa | |
|---|---|---|---|
| A/J | |||
| 0 week | + | − | − |
| 4 week | ++/+++ | − | − |
| 8 week | ++/+++ | − | − |
| BALB/c | |||
| 0 week | + | − | − |
| 4 week | +/++ | − | − |
| 8 week | +/++ | − | − |
| C57BL/6 | |||
| 0 week | + | − | − |
| 4 week | + | − | − |
| 8 week | + | − | − |
| C3H/HeJ | |||
| 0 week | + | − | − |
| 4 week | + | − | − |
| 8 week | + | − | − |
Discussion
Because there is scant information on the description and mechanisms of perivascular inflammation in lung diseases [7,8], we have started a systematic study to document this phenomenon in inflamed lungs. Now we report the results from first such study to show that genetic background influences perivascular inflammation in acute or chronic airway diseases. The data also show strain-dependent differences in VAP-1 expression in chronic airway disease.
Airway inflammation is commonly observed in lung diseases such as asthma and is characterized by peribronchial recruitment of inflammatory cells and proliferation of airway smooth muscle cells [6,26,28]. It is intriguing that periarterial accumulation of inflammatory cells in airway inflammation, though commonly observed in histological sections, has not been addressed [8,14,29]. We examined lung sections from four different strains of mice subjected to chronic airway inflammation and two strains with acute airway inflammation. Histological scores revealed significant time and strain-specific differences in periarterial recruitment of inflammatory cells among the strains. The differences in the recruitment of inflammatory cells suggest the effects of genetic background on periarterial inflammation. More interestingly, strain-dependent differences in periarterial cell recruitment parallel those previously observed for airway remodeling and peribronchial recruitment of inflammatory cells in an OVA model of chronic airway disease [17–19]. Currently, the relationship between peribronchial and periarterial recruitment of inflammatory cells remains unknown. However, we observed merging of highly enlarged pools of peribronchial and periarterial inflammatory cells especially in A/J strain. Furthermore, the perivascular recruitment of inflammatory cells in different strains of mice observed in the present work mimics previously reported peribronchial recruitment [17]. It is possible that these peribronchial and periarterial compartments communicate with each other during recruitment of inflammatory cells in lung inflammation.
To develop a better understanding of molecular mechanisms that regulate peri-arterial accumulation of inflammatory cells, we examined expression of VAP-1 in lungs with chronic airway inflammation only. Similar to our previous report [24], VAP-1 was restricted to the endothelium of large blood vessels in the lung. There were no differences among various strains of normal mice in the lung expression of VAP-1. However, there were differences among the mice strains following chronic challenges with OVA. VAP-1 expression was most intense in A/J strain compared to BALB/c, C57B/6 and C3H/HeJ. Interestingly, A/J and BALB/c strain showed similar peri-arterial accumulation of inflammatory cells at 4 weeks of the challenge but A/J mice developed significantly more peri-arterial recruitment at 8 weeks. Therefore, increased vascular expression of VAP-1 in A/J mice may contribute to more perivascular recruitment of inflammatory cells at 8 weeks compared to the other strains. In the OVA-challenged A/J mice, intense VAP-1 staining was observed in the whole wall of pulmonary arteries. Compared to this, the expression of VAP-1 in C3H/HeJ remained unchanged; this strain also did not develop any peri-vascular inflammation following chronic OVA challenges. VAP-1 is a novel amine oxidase expressed on vascular endothelium, which mediates arrest of leucocytes in various inflammatory conditions [22–24]. VAP-1 is normally present in resting blood vessels and its expression is enhanced in inflammation. Compared to other adhesion molecules such as P-selectin, the expression of VAP-1 is sustained over prolonged periods during inflammation, which makes it a highly desirable therapeutic target [24]. Based on differences in VAP-1 expression among the strains, our data show a putative link between expression of VAP-1 and the extent of OVA-induced perivascular inflammation in the lungs.
Typically, migration of inflammatory cells occurs in postcapillary venules in most of the organs but in capillaries in the lungs, and is mediated by the adhesion molecules and chemoattractants expressed by the endothelial and inflammatory cells [5,30–32]. Therefore, accumulation of inflammatory cell around thick-walled blood vessels as observed in our study is intriguing. It is possible that in response to adhesive proteins and chemoattractants, inflammatory cells migrate across the thick wall of the arteries in the inflamed lungs. However, this possibility seems unlikely because we did not observe transmigrating leucocytes in the arterial walls. Inflammatory cells may also infiltrate from peri-arterial microvessels including those in the alveolar septa in the inflamed lungs. In addition to the unresolved question of the route of migration of inflammatory cells, we also do not know their fate. The perivascular cells, recruited in response to OVA challenge, may produce inflammatory mediators, as well as migrate into peribronchial areas to eventually escape into the airways. The recruited cells may die via apoptosis in the perivascular compartment. Because perivascular inflammatory cells represent a large pool of immune cells in inflamed lungs, it is important address their function and fate in future studies.
We conclude that perivascular inflammation, similar to peribronchial inflammation, is an integral component of lung immune response and is influenced by genetic background in acute or chronic lung inflammation induced by OVA. The data also show that intensity but not cellular localization of VAP-1 protein expression was more in A/J mice, which also showed more perivascular inflammation, compared to the other strains in chronic airway inflammation.
Acknowledgments
This study was supported with funding from German Research Foundation (DFG) SFB 587 (Immune reactions of the lung in infection and allergy: Project B1). Dr Singh was a Visiting Scientist supported by the SFB and the Natural Sciences and Engineering Research Council of Canada (Dr Singh). The authors thank Dr M. Salmi and Dr S. Jalkanen for kind gift of VAP-1 antibody and Ms. Karin Westermann for her technical assistance.
References
- 1.Ryan GB, Majno G. Acute inflammation. Am J Pathol. 1977;86:185–274. [PMC free article] [PubMed] [Google Scholar]
- 2.Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol. 2002;14:123–32. doi: 10.1006/smim.2001.0349. [DOI] [PubMed] [Google Scholar]
- 3.Kubes P, Jutila M, Payne D. Therapeutic potential of inhibiting leukocyte rolling in ischemia/reperfusion. J Clin Invest. 1999;95:2510–9. doi: 10.1172/JCI117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuebler WM, Kuhnle GE, Groh J, et al. Leukocyte kinetics in pulmonary microcirculation: intravital fluorescence microscopic study. J Appl Physiol. 1994;76:65–71. doi: 10.1152/jappl.1994.76.1.65. [DOI] [PubMed] [Google Scholar]
- 5.Larsen GL, Holt PG. The concept of airway inflammation. Am J Respir Crit Care Med. 2000;162:S2–S6. doi: 10.1164/ajrccm.162.supplement_1.maic-1. [DOI] [PubMed] [Google Scholar]
- 6.O'Byrne PM, Postma DS. The many faces of airway inflammation: asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:S41–S66. [PubMed] [Google Scholar]
- 7.Pabst R, Tschernig T. Perivascular capillaries in the lung: an important but neglected vascular bed in immune reactions? J Allergy Clin Immunol. 2002;110:209–14. doi: 10.1067/mai.2002.126836. [DOI] [PubMed] [Google Scholar]
- 8.Pabst R. The periarterial space in the lung: its important role in lung edema, transplantation, and microbial or allergic inflammation. Pathobiology. 2004;71:287–94. doi: 10.1159/000081723. [DOI] [PubMed] [Google Scholar]
- 9.Vernooy JH, Dentener MA, van Suylen RJ, et al. Long-term intratracheal lipopolysaccharide exposure in mice results in chronic lung inflammation and persistent pathology. Am J Respir Cell Mol Biol. 2002;26:152–9. doi: 10.1165/ajrcmb.26.1.4652. [DOI] [PubMed] [Google Scholar]
- 10.Barett EG, Wilder JA, March TH, et al. Cigarette smoke-induced airway hyperresponsiveness is not dependent on elevated immunoglobulin and eosinophilic inflammation in a mouse model of allergic airway disease. Am J Resp Crit Care Med. 2002;165:1410–8. doi: 10.1164/rccm.2106029. [DOI] [PubMed] [Google Scholar]
- 11.Toward TJ, Broadley KJ. Goblet cell hyperplasia, airway function and leukocyte infiltration after chronic lipopolysaccharide exposure in conscious guinea pigs. effect of rolipram and dexamethason. J Pharmacol Exp Ther. 2002;302:814–21. doi: 10.1124/jpet.102.033951. [DOI] [PubMed] [Google Scholar]
- 12.Zheng J, Plopper CG, Lakritz J, et al. Leukotoxin-diol: a putative toxic mediator involved in acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2001;25:434–8. doi: 10.1165/ajrcmb.25.4.4104. [DOI] [PubMed] [Google Scholar]
- 13.Papouchado BG, Chapoval SP, Marietta EV, et al. Cockroach allergen-induced eosinophilic airway inflammation in HLA-DQ/human CD4(+) transgenic mice. J Immunol. 2001;167:4627–34. doi: 10.4049/jimmunol.167.8.4627. [DOI] [PubMed] [Google Scholar]
- 14.Saetta M, Baraldo S, Corbino L, et al. CD8+ cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Resp Crit Care Med. 1999;160:711–7. doi: 10.1164/ajrccm.160.2.9812020. [DOI] [PubMed] [Google Scholar]
- 15.Rafi AQ, Zeytun A, Bradley MJ, et al. Evidence for the involvement of Fas ligand and perforin in the induction of vascular leak syndrome. J Immunol. 1998;161:3077–86. [PubMed] [Google Scholar]
- 16.Workman DL, Clancy J., Jr Phenotypic analysis of pulmonary perivascular mononuclear infiltrates that occur as a direct result of acute lethal graft-versus-host disease describes the onset of interstitial pneumonia. Am J Pathol. 1995;147:1350–60. [PMC free article] [PubMed] [Google Scholar]
- 17.Shinagawa K, Kojima M. Mouse model of airway remodeling: Strain differences. Am J Resp Crit Care Med. 2003;168:959–67. doi: 10.1164/rccm.200210-1188OC. [DOI] [PubMed] [Google Scholar]
- 18.Levitt RC, Mitzner W. Expression of airway hyperreactivity to acetylecholine as a simple autosomal recessive trait in mice. FASEB J. 1988;10:2605–8. doi: 10.1096/fasebj.2.10.3384240. [DOI] [PubMed] [Google Scholar]
- 19.Levitt RC, Mitzner W. Autosomal recessive inheritance of airway hyperreactivity to 5-hydroxytryptamine. J Appl Physiol. 1989;67:1125–32. doi: 10.1152/jappl.1989.67.3.1125. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Haczku A, Lee JJ, et al. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol. 2001;281:L394–L402. doi: 10.1152/ajplung.2001.281.2.L394. [DOI] [PubMed] [Google Scholar]
- 21.Burke-Gaffney A, Griffiths M. Adhesion Molecules in Acute Lung Injury. In: Evans TW, Griffiths MJD, Keogh BF, editors. ARDS. Sheffield: European Respiratory Society Monograph; 2002. pp. 83–104. [Google Scholar]
- 22.Tohka S, Laukkanen M, Jalkanen S, et al. Vascular adhesion protein 1 (VAP-1) functions as a molecular brake during granulocyte rolling and mediates recruitment in vivo. FASEB J. 2001;15:373–82. doi: 10.1096/fj.00-0240com. [DOI] [PubMed] [Google Scholar]
- 23.Salmi M, Tohka S, Jalkanen S. Human vascular adhesion protein-1 (VAP-1) plays a critical role in lymphocyte-endothelial cell adhesion cascade under shear. Circ Res. 2000;86:1245–51. doi: 10.1161/01.res.86.12.1245. [DOI] [PubMed] [Google Scholar]
- 24.Singh B, Tschernig T, van Griensven M, et al. Expression of vascular adhesion protein-1 in normal and inflamed mice lungs and normal human lungs. Virchows Arch. 2003;442:491–5. doi: 10.1007/s00428-003-0802-6. [DOI] [PubMed] [Google Scholar]
- 25.Lalor PF, Edwards S, McNab G, et al. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol. 2002;169:983–92. doi: 10.4049/jimmunol.169.2.983. [DOI] [PubMed] [Google Scholar]
- 26.Tomkinson A, Duez C, Lahn M, et al. Adoptive transfer of T cells induces airway hyperresponsiveness independently of airway eosinophilia but in a signal transducer and activator of transcription 6-dependent manner. J Allergy Clin Immunol. 2002;109:810–6. doi: 10.1067/mai.2002.123531. [DOI] [PubMed] [Google Scholar]
- 27.Takeda K, Hamelmann E, Joetham A, et al. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med. 1997;186:449–54. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 29.Korsgren M, Erjefalt JS, Korsgren O, et al. Allergic eosinophil-rich inflammation develops in lungs and airways of B cell-deficient mice. J Exp Med. 1997;185:885–92. doi: 10.1084/jem.185.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doerschuk CM. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation. 2001;8:71–88. [PubMed] [Google Scholar]
- 31.Gahmberg CG, Valmu L, Kotovuori P, et al. Leukocyte integrins in inflammation. Cell Mol Life Sci. 1998;54:549–55. doi: 10.1007/s000180050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel KD, Cuvelier SL, Wiehler S. Selectins: critical mediators of leukocyte recruitment. Semin Immunol. 2002;14:73–81. doi: 10.1006/smim.2001.0344. [DOI] [PubMed] [Google Scholar]