Abstract
Morphine analgesia is mediated principally by the μ-opioid receptor (MOR). Since morphine and other opiates have been shown to influence glucose homeostasis, we investigated the hypothesis of direct cross talk between the MOR and the insulin receptor (IR) signaling cascades. We show that prolonged morphine exposure of cell lines expressing endogenous or transfected MOR, IR, and the insulin substrate 1 (IRS-1) protein specifically desensitizes IR signaling to Akt and ERK cascades. Morphine caused serine phosphorylation of the IR and impaired the formation of the signaling complex among the IR, Shc, and Grb2. Morphine also resulted in IRS-1 phosphorylation at serine 612 and reduced tyrosine phosphorylation at the YMXM p85-binding motifs, weakening the association of the IRS-1/p85 phosphatidylinositol 3-kinase complex. However, the IRS-1/Grb2 complex was unaffected by chronic morphine treatment. These results suggest that morphine attenuates IR signaling to Akt by disrupting the IRS-1-p85 interaction but inhibits signaling to ERK by disruption of the complex among the IR, Shc, and Grb2. Finally, we show that systemic morphine induced IRS-1 phosphorylation at Ser612 in the hypothalamus and hippocampus of wild type, but not MOR knockout, mice. Our results demonstrate that opiates can inhibit insulin signaling through direct cross talk between the downstream signaling pathways of the MOR and the IR.
The clinically useful properties of morphine are often overshadowed by the development of tolerance and dependence following chronic use. The mechanisms of morphine's acute and chronic actions have therefore been the focus of intense research. Mouse gene-targeting studies have confirmed that morphine-induced analgesia and dependence are mediated by μ-opioid receptors (MORs) (40). MOR signaling can be regulated at several levels, namely, receptor homo- and heterodimerization (14), MOR desensitization and trafficking (8, 20, 70), or the downstream signaling pathways (36). It is not fully understood how these different mechanisms regulating receptor signaling are coordinated. The MOR is typically coupled to Gi/o proteins, which inhibit adenylyl cyclase and modulate both inwardly rectifying K+ and voltage-dependent calcium channels (36). Evidence has recently emerged that binding of MOR by agonists, including morphine, leads to the activation of Gβγ- and phosphatidylinositol 3-kinase (PI3K)-dependent signaling cascades (22, 36, 48, 49). These include the stimulation of serine/threonine kinases such as ERK, Akt, and p70S6 kinase (22, 48, 49). However, the functional consequences of MOR activation of ERK or Akt signaling pathways in vivo are unclear.
Activation of the Akt and ERK pathways is typically observed upon mitogenic stimulation of receptor tyrosine kinases (RTKs), including the insulin receptor (IR). Binding of insulin to its receptor leads to activation of IR tyrosine kinase activity and consequent tyrosine phosphorylation of several IR substrates (IRS), including IRS-1 and IRS-2, and the adaptor protein Shc (58, 66, 71). IRS proteins interact with Src homology domain (SH2)-containing proteins such as the p85 subunit of PI3K, Grb2, SHP2, Nck, and others (71). Activation of PI3K leads to stimulation of Akt, which contributes to the stimulation of glucose uptake, glycogen synthesis, and protein synthesis (58, 66, 71). Association of IR with Shc and/or association of IRS with Grb2 and consequent recruitment of SOS and Ras lead to activation of the ERK pathway, resulting in mitogenic effects and changes in gene expression (58, 66, 71). Sustained activation of the IR or stress-activated pathways can result in serine phosphorylation of IRS-1 and consequent attenuation of insulin signaling (77). This mechanism is thought to contribute to acute and chronic insulin resistance.
There is evidence that signaling pathways activated by G-protein-coupled receptors (GPCRs) and RTKs can be highly coordinated (25). One of the most extensively studied cases of such cross-regulation is the transactivation of the epidermal growth factor receptor by different GPCRs, including MOR (5, 25). Cross talk between the IR and GPCRs appears to be bidirectional and complex. For example, insulin attenuates catecholamine actions through tyrosine phosphorylation of the β2-adrenergic receptor (β2AR), diminishing its ability to induce Gs-mediated accumulation of cyclic AMP (32). Insulin also induces Akt-mediated serine phosphorylation of β2AR, and both tyrosine and serine phosphorylations of β2AR contribute to its rapid sequestration (15, 16). Other evidence suggests that GPCR activation can attenuate insulin signaling. Stimulation of β3AR in adipocytes inhibits IR and IRS-1 signaling to PI3K and glucose uptake (35), and in the heart and vascular tissues, two distinct GPCR agonists, angiotensin II and endothelin, inhibit insulin signaling (29, 68).
It is well established that opioids influence glucose homeostasis (18, 19). Early studies documented the hyperglycemic effects of morphine and methadone when administered centrally (18, 23, 30, 56) and suggested that heroin addiction produces a metabolic state similar to that of non-insulin-dependent diabetes mellitus (24). Although morphine and other opiates may act indirectly via the sympathetic nervous system to cause hyperglycemia, the possibility of a direct interaction between opioid and insulin signaling pathways has not been well explored. The present study addressed the question of direct signaling interactions between the MOR and the IR-signaling pathways. Our results demonstrate that morphine stimulates serine phosphorylation of IRS-1 and the IR, resulting in disruption of functional signaling complexes that couple the insulin response to the ERK and Akt pathways.
MATERIALS AND METHODS
Reagents.
DAMGO, morphine, naloxone, and insulin were purchased from Sigma. Puromycin was from Calbiochem. Protein A-agarose was purchased from Roche. Glutathione-Sepharose was from Amersham-Pharmacia. A PolyFect transfection kit was obtained from Qiagen. All of the antibodies used in this study were from Cell Signaling Technology Inc. (Beverly, Mass.), except for the anti-IR, anti-Grb2, and anti-Shc antibodies from Transduction Laboratories (Lexington, Ky.). The protein kinase C (PKC) substrate antibody was raised and characterized as previously described (75). The p85-binding motif antibody was raised and characterized by following essentially the same procedures and assays as for the PKC substrate antibody except for the sequences in the peptide libraries used as antigens. Wild-type IRS-1-hemagglutinin (HA) and F6-IRS-1-HA constructs were kindly provided by Michael J. Quon.
Cell culture.
A stable Chinese hamster ovary cell line expressing the IR and IRS-1 (CHO-IR-IRS-1) was transfected with a DNA construct expressing the HA-tagged ΜΟR cDNA. This cDNA was originally amplified by PCR from an embryonic mouse brain cDNA library and cloned into a PEAK-10 vector (Edge Biosystems). Transfected cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum and puromycin (1.5 μg/ml) and were shown to be responsive to insulin and morphine (measured by Akt and ERK activation), respectively. SK-N-SH human neuroblastoma cells were grown in Dulbecco modified Eagle medium and 10% fetal bovine serum. In a typical experiment, cells were grown in six-well plates for 24 h prior to treatment, washed, and then incubated in serum-free medium overnight prior to ligand stimulation as indicated in the figure legends. Incubation with inhibitors was initiated 30 min (U0126, PD98059) and 5 min (naloxone) prior to ligand stimulation. For desensitization experiments, cells were incubated overnight in serum-free medium before morphine, insulin, and different inhibitors were added to the medium for an additional 1.5 h. The cells were then washed three times in phosphate-buffered saline and subjected to a second ligand stimulus for 5 min. Extracts were then prepared and analyzed by immunoblotting as described below.
Animal experiments.
Male MOR knockout (40) and littermate control mice 10 to 18 weeks old were used for all experiments and housed four per cage with food and water provided ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. Morphine sulfate and naloxone were obtained from the National Institute on Drug Abuse (Bethesda, Md.) drug supply program. Mice were habituated to the injection procedure by daily (subcutaneous [s.c.]) injection for 6 days with saline. On day 7, mice were injected s.c. with either saline or 2 mg of naloxone per kg and then injected s.c. with either saline or 10 mg of morphine per kg 15 min later. Thirty minutes following the morphine injection, mice were sacrificed and their brains were removed. Brains were dissected on ice and immediately frozen at −70°C.
Immunoprecipitation.
Cells were grown in 10-cm-diameter plates for 24 h, serum starved overnight, and treated as indicated in the figure legends. After treatment, cells were scraped into 0.5 ml of lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, protease and phosphatase inhibitors). Brain tissue extracts were prepared in the same buffer with a Polytron homogenizer. Extracts were incubated on ice for 10 min and centrifuged at 14,000 × g for 10 min at 4°C. The supernatant was incubated with the indicated antibodies at 4°C for 2 h. Protein A-agarose was then added, and incubation under the same conditions was continued for 1 h more. Immune complexes were washed three times with lysis buffer, and the pellets were resuspended in 3× sodium dodecyl sulfate (SDS) sample buffer (187.5 mM Tris-HCl [pH 6.8], 6% SDS, 30% glycerol, 150 mM dithiothreitol, 0.03% bromophenol blue) and boiled for 5 min. These samples were then subjected to immunoblotting with the indicated antibodies.
Extract preparation and immunoblotting.
For Western blotting experiments, cell extracts were prepared by lysing the cells immediately after treatment in Laemmli sample buffer and subjected to SDS-10% polyacrylamide gel electrophoresis and immunoblotted as previously described (48, 49). Peptide competition experiments were done with p85-binding motif antibody and PY-100 antibody alone or together with the following peptides at 1 μg/ml: Y608-P (CLHTDDGY[PO3]MPMS), Y608 (CLHTDDGY[PO3]MPMS), and Y891-P (CPKSPGEY[PO3]VNIEFGS).
GST-Grb2 pulldown assay.
Cells grown on 10-cm-diameter plates were lysed in 0.5 ml of lysis buffer. Extracts were incubated on ice for 10 min and centrifuged at 14,000 × g for 10 min at 4°C. Pulldown assays were conducted by adding 2 μg of glutathione S-transferase (GST) fusion protein-Sepharose slurry to 0.5 ml of cell lysate. After 1 h of incubation at 4°C on a rotator, beads were washed three times with lysis buffer, resolved by SDS-polyacrylamide gel electrophoresis, and subjected to immunoblotting.
RESULTS
Morphine and insulin activate similar signaling cascades.
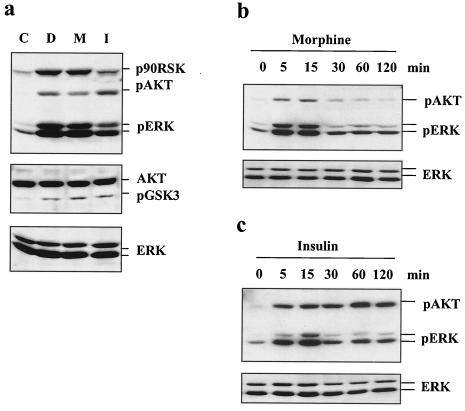
We have shown in previous studies that, similar to the effects of insulin, MOR agonists such as DAMGO and morphine lead to rapid activation of PI3K and ERK signaling cascades (48, 49). This was determined by measuring the enzymatic activities, as well as the phosphorylation status, of Akt, p70S6K, MEK, and ERK (48, 49). In order to study the possible signaling cross talk between the IR and MOR signaling pathways, a construct expressing murine HA-tagged MOR cDNA was stably transfected into a CHO cell line overexpressing the IR and IRS-1 (67). This cell line expressed a number of MORs similar to that of the CHO-MOR cell line we used in earlier studies (data not shown) (48). With this CHO-MOR/IR/IRS-1 cell line, we first confirmed that DAMGO and morphine induced the phosphorylation of ERK and Akt, as well as phosphorylation of their downstream in vivo substrates p90RSK (at T573) and GSK-3β (at S9), respectively (Fig. 1a). Exposure of the CHO-MOR/IR/IRS-1 cells to insulin provoked robust phosphorylation of Akt, ERK, p90RSK, and GSK3β (Fig. 1a). Insulin also activated these cascades in a CHO-MOR cell line not overexpressing the IR and IRS-1 but with less efficacy, presumably because of the lower number of IRs (data not shown). The rapid induction of the Akt and ERK cascades by either morphine (Fig. 1b) or insulin (Fig. 1c) followed similar kinetics, except for the increased duration of phosphorylation of Akt at S473 following insulin (Fig. 1c).
FIG. 1.
Induction of Akt and ERK phosphorylation by morphine and insulin. (a) Cells expressing the IR, IRS-1, and the MOR were left untreated (lane C) or treated with DAMGO (lane D; 1 μM), morphine (M; 1 μM), or insulin (I; 200 nM) for 5 min, extracted, and assayed by Western blotting for levels of phospho-Akt (S473), phospho-p90RSK (T573), phospho-ERK (T202/Y204), and phospho-GSK3β (S9), as well as total Akt and ERK. The time courses of Akt and ERK induction by morphine (1 μM; b) and insulin (200 nM; c) are also shown.
Morphine desensitizes IR signaling.
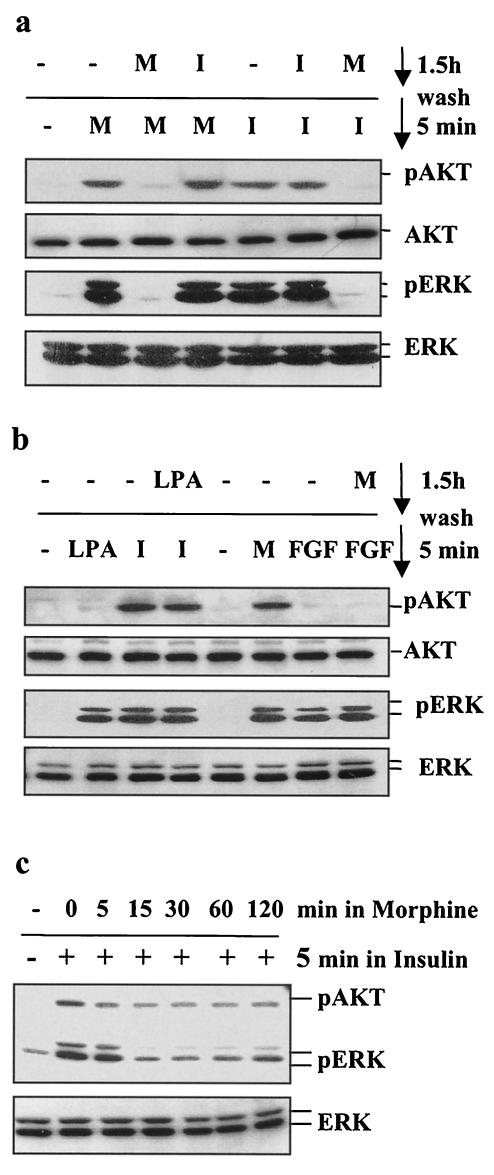
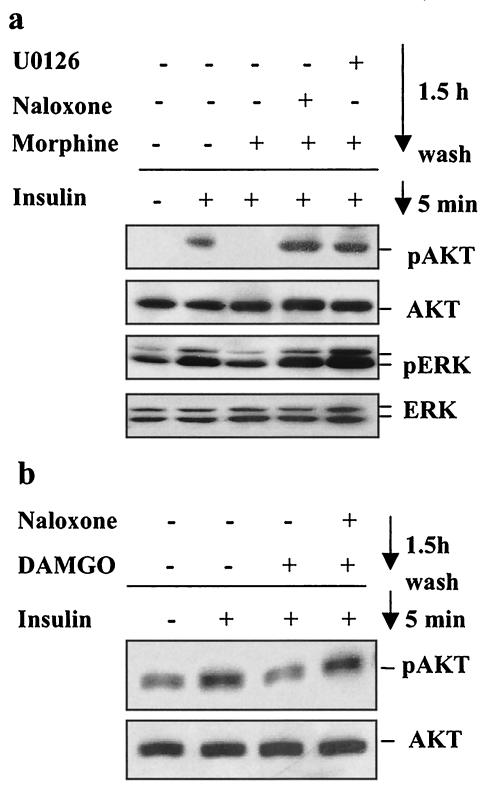
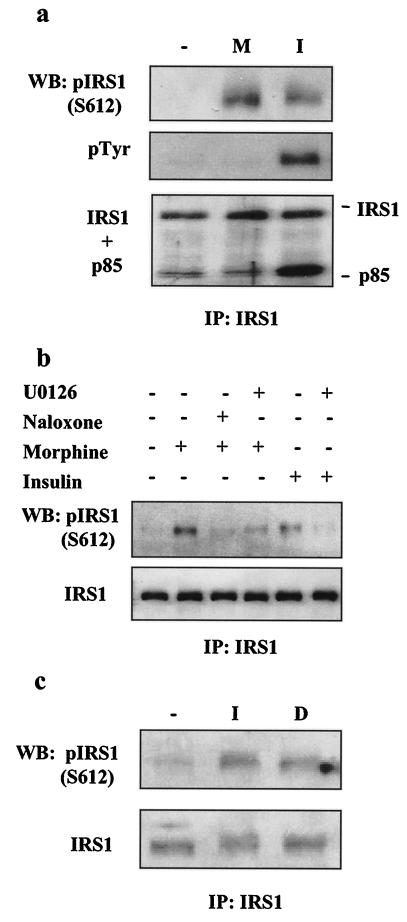
Multiple lines of evidence suggest a functional interaction between MOR and IR signaling cascades: the existence of signaling cross talk between the IR and other GPCRs (29, 31, 35, 68), a recent report indicating that insulin pretreatment of Xenopus oocytes expressing the rat MOR increases the intrinsic efficacy of MOR agonists (41), and the reported effects of opiates on glucose metabolism (18, 19, 23, 24, 30, 56). To examine possible cross talk between MOR and IR signaling, we exposed the CHO-MOR/IR/IRS-1 cells to either morphine or insulin for 1.5 h, washed them three times in serum-free medium, and then exposed them again to either morphine or insulin for 5 min. Figure 2a shows that preincubation of the cells with insulin had little, if any, effect on MOR-induced phosphorylation of Akt and ERK. Compared to cells stimulated with morphine alone, a slight enhancement of the phosphorylated Akt and ERK signal was observed in cells preexposed to insulin. This enhancement is consistent with the results showing that insulin pretreatment enhances MOR activation in Xenopus oocytes (41). Alternatively, this enhancement could be explained by prolonged residual insulin-induced phosphorylation of Akt and ERK (Fig. 1c). In contrast to the weak effects of insulin on MOR signaling, preexposure of the cells to morphine resulted in dramatic modulation of insulin signaling. Incubation of CHO-MOR/IR/IRS-1 cells with 1 μM morphine for 1.5 h completely abolished the ability of insulin to induce the phosphorylation of both Akt and ERK (Fig. 2a). This attenuation of IR signaling was observed as soon as 15 min following morphine exposure (Fig. 2c). In the same cells, ERK activation by bFGF was unaffected by morphine pretreatment (Fig. 2b), indicating that this receptor cross talk does not represent a general effect of morphine exposure on the signaling of all RTKs. Like morphine, ERK phosphorylation can also be induced in CHO-MOR/IR/IRS-1 cells by another Gi-coupled receptor ligand, lysophosphatidic acid (LPA). However, preexposure of the cells to LPA for 1.5 h did not cause desensitization of the insulin response (Fig. 2b). This suggests that MOR modulation of IR signaling cannot be mediated by all GPCRs that couple to inhibitory G proteins. The desensitizing effect of morphine on the insulin response is blocked by the opioid antagonist naloxone, as well as the MEK inhibitors U0126 (Fig. 3a) and PD98059 (data not shown). Similar modulation of IR signaling was observed with other MOR selective agonists, such as DAMGO, as well as in other cell lines expressing endogenous MOR and IR, such as the neuroblastoma cell line SK-N-SH (4, 46) (Fig. 3b).
FIG. 2.
Desensitization of insulin signaling to Akt and ERK by morphine. (a) Cells were treated with morphine (M; 1 μM) or insulin (I; 200 nM) for 1.5 h, washed, subjected to either morphine or insulin for 5 min, and then assayed for levels of phospho-Akt, phospho-ERK, and total Akt and ERK. (b) Cells were treated with morphine (1 μM) or LPA (10 μM) for 1.5 h; washed; exposed to morphine (1 μM), LPA (10 μM), insulin (200 nM), or bFGF (100 ng/ml) for 5 min; and then analyzed for kinase activation. (c) Time course of MOR-mediated desensitization of IR signaling via Akt and ERK. Cells were incubated for various periods of time with 1 μM morphine, washed, subjected to 200 nM insulin for 5 min, and then assayed for phospho-Akt, total Akt, phospho-ERK, and total ERK.
FIG. 3.
Inhibition of the ERK pathway blocks desensitization of insulin signaling by morphine. CHO-MOR/IR/IRS-1 (a) or SK-N-SH (b) cells were serum starved overnight and then pretreated with 1 μM morphine (a) or 200 nM DAMGO (b). Morphine and DAMGO pretreatments were performed in the absence or presence of 10 μM naloxone (a and b) or 10 μM U0126 (a). After 1.5 h, the cells were washed three times with serum-free medium and exposed to 200 nM insulin for 5 min. Extracts were then assayed for levels of phospho-Akt (S473), total Akt, phospho-ERK(T202/Y204), and total ERK.
Morphine induces serine phosphorylation of the IR and IRS-1.
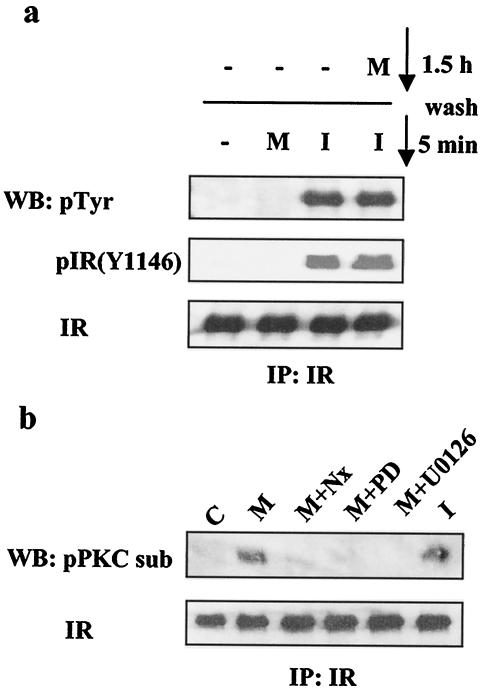
Insulin resistance can be caused by multiple mechanisms, including elevated activity of protein or lipid phosphatases like PTEN, PTP1B, and SHIP2 (9, 58). Insulin resistance can also result from serine/threonine phosphorylation of the IR itself (6, 37, 65) and/or its main substrates, such as the IRS docking proteins (77). Work by several groups has documented the negative effects of serine phosphorylation on IRS-1 function (77), and serine phosphorylation of IRS proteins has been implicated in insulin resistance associated with obesity and trauma (2, 3, 52, 55). In one example, tumor necrosis factor alpha signaling leads to JNK-dependent phosphorylation of rat IRS-1 at S307 and consequent uncoupling of insulin signaling (2, 3, 26, 55). Endothelin, a GPCR ligand, causes similar effects by stimulating the PKC/ERK-mediated IRS-1 phosphorylation at S612 (29, 42). IRS-1 phosphorylation at serines 632, 662, and 731 is induced by platelet-derived growth factor and negatively regulates the induction of an IRS-1-PI3K complex in a mechanism involving a PI3K/Akt/mTOR cascade (38, 54, 64). Other studies have implicated a negative feedback signal involving IRS-1 phosphorylation by PKCζ (53, 57), as well as by other PI3K-dependent kinases leading to insulin-dependent proteosome degradation of IRS-1 (47, 74). We thus investigated the effects of acute and prolonged exposure to morphine on IR and IRS-1 tyrosine and serine phosphorylation. CHO-MOR/IR/IRS-1 cells were either treated with morphine or insulin for 5 min or treated with morphine for 1.5 h prior to stimulation with insulin for 5 min. Cell extracts were prepared, the IR was immunoprecipitated with an IR antibody, and tyrosine and serine phosphorylation status was analyzed by Western blotting (Fig. 4). Stimulation with morphine had no detectable effect on the general tyrosine phosphorylation status of the IR, as measured by a general phosphotyrosine monoclonal antibody (PY-100) or more specifically measured by an antibody directed against the phosphorylated Y1146 residue located in the activation loop of the IR kinase domain (Fig. 4a). This result indicates that MOR stimulation cannot transactivate the IR. Furthermore, prolonged stimulation with morphine did not attenuate the tyrosine phosphorylation of the IR induced by insulin (Fig. 4a). These results and the fact that general tyrosine phosphorylation of IRS-1 is also not diminished following morphine treatment (see Fig. 6) suggest that the IR intrinsic kinase activity stimulated by insulin is not significantly affected by MOR activation. However, since a general phosphotyrosine antibody was used, we cannot rule out possible alteration of the phosphorylation state of individual tyrosine residues in the IR that are involved in the binding of specific adaptor proteins. Reduced phosphorylation of some sites could escape detection in the background of the phosphorylation of other tyrosine residues in the IR when detected with a general phosphotyrosine antibody such as PY-100 (see text below and Fig. 6). Interestingly, both insulin and morphine markedly induced serine phosphorylation of the IR as probed with an antibody designed to detect serine phosphorylation within a motif defined as phospho-S-X-R/K (Fig. 4b) (75). Serine phosphorylation of the IR was blocked by preincubation with either the MOR antagonist naloxone (10 μM) or the MEK inhibitors PD98059 (10 μM) and U0126 (10 μM). This is consistent with the notion that MOR agonists can induce IR phosphorylation at serine residues by kinases that phosphorylate serine in the context of such a motif. Earlier studies suggested that serine phosphorylation of the IR can be mediated by PKCs (6, 37, 65). Our results indicate that a kinase, capable of phosphorylating a motif defined as pS-X-R/K and activated downstream of ERK, is responsible for the serine phosphorylation of the IR induced by morphine. The precise identity of such a kinase remains to be determined.
FIG. 4.
Effect of morphine on IR phosphorylation. (a) CHO-MOR/IR/IRS-1 cells were serum starved overnight, exposed to morphine (M; 1 μM) or insulin (I; 200 nM) for 5 min or exposed to 1 μM morphine for 1.5 h, washed, and then stimulated with 200 nM insulin for 5 min. Cell lysates were subjected to immunoprecipitation with an anti-IR antibody and then assayed for levels of phospho-Tyr (PY-100) (top), phospho-IR (Y1146) (middle), and total IR (bottom). (b) Cells were serum starved overnight and then treated with 200 nM insulin for 5 min or 1 μM morphine for 1.5 h alone or together with naloxone (Nx; 10 μM) or U0126 (10 μM). Cell lysates were subjected to immunoprecipitation (IP) with anti-IR antibody and then analyzed by Western blotting (WB) with the phospho-PKC substrate (sub) and anti-IR antibodies. C, control; PD, PD98059.
FIG. 6.
Effect of morphine on the tyrosine phosphorylation of IRS-1. (a) Specificity of p85-binding motif antibody. NIH 3T3 cells were transfected with wild-type and mutant F6-HA-IRS-1 constructs, starved overnight, and treated with insulin (I; 200 nM) for 5 min. Transfected IRS-1 proteins were immunoprecipitated with anti-HA antibodies and analyzed with p85-binding motif (pYMXM; top), general phosphotyrosine (PY-100; middle), or HA (bottom) antibodies. (b) Cell lysates were prepared as described in the legend to Fig. 4a and then subjected to immunoprecipitation (IP) with IRS-1 antibodies, followed by analysis with phospho-p85-binding motif (pYMXM), phosphotyrosine (pY-100), and IRS-1 antibodies. (c) Cell lysates from panel b were analyzed for levels of phospho-Akt (S473) and total Akt. WB, Western blotting.
We then asked whether acute or prolonged treatment with morphine would result in serine phosphorylation of IRS-1. CHO-MOR/IR/IRS-1 cells were treated with either morphine or insulin for 5 min. Following treatment, extracts were prepared and IRS-1 was immunoprecipitated with IRS-1-specific antibodies, followed by Western blotting to assess the tyrosine phosphorylation state of IRS-1 and phosphorylation at specific serine residues known to affect IRS-1 function. Consistent with the results shown in Fig. 4, morphine did not induce tyrosine phosphorylation of IRS-1 or binding of IRS-1 to the p85 subunit of PI3K in CHO-MOR/IR/IRS-1 cells (Fig. 5a, middle and bottom). However, Fig. 5a shows that, like insulin treatment (11, 12), acute treatment with morphine induces IRS-1 phosphorylation at S612. Similarly, prolonged stimulation with morphine (1.5 h) led to IRS-1 phosphorylation at S612 (Fig. 5b). This phosphorylation could be fully reversed by naloxone (10 μM) (Fig. 5b) and the MEK inhibitors U0126 (10 μM; Fig. 5b) and PD98059 (10 μM; data not shown). Also, in SK-N-SH cells, acute treatment with insulin or prolonged treatment (1.5 h) with the MOR selective agonist DAMGO induced IRS-1 phosphorylation at S612 (Fig. 5c). These results indicate that the ERK cascade likely mediates MOR-induced IRS-1 phosphorylation at S612. IR-induced tyrosine phosphorylation of IRS-1 creates active binding sites for various SH2 domain-containing proteins, including p85, the regulatory subunit of PI3K. Studies on the effects of IRS-1 phosphorylation at S612 have shown that this modification contributes to the diminished activation of PI3K (12, 38), which ultimately results in diminished activation of Akt. Morphine-induced serine phosphorylation of IRS-1 could therefore have a role in the attenuation of the insulin response, as shown in Fig. 2 and 3.
FIG. 5.
Serine phosphorylation of IRS-1 by morphine. CHO-MOR/IR/IRS-1 (a and b) or SK-N-SH (c) cells were serum starved overnight and then stimulated with morphine (M; 1 μM) or insulin (I; 200 nM) for 5 min (a), with morphine alone or in the absence or presence of naloxone (10 μM) or U0126 (10 μM) for 1.5 h; or with insulin for 5 min, with or without preincubation with U0126 (10 μM) (b); and with insulin for 5 min or with 200 nM DAMGO (D) for 1.5 h (c). Cell lysates then were immunoprecipitated (IP) with IRS-1 antibodies and analyzed with phospho-IRS1 (S612), phosphotyrosine (PY-100), PI3-kinase p85, and IRS-1 antibodies. WB, Western blot.
Prolonged morphine exposure disrupts the complex between IRS-1 and p85 PI3K but not that between IRS-1 and Grb2.
We next investigated whether prolonged exposure to morphine affects insulin-induced tyrosine phosphorylation of IRS-1 and the integrity of the IR-IRS-1 complex. IRS-1 couples the insulin response to different pathways, including the Akt and ERK pathways. Upon IR-mediated phosphorylation, specific tyrosine residues in IRS-1 become docking sites for other adaptors and effector molecules, such as p85, the regulatory subunit of PI3K, and Grb2. To directly probe the tyrosine phosphorylation of IRS-1 at the p85-binding site, we raised a novel phosphospecific antibody that recognizes phosphotyrosine only in the context of the SH2 domain p85-binding motif, phospho-YMXM (where X indicates any amino acid). We prepared this antibody with a peptide library containing this motif by following principles we have described recently (75). The specificity of this antibody was assessed with an HA-tagged IRS-1 mutant construct, IRS-1-F6, with six phenylalanine residues substituted for Y465, Y612, Y632, Y662, Y989, and Y941 in YMXM motifs of human IRS-1 (corresponding to Y460, Y608, Y628, Y658, Y935, and Y983 in mouse IRS-1) (17). NIH 3T3 cells were transfected with either the IRS-1-F6 construct or a wild-type HA-IRS-1 construct and then stimulated with insulin for 15 min. Epitope-tagged IRS-1 proteins were immunoprecipitated with an anti-HA antibody and analyzed by Western blotting with antibodies directed against the p85-binding motif (pYMXM), phosphotyrosine (PY-100), or the HA epitope. Figure 6a shows that following insulin treatment, the p85-binding motif antibody and the general phosphotyrosine antibody, PY-100, detect wild-type IRS-1. In contrast, the IRS-1-F6 protein was not detected by the p85-binding motif antibody following insulin treatment although residual tyrosine phosphorylation was still detected by PY-100, presumably because of phosphorylated tyrosines not embedded in the YMXM motif (Fig. 6a, middle). To further establish the specificity of the antibody, competition experiments were performed with a phosphorylated peptide containing the sequence corresponding to the p85-binding site Y608 in mouse IRS-1. As controls, the unphosphorylated peptide with the same Y608 sequence or a phosphorylated peptide containing Grb2-binding site Y891 were used (data not shown). These experiments demonstrated that the p85-binding motif antibody had no general phosphotyrosine immunoreactivity but reacted specifically with phosphorylated tyrosine in the context of a YMXM sequence.
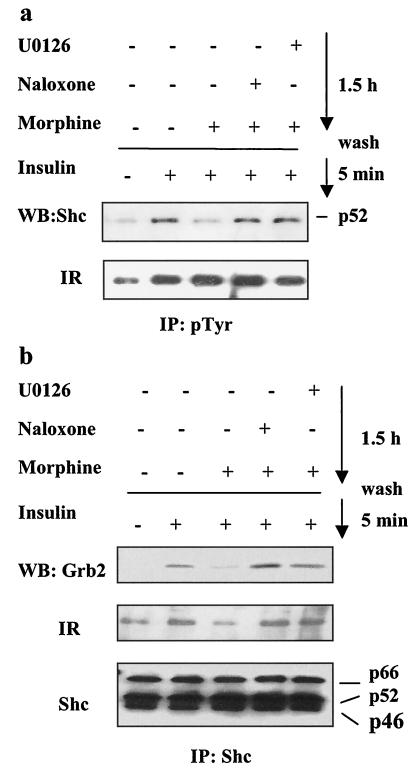
To determine the regulation of the p85-binding motif, CHO-MOR/IR/IRS-1 cells were treated with morphine or insulin acutely or with morphine for 1.5 h and then stimulated with insulin. Cell extracts were then immunoprecipitated with IRS-1 antibodies and analyzed by Western blotting as described in the legend to Fig. 6b. Receptor activation was confirmed by induction of Akt phosphorylation (Fig. 6c). Although morphine pretreatment did not reduce the general phosphotyrosine content of IRS-1 (Fig. 6b, middle), morphine pretreatment caused a significant reduction in the tyrosine phosphorylation of IRS-1 at the p85-binding motif pYMXM (Fig. 6b, top). Similar results were obtained with SK-N-SH cells (Fig. 7c). These results underscore the importance of assessing the phosphorylation state of specific tyrosine residues and their respective docking proteins to obtain more precise data on pathway activation. Consistent with the reduction in phosphorylation at the pYMXM motif, prolonged exposure to morphine significantly reduced the amounts of the IR and p85 pulled down with an IRS-1 antibody in either CHO-MOR/IR/IRS-1 cells or SK-N-SH neuroblastoma cells (Fig. 7a and c, respectively). This effect was blocked by naloxone (10 μM) or the MEK inhibitor U0126 (10 μM). Morphine pretreatment, however, had no effect on the association between IRS-1 and Grb2 (Fig. 7b), the SH2 domain-containing adaptor protein that typically connects IRS-1 to the Ras/ERK cascade (43, 62, 63). This was consistent with our peptide competition results indicating that IRS-1 phosphorylation at Grb2-binding sites remained intact upon morphine exposure (data not shown). Disruption of the IR/IRS-1/p85 complex could explain a mechanism for morphine desensitization of the insulin-mediated activation of Akt (by disruption of the IRS-1-p85 complex) but cannot entirely account for morphine attenuation of insulin signaling via ERK (Fig. 2 and 3).
FIG. 7.
Effect of morphine on IRS-1, IR, PI3K, and Grb2 interactions. CHO-MOR/IR/IRS-1 (a) or SK-N-SH (c) cells were treated with morphine or DAMGO, respectively. Cell lysates were then prepared as described in the legend to Fig. 4a, and IRS-1 was immunoprecipitated with IRS-1 antibodies, followed by analysis with IR, p85 PI3-kinase, p85-binding motif, and IRS-1 antibodies. (b) Cell lysates were prepared as described in the legend to Fig. 4a, and IRS-1 was immunoprecipitated (IP) with IRS-1 antibodies and analyzed with anti-Grb2 and IRS-1 antibodies. The same cell lysates were analyzed for levels of phospho-ERK and total ERK (bottom). WB, Western blotting; M, morphine; I, insulin.
Prolonged morphine exposure disrupts the complex among IR, Shc, and Grb2.
IR activation leads to tyrosine phosphorylation of the phosphotyrosine-binding (PTB) domains in both IRS-1 and Shc, and Grb2 subsequently binds to both docking proteins (33, 71, 72). The Shc-Grb2-Sos complex formed upon Shc phosphorylation may constitute the dominant pathway coupling the IR to the Ras/ERK-mediated mitogenic effects of insulin (59). Because morphine treatment did not disrupt the complex between IRS-1 and Grb2 (Fig. 7b), we asked whether morphine interferes with the tyrosine phosphorylation of Shc and with the interaction among Shc, the IR, and Grb2. CHO-MOR/IR/IRS-1 cells were exposed to morphine alone or in the presence of naloxone or U0126 for 1.5 h and subsequently stimulated with insulin for 5 min. To assess tyrosine phosphorylation of Shc, we prepared cell extracts, immunoprecipitated them with either PY-100 or Shc antibodies, and then subjected them to Western blotting with Shc or IR antibodies. Figure 8a shows that preincubation with morphine for 1.5 h attenuated the tyrosine phosphorylation of Shc by insulin, an effect that could be blocked by naloxone and U0126. In contrast, tyrosine phosphorylation of the IR was not reduced by naloxone and U0126. We then tested the levels of the IR and Grb2 coimmunoprecipitating with Shc. As anticipated, insulin induced the formation of a complex containing the IR, Shc, and Grb2, and morphine preincubation reduced the levels of the IR and Grb2 coimmunoprecipitated by the Shc-specific antibody (Fig. 8b). The effect of morphine was blocked by naloxone (10 μM) or U0126 (10 μM). To confirm this result, we tested the ability of a GST-Grb2 fusion protein to pull down the IR, Shc, or IRS-1 from extracts of cells stimulated with insulin alone or exposed to morphine prior to insulin stimulation. GST-Grb2 was able to efficiently pull down the IR, Shc, and IRS-1 from extracts of cells stimulated with insulin alone. However, preincubation with morphine reduced the levels of the IR and Shc, but not that of IRS-1, pulled down by GST-Grb2 (data not shown). Together, these results support the hypothesis that morphine pretreatment prevents the formation of an active complex among the IR, Shc, and Grb2 but does not affect the interaction between IRS-1 and Grb2 (Fig. 7). Thus, the effect of morphine on the IR-Shc interaction is not due to the impairment of the IR intrinsic kinase activity (Fig. 4) but instead is likely a result of morphine-stimulated phosphorylation of IR serine residues (Fig. 4b) that reduce the docking and phosphorylation of Shc (Fig. 8). Morphine-stimulated phosphorylation of IR-specific serine residues may also explain the attenuated tyrosine phosphorylation of IRS-1 at YMXM motifs, thus reducing IRS-1 interaction with p85 but not with Grb2 (Fig. 6 and 7).
FIG. 8.
Effect of morphine on Shc, IR, and Grb2 interactions. (a) Cell lysates were prepared as described in the legend to Fig. 4b. The lysates were immunoprecipitated with phosphotyrosine antibodies (PY-100) and then analyzed with anti-Shc and anti-IR antibodies. (b) The same cell lysates as in panel a were immunoprecipitated (IP) with anti-Shc antibody and then analyzed with Grb2, IR, and Shc antibodies. WB, Western blotting.
Morphine induces IRS-1 phosphorylation at S612 in discrete brain regions.
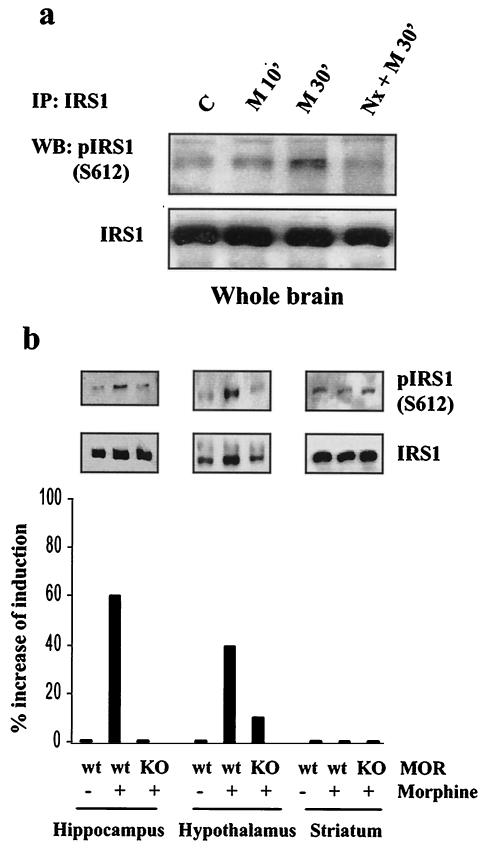
To assess the physiological relevance of our findings, we explored whether acute administration of morphine to mice induces serine phosphorylation of IRS-1 at the S612 site as determined in cell culture. It has been documented that the MOR mediates most of the analgesic effects of morphine (40). However, since morphine can activate the δ- and κ-opioid receptors, we compared the effects of morphine administration in wild-type and MOR knockout mice. Mice were habituated to the injection procedure by being injected s.c. daily for 6 days with saline. On day 7, mice were injected s.c. with either saline or naloxone and then injected s.c. with either saline or morphine at 10 mg/kg 15 min later. Thirty minutes later, mice were sacrificed and their brains were removed. The brains were dissected on ice and immediately frozen at −70°C. The whole brains, hypothalamus, hippocampus, and striatum were homogenized in lysis buffer. These brain regions were selected because of the relative abundance of MOR and IRS-1 expression (21). Tissue extracts were used to immunoprecipitate IRS-1 as described previously, followed by Western blotting with total and phospho-S612 IRS-1 antibodies. Increased IRS-1 phosphorylation at S612 in whole brain extracts was observed following acute morphine administration, and this effect could be blocked by naloxone (Fig. 9a). Furthermore, morphine-induced IRS-1 phosphorylation at S612 was observed in the hypothalamus and hippocampus of wild-type but not MOR knockout mice (Fig. 9b). No induction of IRS-1 phosphorylation could be detected in the striatum despite a high abundance of IRS-1 (Fig. 9b, bottom) and the MOR. This demonstrates MOR modulation of IRS-1 serine phosphorylation in specific brain areas.
FIG. 9.
Morphine induces IRS-1 phosphorylation at S612 in the mouse brain. Mice were injected s.c. with either saline or 2 mg of naloxone (Nx) per kg, followed by s.c. injection of either saline or 10 mg of morphine (M) per kg 15 min later as described in Materials and Methods. Extracts of whole brains from wild-type mice (a) or hypothalamus, hippocampus, and striatum tissues from wild type (wt) or MOR knockout (KO) mice (b) were prepared, and IRS-1 was immunoprecipitated (IP) with an IRS-1 antibody, followed by analysis with anti-IRS-1 or phospho-IRS1 S612 antibodies. Histograms represent the average percent induction of IRS-1 S612 phosphorylation of two independent experiments normalized for total immunoprecipitated IRS-1. WB, Western blotting; C, control.
DISCUSSION
This study demonstrates unidirectional cross talk between MOR and IR signaling whereby opiate agonists like morphine activate MOR signaling cascades that lead to an insulin-resistant state. Attenuation of insulin signaling was measured by the lack of insulin-induced activation of both Akt and ERK cascades in transfected CHO (CHO-MOR/IR/IRS-1) cells and in neuroblastoma cells endogenously expressing MOR and IR (SK-N-SH cells). The specificity of the cross talk between the MOR and the IR was established by a lack of MOR modulation of another RTK signaling pathway (bFGF) and by the fact that activation of a different Gi-coupled receptor (the LPA receptor) did not desensitize IR signaling. Unlike the reported transactivation of the epidermal growth factor receptor by opioid receptor ligands (5), morphine did not modulate IR tyrosine phosphorylation and activity. The desensitizing effects of morphine on insulin signaling were mediated by the ERK signaling cascade, as they were effectively abolished by both MEK1/2 inhibitors in a fashion similar to the homologous desensitization of the MOR (48, 60). Our results also suggest that the mechanism underlying the desensitization of insulin signaling involves increased serine phosphorylation of both the IR and IRS-1, resulting in uncoupling of the IR from its major adaptor signaling complexes. In the case of IRS-1, we demonstrate that morphine induced phosphorylation at Ser612 via an ERK-dependent pathway. Accordingly, morphine pretreatment had a profound effect on IRS-1 function: it diminished tyrosine phosphorylation at the p85-binding motif YMXM sites and the binding of p85 and the IR to IRS-1 without detectable alteration of phosphorylation at other tyrosine residues. Our preliminary results indicate that morphine stimulates the phosphorylation of other serine residues in IRS-1, but the effect of these phosphorylation events on insulin signaling is not clear. Morphine-dependent IRS-1 phosphorylation at S612 is reminiscent of endothelin-induced IRS-1 phosphorylation at S612 via a PKC/ERK-dependent pathway (38). Because of their close proximity, phosphorylation of S612 may hinder the binding of p85 to Y608 (12), one of the major p85-binding sites in mouse IRS-1. A different study demonstrated the role of the phosphorylation of serine residues adjacent to YMXM motifs in the negative regulation of PI3K and Akt (13). MOR-induced reduction of YMXM motif phosphorylation and the reduced binding of p85 to IRS-1 are consistent with an attenuated Akt response. Our results support the premise that phosphorylation at S612 leads to the dissociation of IRS-1 from IR and p85, the regulatory subunit of PI3K. MOR activation most likely affects the binding of only a subset of IRS-1 binding partners containing an SH2 domain, as evidenced by the lack of modulation of the interaction between IRS-1 and Grb2 by morphine. Whether morphine stimulation also disrupts the association of IRS-1 with other binding partners, such as Nck, Crk, Fyn, and SHP-2, remains unanswered.
How does morphine desensitize insulin signaling to ERK? Following insulin stimulation, the IRS-1 and Shc adaptor proteins bind the IR through their PTB domains. IRS-1 and Shc have been shown to bind Grb2, relaying the insulin signal to the Ras/ERK cascade (43, 62, 63). However, the relative contribution of either Shc or IRS-1 to ERK activation has not been fully characterized and may differ from one cell type to another. A recent report suggested that the Shc/Grb2 complex, rather than IRS-1/Grb2, may actually be the adaptor system predominantly transmitting insulin signaling to the ERK pathway (59). In our system, the interaction between IRS-1 and Grb2 remained intact following MOR activation. This observation and the fact that morphine induced serine phosphorylation of the IR prompted us to test the integrity of the complex among the IR, Shc, and Grb2. Our analysis of IR-Shc-Grb2 complexes demonstrated that morphine pretreatment effectively prevented the association of Shc with the IR and that of Shc with Grb2. This result indicates that disruption of the IR/Shc/Grb2 complexes plays a major role in the desensitization of insulin signaling to the ERK pathway following MOR activation.
Several studies have reported a role for PKC phosphorylation in the inhibition of IR kinase activity (6, 37, 65). One of these studies postulated S994 as a possible phosphate acceptor site for PKCs (65). Ser994 in the human IR is in close proximity to Y999, a putative Shc PTB site, although it is not contained within a prototypical PKC site. Morphine had little effect on IR kinase activity; at least as measured by autophosphorylation of the IR and tyrosine phosphorylation of IRS-1. However, morphine induced marked serine phosphorylation of the IR, as demonstrated by an antibody that detects phosphorylation of consensus “PKC motifs” (75). These motifs, namely, phospho-S-X-R/K, are found within the IR sequence in close proximity to serine residues 727 and 1064. Serine phosphorylation of these IR residues may hinder the binding and phosphorylation of Shc, thus preventing the formation of an active complex with Grb2. The reduced binding of Shc to the IR and the diminished tyrosine phosphorylation of Shc induced by morphine support this hypothesis. In future studies, it will be important to determine the precise IR serine phosphorylation site(s) following morphine or insulin stimulation and identify the relevant kinase(s) downstream from ERK involved in this event. Another layer of regulation that fits well with our observations involves potential modulation of Shc interaction with CEACAM1. CEACAM1 is tyrosine phosphorylated by the IR, and upon binding to Shc, this results in sequestration and uncoupling of the IR from the Ras/ERK pathway (50). Phosphorylation of CEACAM1 at S503 enhances this inhibitory activity (50). It is tempting to speculate that a mechanism by which morphine, or perhaps other stimuli, could enhance insulin resistance may involve CEACAM1 phosphorylation of S503 (51).
This report demonstrates that MOR agonists can dramatically modulate insulin signaling, at least at the level of the Akt and ERK pathways. Further studies aimed at exploring the direct interaction between opioid and insulin signaling systems are critical to determining whether the hyperglycemic effects of opiates can be explained by receptor cross talk. Where may a physiologically relevant interaction between insulin and opioid receptor signaling occur in vivo? IRs are expressed in most tissues of the body, including “insulin-insensitive” tissues such as the brain (21), and the IR, IRS-1, and the MOR are abundantly expressed in many brain areas. Following systemic morphine administration, we observed increased IRS-1 phosphorylation at S612 in the hypothalamus and hippocampus of wild-type but not MOR knockout mice. IRS-1 phosphorylation at this site was blocked by naloxone. These results indicate that the effect of morphine on IRS-1 phosphorylation in vivo was mediated by the MOR and not by other opioid receptors. These results also support the physiological relevance of our results obtained with cell culture systems. It is noteworthy that morphine-induced IRS-1 phosphorylation occurred in discrete brain regions such as the hypothalamus and hippocampus but not in the striatum, where there is also abundant MOR and IRS-1 expression. It remains to be determined whether the MOR and the IR or IRS-1 have the same cellular localization in different brain regions. It has recently been proposed that the IR, together with leptin signaling, activates IRS-1and PI3K cascades in the arcuate nucleus of the hypothalamus, thereby contributing to the control of energy homeostasis (45). Inhibition of PI3K activity in the hypothalamus reverses the food intake-lowering effects of insulin (44). An elegant study in which the gene for the IR was specifically disrupted in neurons showed that mutant mice displayed diet-sensitive obesity, with increases in body fat and plasma leptin levels, mild insulin resistance, elevated insulin levels and hypertriglyceridemia (7). Interestingly, early studies documented that opiate antagonists like naloxone and naltrexone significantly reduced food consumption in both genetically and dietarily obese animals, leading to the concept that endogenous opioid peptides have a role in regulating food intake (27, 39, 61). A more detailed analysis of the effects of morphine on hypothalamic insulin signaling is required to better understand how these two systems interact and how this interaction affects energy metabolism.
Opiates may also modulate insulin-like growth factorI receptor signaling through serine phosphorylation of IRS-1, thus neutralizing the neuroprotective and neurogenic effects of insulin-like growth factor I on hippocampal neurons (1, 76). This could have important implications when opiate drugs are used chronically. Finally, the presence of opioid peptides and opioid receptors in the pancreas, as well as the influence of β-endorphin on endocrine pancreas function, has been reported (10, 28, 34). Whether morphine has any effect on pancreatic islets through the MOR remains controversial (28, 69). Nonetheless, these observations raise overall the possibility that endogenous opioids modulate insulin gene expression and secretion through inhibition of IR signaling in islet β cells (73). The use of MOR and IR knockout mice may help confirm our hypothesis that signaling triggered by activation of these receptors and their physiological functions are regulated in a coordinated fashion.
Acknowledgments
We thank Sandra Schieferl for technical assistance. HA-tagged IRS-1 wild-type and mutant constructs and the SK-N-SH neuronal cell line were provided by Michael J. Quon and Lakshmi Devi, respectively. We also thank Morris Birnbaum and Al Moritz for critical reading of the manuscript. We specially thank Michael Comb for encouragement and helpful discussions.
REFERENCES
- 1.Aberg, M. A., N. D. Aberg, H. Hedbacker, J. Oscarsson, and P. S. Eriksson. 2000. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 20:2896-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre, V., T. Uchida, L. Yenush, R. Davis, and M. F. White. 2000. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J. Biol. Chem. 275:9047-9054. [DOI] [PubMed] [Google Scholar]
- 3.Aguirre, V., E. D. Werner, J. Giraud, Y. H. Lee, S. E. Shoelson, and M. F. White. 2002. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 277:1531-1537. [DOI] [PubMed] [Google Scholar]
- 4.Baumhaker, Y., M. Gafni, O. Keren, and Y. Sarne. 1993. Selective and interactive down-regulation of μ- and δ-opioid receptors in human neuroblastoma SK-N-SH cells. Mol. Pharmacol. 44:461-467. [PubMed] [Google Scholar]
- 5.Belcheva, M. M., M. Szucs, D. Wang, W. Sadee, and C. J. Coscia. 2001. μ-Opioid receptor-mediated ERK activation involves calmodulin-dependent epidermal growth factor receptor transactivation. J. Biol. Chem. 276:33847-33853. [DOI] [PubMed] [Google Scholar]
- 6.Bollag, G. E., R. A. Roth, J. Beaudoin, D. Mochly-Rosen, and D. E. Koshland, Jr. 1986. Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc. Natl. Acad. Sci. USA 83:5822-5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruning, J. C., D. Gautam, D. J. Burks, J. Gillette, M. Schubert, P. C. Orban, R. Klein, W. Krone, D. Muller-Wieland, and C. R. Kahn. 2000. Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122-2125. [DOI] [PubMed] [Google Scholar]
- 8.Chavkin, C., J. P. McLaughlin, and J. P. Celver. 2001. Regulation of opioid receptor function by chronic agonist exposure: constitutive activity and desensitization. Mol. Pharmacol. 60:20-25. [DOI] [PubMed] [Google Scholar]
- 9.Clement, S., U. Krause, F. Desmedt, J. F. Tanti, J. Behrends, X. Pesesse, T. Sasaki, J. Penninger, M. Doherty, W. Malaisse, J. E. Dumont, Y. Marchand-Brustel, C. Erneux, L. Hue, and S. Schurmans. 2001. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature 409:92-97. [DOI] [PubMed] [Google Scholar]
- 10.Curry, D. L., L. L. Bennett, and C. H. Li. 1987. Stimulation of insulin secretion by β-endorphins (1-27 & 1-31). Life Sci. 40:2053-2058. [DOI] [PubMed] [Google Scholar]
- 11.De Fea, K., and R. A. Roth. 1997. Modulation of insulin receptor substrate-1 tyrosine phosphorylation and function by mitogen-activated protein kinase. J. Biol. Chem. 272:31400-31406. [DOI] [PubMed] [Google Scholar]
- 12.De Fea, K., and R. A. Roth. 1997. Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry 36:12939-12947. [DOI] [PubMed] [Google Scholar]
- 13.Delahaye, L., I. Mothe-Satney, M. G. Myers, M. F. White, and E. Van Obberghen. 1998. Interaction of insulin receptor substrate-1 (IRS-1) with phosphatidylinositol 3-kinase: effect of substitution of serine for alanine in potential IRS-1 serine phosphorylation sites. Endocrinology 139:4911-4919. [DOI] [PubMed] [Google Scholar]
- 14.Devi, L. A. 2001. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol. Sci. 22:532-537. [DOI] [PubMed] [Google Scholar]
- 15.Doronin, S., E. Shumay, H. H. Wang, and C. C. Malbon. 2002. Akt mediates sequestration of the b2-adrenergic receptor in response to insulin. J. Biol. Chem. 277:15124-15131. [DOI] [PubMed] [Google Scholar]
- 16.Doronin, S., H. H. Wang, and C. C. Malbon. 2002. Insulin stimulates phosphorylation of the b2-adrenergic receptor by the insulin receptor, creating a potent feedback inhibitor of its tyrosine kinase. J. Biol. Chem. 277:10698-10703. [DOI] [PubMed] [Google Scholar]
- 17.Esposito, D. L., Y. Li, A. Cama, and M. J. Quon. 2001. Tyr612 and Tyr632 in human insulin receptor substrate-1 are important for full activation of insulin-stimulated phosphatidylinositol 3-kinase activity and translocation of GLUT4 in adipose cells. Endocrinology 142:2833-2840. [DOI] [PubMed] [Google Scholar]
- 18.Feldberg, W., and K. P. Gupta. 1974. Morphine hyperglycaemia. J. Physiol. 238:487-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman, M., R. S. Kiser, R. H. Unger, and C. H. Li. 1983. Beta-endorphin and the endocrine pancreas. Studies in healthy and diabetic human beings. N. Engl. J. Med. 308:349-353. [DOI] [PubMed] [Google Scholar]
- 20.Finn, A. K., and J. L. Whistler. 2001. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron 32:829-839. [DOI] [PubMed] [Google Scholar]
- 21.Folli, F., L. Bonfanti, E. Renard, C. R. Kahn, and A. Merighi. 1994. Insulin receptor substrate-1 (IRS-1) distribution in the rat central nervous system. J. Neurosci. 14:6412-6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda, K., S. Kato, H. Morikawa, T. Shoda, and K. Mori. 1996. Functional coupling of the delta-, mu-, and kappa-opioid receptors to mitogen-activated protein kinase and arachidonate release in Chinese hamster ovary cells. J. Neurochem. 67:1309-1316. [DOI] [PubMed] [Google Scholar]
- 23.Giugliano, D. 1984. Morphine, opioid peptides, and pancreatic islet function. Diabetes Care 7:92-98. [DOI] [PubMed] [Google Scholar]
- 24.Giugliano, D., A. Ceriello, A. Quatraro, and F. D'Onofrio. 1985. Endogenous opiates, heroin addiction, and non-insulin-dependent diabetes.Lancet ii:769-770. [DOI] [PubMed] [Google Scholar]
- 25.Gschwind, A., E. Zwick, N. Prenzel, M. Leserer, and A. Ullrich. 2001. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene 20:1594-1600. [DOI] [PubMed] [Google Scholar]
- 26.Hirosumi, J., G. Tuncman, L. Chang, C. Z. Gorgun, K. T. Uysal, K. Maeda, M. Karin, and G. S. Hotamisligil. 2002. A central role for JNK in obesity and insulin resistance. Nature 420:333-336. [DOI] [PubMed] [Google Scholar]
- 27.Holtzman, S. G. 1975. Effects of narcotic antagonists on fluid intake in the rat. Life Sci. 16:1465-1470. [DOI] [PubMed] [Google Scholar]
- 28.Ipp, E., R. Dobbs, and R. H. Unger. 1978. Morphine and beta-endorphin influence the secretion of the endocrine pancreas. Nature 276:190-191. [DOI] [PubMed] [Google Scholar]
- 29.Jiang, Z. Y., Q. L. Zhou, A. Chatterjee, E. P. Feener, M. G. Myers, Jr., M. F. White, and G. L. King. 1999. Endothelin-1 modulates insulin signaling through phosphatidylinositol 3-kinase pathway in vascular smooth muscle cells. Diabetes 48:1120-1130. [DOI] [PubMed] [Google Scholar]
- 30.Johansen, O., T. Tonnesen, T. Jensen, R. Jorde, P. G. Burhol, and O. Reikeras. 1992. Increments in glucose, glucagon and insulin after morphine in rats, and naloxone blocking of this effect. Life Sci. 51:1237-1242. [DOI] [PubMed] [Google Scholar]
- 31.Karoor, V., and C. C. Malbon. 1998. G-protein-linked receptors as substrates for tyrosine kinases: cross-talk in signaling. Adv. Pharmacol. 42:425-428. [DOI] [PubMed] [Google Scholar]
- 32.Karoor, V., L. Wang, H. Y. Wang, and C. C. Malbon. 1998. Insulin stimulates sequestration of beta-adrenergic receptors and enhanced association of beta-adrenergic receptors with Grb2 via tyrosine 350. J. Biol. Chem. 273:33035-33041. [DOI] [PubMed] [Google Scholar]
- 33.Kasus-Jacobi, A., D. Perdereau, S. Tartare-Deckert, E. Van Obberghen, J. Girard, and A. F. Burnol. 1997. Evidence for a direct interaction between insulin receptor substrate-1 and Shc. J. Biol. Chem. 272:17166-17170. [DOI] [PubMed] [Google Scholar]
- 34.Khawaja, X. Z., I. C. Green, J. R. Thorpe, and M. A. Titheradge. 1990. The occurrence and receptor specificity of endogenous opioid peptides within the pancreas and liver of the rat. Comparison with brain. Biochem. J. 267:233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein, J., M. Fasshauer, M. Ito, B. B. Lowell, M. Benito, and C. R. Kahn. 1999. β3-Adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J. Biol. Chem. 274:34795-34802. [DOI] [PubMed] [Google Scholar]
- 36.Law, P. Y., Y. H. Wong, and H. H. Loh. 2000. Molecular mechanisms and regulation of opioid receptor signaling. Annu. Rev. Pharmacol. Toxicol. 40:389-430. [DOI] [PubMed] [Google Scholar]
- 37.Lewis, R. E., D. J. Volle, and S. D. Sanderson. 1994. Phorbol ester stimulates phosphorylation on serine 1327 of the human insulin receptor. J. Biol. Chem. 269:26259-26266. [PubMed] [Google Scholar]
- 38.Li, J., K. DeFea, and R. A. Roth. 1999. Modulation of insulin receptor substrate-1 tyrosine phosphorylation by an Akt/phosphatidylinositol 3-kinase pathway. J. Biol. Chem. 274:9351-9356. [DOI] [PubMed] [Google Scholar]
- 39.Marin-Bivens, C. L., and D. H. Olster. 1999. Opioid receptor blockade promotes weight loss and improves the display of sexual behaviors in obese Zucker female rats. Pharmacol. Biochem. Behav. 63:515-520. [DOI] [PubMed] [Google Scholar]
- 40.Matthes, H. W., R. Maldonado, F. Simonin, O. Valverde, S. Slowe, I. Kitchen, K. Befort, A. Dierich, M. Le Meur, P. Dolle, E. Tzavara, J. Hanoune, B. P. Roques, and B. L. Kieffer. 1996. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383:819-823. [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin, J. P., and C. Chavkin. 2001. Tyrosine phosphorylation of the mu-opioid receptor regulates agonist intrinsic efficacy. Mol. Pharmacol. 59:1360-1368. [DOI] [PubMed] [Google Scholar]
- 42.Mothe, I., and E. Van Obberghen. 1996. Phosphorylation of insulin receptor substrate-1 on multiple serine residues, 612, 632, 662, and 731, modulates insulin action. J. Biol. Chem. 271:11222-11227. [DOI] [PubMed] [Google Scholar]
- 43.Myers, M. G., Jr., L. M. Wang, X. J. Sun, Y. Zhang, L. Yenush, J. Schlessinger, J. H. Pierce, and M. F. White. 1994. Role of IRS-1-GRB-2 complexes in insulin signaling. Mol. Cell. Biol. 14:3577-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niswender, K. D., C. D. Morrison, D. J. Clegg, R. Olson, D. G. Baskin, M. G. Myers, Jr., R. J. Seeley, and M. W. Schwartz. 2003. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52:227-231. [DOI] [PubMed] [Google Scholar]
- 45.Niswender, K. D., and M. W. Schwartz. 2003. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front. Neuroendocrinol. 24:1-10. [DOI] [PubMed] [Google Scholar]
- 46.Ota, A., J. Shemer, R. M. Pruss, W. L. Lowe, Jr., and D. LeRoith. 1988. Characterization of the altered oligosaccharide composition of the insulin receptor on neural-derived cells. Brain Res. 443:1-11. [DOI] [PubMed] [Google Scholar]
- 47.Pederson, T. M., D. L. Kramer, and C. M. Rondinone. 2001. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes 50:24-31. [DOI] [PubMed] [Google Scholar]
- 48.Polakiewicz, R. D., S. M. Schieferl, L. F. Dorner, V. Kansra, and M. J. Comb. 1998. A mitogen-activated protein kinase pathway is required for mu-opioid receptor desensitization. J. Biol. Chem. 273:12402-12406. [DOI] [PubMed] [Google Scholar]
- 49.Polakiewicz, R. D., S. M. Schieferl, A. C. Gingras, N. Sonenberg, and M. J. Comb. 1998. μ-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J. Biol. Chem. 273:23534-23541. [DOI] [PubMed] [Google Scholar]
- 50.Poy, M. N., R. J. Ruch, M. A. Fernstrom, Y. Okabayashi, and S. M. Najjar. 2002. Shc and CEACAM1 interact to regulate the mitogenic action of insulin. J. Biol. Chem. 277:1076-1084. [DOI] [PubMed] [Google Scholar]
- 51.Poy, M. N., Y. Yang, K. Rezaei, M. A. Fernstrom, A. D. Lee, Y. Kido, S. K. Erickson, and S. M. Najjar. 2002. CEACAM1 regulates insulin clearance in liver. Nat. Genet. 30:270-276. [DOI] [PubMed] [Google Scholar]
- 52.Qiao, L. Y., J. L. Goldberg, J. C. Russell, and X. J. Sun. 1999. Identification of enhanced serine kinase activity in insulin resistance. J. Biol. Chem. 274:10625-10632. [DOI] [PubMed] [Google Scholar]
- 53.Ravichandran, L. V., D. L. Esposito, J. Chen, and M. J. Quon. 2001. Protein kinase C-ζ phosphorylates insulin receptor substrate-1 and impairs its ability to activate phosphatidylinositol 3-kinase in response to insulin. J. Biol. Chem. 276:3543-3549. [DOI] [PubMed] [Google Scholar]
- 54.Ricort, J. M., J. F. Tanti, E. Van Obberghen, and Y. Marchand-Brustel. 1997. Cross-talk between the platelet-derived growth factor and the insulin signaling pathways in 3T3-L1 adipocytes. J. Biol. Chem. 272:19814-19818. [DOI] [PubMed] [Google Scholar]
- 55.Rui, L., V. Aguirre, J. K. Kim, G. I. Shulman, A. Lee, A. Corbould, A. Dunaif, and M. F. White. 2001. Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J. Clin. Investig. 107:181-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadava, D., D. Alonso, H. Hong, and D. P. Pettit-Barrett. 1997. Effect of methadone addiction on glucose metabolism in rats. Gen. Pharmacol. 28:27-29. [DOI] [PubMed] [Google Scholar]
- 57.Sajan, M. P., M. L. Standaert, G. Bandyopadhyay, M. J. Quon, T. R. Burke, Jr., and R. V. Farese. 1999. Protein kinase C-ζ and phosphoinositide-dependent protein kinase-1 are required for insulin-induced activation of ERK in rat adipocytes. J. Biol. Chem. 274:30495-30500. [DOI] [PubMed] [Google Scholar]
- 58.Saltiel, A. R., and C. R. Kahn. 2001. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799-806. [DOI] [PubMed] [Google Scholar]
- 59.Sasaoka, T., and M. Kobayashi. 2000. The functional significance of Shc in insulin signaling as a substrate of the insulin receptor. Endocr. J. 47:373-381. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt, H., S. Schulz, M. Klutzny, T. Koch, M. Handel, and V. Hollt. 2000. Involvement of mitogen-activated protein kinase in agonist-induced phosphorylation of the mu-opioid receptor in HEK 293 cells. J. Neurochem. 74:414-422. [DOI] [PubMed] [Google Scholar]
- 61.Shimomura, Y., J. Oku, Z. Glick, and G. A. Bray. 1982. Opiate receptors, food intake and obesity. Physiol. Behav. 28:441-445. [DOI] [PubMed] [Google Scholar]
- 62.Skolnik, E. Y., A. Batzer, N. Li, C. H. Lee, E. Lowenstein, M. Mohammadi, B. Margolis, and J. Schlessinger. 1993. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science 260:1953-1955. [DOI] [PubMed] [Google Scholar]
- 63.Skolnik, E. Y., C. H. Lee, A. Batzer, L. M. Vicentini, M. Zhou, R. Daly, M. J. Myers, Jr., J. M. Backer, A. Ullrich, and M. F. White. 1993. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 12:1929-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Staubs, P. A., J. G. Nelson, D. R. Reichart, and J. M. Olefsky. 1998. Platelet-derived growth factor inhibits insulin stimulation of insulin receptor substrate-1-associated phosphatidylinositol 3-kinase in 3T3-L1 adipocytes without affecting glucose transport. J. Biol. Chem. 273:25139-25147. [DOI] [PubMed] [Google Scholar]
- 65.Strack, V., A. M. Hennige, J. Krutzfeldt, B. Bossenmaier, H. H. Klein, M. Kellerer, R. Lammers, and H. U. Haring. 2000. Serine residues 994 and 1023/25 are important for insulin receptor kinase inhibition by protein kinase C isoforms β2 and θ. Diabetologia 43:443-449. [DOI] [PubMed] [Google Scholar]
- 66.Summers, S. A., V. P. Yin, E. L. Whiteman, L. A. Garza, H. Cho, R. L. Tuttle, and M. J. Birnbaum. 1999. Signaling pathways mediating insulin-stimulated glucose transport. Ann. N. Y. Acad. Sci. 892:169-186. [DOI] [PubMed] [Google Scholar]
- 67.Sun, X. J., M. Miralpeix, M. G. Myers, Jr., E. M. Glasheen, J. M. Backer, C. R. Kahn, and M. F. White. 1992. Expression and function of IRS-1 in insulin signal transmission. J. Biol. Chem. 267:22662-22672. [PubMed] [Google Scholar]
- 68.Velloso, L. A., F. Folli, X. J. Sun, M. F. White, M. J. Saad, and C. R. Kahn. 1996. Cross-talk between the insulin and angiotensin signaling systems. Proc. Natl. Acad. Sci. USA 93:12490-12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verspohl, E. J., U. Berger, and H. P. Ammon. 1986. The significance of mu- and delta-receptors in rat pancreatic islets for the opioid-mediated insulin release. Biochim. Biophys. Acta. 888:217-224. [DOI] [PubMed] [Google Scholar]
- 70.Whistler, J. L., H. H. Chuang, P. Chu, L. Y. Jan, and M. von Zastrow. 1999. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron 23:737-746. [DOI] [PubMed] [Google Scholar]
- 71.White, M. F. 1998. The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol. Cell. Biochem. 182:3-11. [PubMed] [Google Scholar]
- 72.Wolf, G., T. Trub, E. Ottinger, L. Groninga, A. Lynch, M. F. White, M. Miyazaki, J. Lee, and S. E. Shoelson. 1995. PTB domains of IRS-1 and Shc have distinct but overlapping binding specificities. J. Biol. Chem. 270:27407-27410. [DOI] [PubMed] [Google Scholar]
- 73.Xu, G. G., and P. L. Rothenberg. 1998. Insulin receptor signaling in the beta-cell influences insulin gene expression and insulin content: evidence for autocrine beta-cell regulation. Diabetes 47:1243-1252. [DOI] [PubMed] [Google Scholar]
- 74.Zhande, R., J. J. Mitchell, J. Wu, and X. J. Sun. 2002. Molecular mechanism of insulin-induced degradation of insulin receptor substrate 1. Mol. Cell. Biol. 22:1016-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang, H., X. Zha, Y. Tan, P. V. Hornbeck, A. J. Mastrangelo, D. R. Alessi, R. D. Polakiewicz, and M. J. Comb. 2002. Phosphoprotein analysis using antibodies broadly reactive against phosphorylated motifs. J. Biol. Chem. 277:39379-39387. [DOI] [PubMed] [Google Scholar]
- 76.Zheng, W. H., S. Kar, and R. Quirion. 2002. Insulin-like growth factor-1-induced phosphorylation of transcription factor FKHRL1 is mediated by phosphatidylinositol 3-kinase/Akt kinase and role of this pathway in insulin-like growth factor-1-induced survival of cultured hippocampal neurons. Mol. Pharmacol. 62:225-233. [DOI] [PubMed] [Google Scholar]
- 77.Zick, Y. 2001. Insulin resistance: a phosphorylation-based uncoupling of insulin signaling. Trends Cell Biol. 11:437-441. [DOI] [PubMed] [Google Scholar]