Abstract
The animal model of inflammatory response induced by intratracheal application of lipopolysaccharide includes many typical features of acute lung injury or the acute respiratory distress syndrome. A number of experimental investigations have been performed to characterize the nature of this injury more effectively. In inflammatory conditions, hypoxia occurs frequently before and in parallel with pulmonary and non-pulmonary pathological events. This current study was designed to examine the in vivo effect of hypoxia as a potentially aggravating condition in endotoxin-induced lung injury. Lipopolysaccharide, 150 µg, was instilled intratracheally into rat lungs, and thereafter animals were exposed to either normoxia or hypoxia (10% oxygen). Lungs were collected 2, 4, 6 and 8 h later. Inflammatory response and tissue damage were evaluated by quantitative analysis of inflammatory cells and mediators, surfactant protein and vascular permeability. A significantly enhanced neutrophil recruitment was seen in lipopolysaccharide-animals exposed to hypoxia compared to lipopolysaccharide-animals under normoxia. This increased neutrophil accumulation was triggered by inflammatory mediators such as tumour necrosis factor-α and macrophage inflammatory protein-1β, secreted by alveolar macrophages. Determination of vascular permeability and surfactant protein-B showed enhanced concentrations in lipopolysaccharide-lungs exposed to hypoxia, which was absent in animals previously alveolar macrophage-depleted. This study demonstrates that hypoxia aggravates lipopolysaccharide injury and therefore represents a second hit injury. The additional hypoxia-induced inflammatory reaction seems to be predominantly localized in the respiratory compartment, underlining the compartmentalized nature of the inflammatory response.
Keywords: acute lung injury, acute respiratory distress syndrome, endotoxin, hypoxia, lung inflammation
Introduction
There is increasing evidence that lipopolysaccharide (LPS) induces an inflammatory response in the lung. Enhanced expression of numerous mediators such as adhesion molecules, cytokines and chemokines, increase of vascular permeability and recruitment of polymorphonuclear neutrophils (PMNs) into the extracellular lung matrix and respiratory compartment are characteristics of this inflammatory reaction [1–5]. Endotoxin-induced lung injury is a useful experimental model for the corresponding clinical situation of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Although significant advances have been made in the treatment of these syndromes, mortality associated with ALI/ARDS still exceeds 40%[6,7].
Alveolar macrophages are situated at the air–tissue interface in the lung. During development of an acute inflammatory lung injury, alveolar macrophages play a very important role upon their activation. It has been shown in several lung injury models that activated pulmonary macrophages secrete the cytokines tumour necrosis factor (TNF)-α and interleukin (IL)-1β as well as the chemokines monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein (MIP)-1β) [8–10]. These cytokines and chemokines, together with adhesion molecules, play a crucial role in the orchestration of an inflammatory response, particularly in neutrophil recruitment. A possible relevance of enhanced expression of these inflammatory mediators could be shown in a large number of inflammatory and antigen-induced models of lung diseases such as reperfusion-induced lung injury, asthma, lung damage caused by immunocomplexes and endotoxin-induced lung injury [4,11–13]. While inflammatory mediators play a pivotal role in the evolution of acute lung injury, abnormal surfactant function is thought to have a central role as well [14].
Recent studies indicate that the occurrence of ALI increases with multiple risk factors. The concept of parallel activation of inflammatory processes altering cellular responses to a given stimulus has experimental support from cell and animal studies [15–17]. It is known from previous studies that hypoxia can exert a proinflammatory effect on the lung [18–20]. Hypoxia, however, is also a potent form of cardioprotection in ischaemia/reperfusion [21]. To better understand the role of hypoxia as a potential additional hit, a rat model of LPS-induced lung injury combined with exposure to normobaric hypoxia was used, a model which reflects more clearly a clinical situation. Lung injury was assessed by determination of accumulation of neutrophils, capillary leakage, expression of inflammatory mediators and production of surfactant proteins. The research hypothesis was that hypoxia enhances inflammatory reactions.
Materials and methods
Animals
All animals were housed in individual isolator cages within the Animal Care Facilities at the University of Zurich until the day of experimentation. The experimental protocols were approved by the Swiss Veterinary Health Authorities.
Male Wistar rats, 250–300 g, were anaesthetized with Hypnorm®[fentanyl-fluanisone, 0·25 ml/kg subcutaneously (s.c.)] and Domitor® (medetomidine hydrochloridum, 0·25 ml/kg s.c.) according to a protocol used previously [20]. Immediately after induction of anaesthesia, 150 µg of LPS were instilled intratracheally in 300 µl phosphate-buffered saline (PBS) [4]. Hypoxia and LPS-hypoxia animals were then placed into a hypoxic chamber with decreasing oxygen concentrations from 21% to 10% within 60 min, as described previously [20]. Anaesthetized animals were exposed to 10% oxygen for 2, 4, 6 and 8 h. LPS-normoxia animals remained at normoxic conditions. Rats were breathing spontaneously. A modest increase (20%) in breathing rate was observed. As arterial blood gases were not measured during our studies, no information is available about pCO2 in our model. Rehydration was assured with intraperitoneal application of ringer lactate (10 ml/kg every 2 h). At predefined time-points, animals were exsanguinated. For bronchoalveolar lavage (BAL), 10 ml of cold PBS were gently instilled into the lungs, withdrawn and re-instilled four times and collected. For the various experiments (determination of extravascular albumin, interstitial neutrophil accumulation, expression of transcriptional factors and mRNA of various genes) different lobes from the lung were used.
Alveolar macrophage depletion
Clodronate-liposomes were prepared as described previously [20,22]. Briefly, liposomes composed of soy phosphatidylcholine (880 mg), cholesterol (132 mg) and DL-α-tocopherol (5 mg) were added to a clodronate solution (375 mg clodronate in 10 ml, Ostac®, Boehringer Mannheim, Germany) by freeze-thawing and filter extrusion. Unencapsulated clodronate was removed with an Amicon ultrafiltration cell, followed by size exclusion chromatography on a Sephadex G25 column. For the application of liposomes, animals were anaesthetized and placed in a supine position. The trachea was exposed surgically and a 25-gauge needle was inserted into the trachea. Control liposomes or liposomes containing 500 µg clodronate were injected in a volume of 300 µl into the lungs; 72 h later, depletion rate varied between 70 and 80%[20]. Control animals received liposomes without clodronate. Before experiments with clodronate-liposomes were started, different depletion conditions were evaluated. Depletion time was varied between 24 and 72 h, with the most effective depletion observed after 72 h. Several concentrations of clodronate were used (100 µg, 500 µg, 1000 µg, 1500 µg). Optimal depletion was seen at a dose of 500 µg, whereas with increased concentrations of clodronate enhanced neutrophil accumulation was observed. This phenomenon of neutrophil recruitment induced by clodronate is a known observation [23]. Depletion was verified with macrophage staining in bronchoalveolar lavage fluid (BALF) and tissue (data not shown).
Bronchoalveolar lavage
Upon collecting lung material, the vascular system of the lungs was carefully flushed with PBS. Lungs were then lavaged four times with 10 ml PBS, always applying a constant pressure while lavaging. BAL fluid was centrifuged at 400 g. Supernatant was aliquoted and frozen at −20°C. Cell pellets from centrifuged BAL fluid were assessed for differential cell counts using cytospins and Diff-Quick (Dade Behring, Duedingen, Switzerland).
Tissue myeloperoxidase (MPO) content
In order to determine the parenchymal content of neutrophils, a MPO assay was performed as described previously [20]. Briefly, lungs were flushed and BAL was performed as described. Lungs were then homogenized in a buffer containing 50 m M potassium phosphate, 0·5% hexadecyltrimethylammonium bromide and 5 mM ethylenediamine tetra-acetic acid (EDTA), sonicated and centrifuged as described earlier. Fifty µl of supernatant was added to 1450 µl of assay buffer, consisting of 100 m M potassium phosphate, o-dianisidine hydrochloride and 30% H2O2. The reaction was assayed by UV at 420 nm and the data were evaluated by measuring the change of optical density over 360 s.
mRNA extraction and reverse transcription polymerase chain reaction (RT-PCR)
The vascular and respiratory compartments were flushed before using the lungs for determination of mRNA of various genes. Total mRNA from lungs was extracted as described previously and analysed by RT-PCR (Perkin-Elmer, Branchburg, NJ, USA) [19]. The primers used for gene analysis are summarized in Table 1. PCR was also performed with 18S primers to ensure equal loading.
Table 1.
Optimized conditions for reverse transcription-polymerase chain reaction (RT-PCR).
| Gene | Primers | Size of PCR fragments (bp) | Thermocycle conditions | Tm |
|---|---|---|---|---|
| ICAM-1 | Sense 5′-AGG TAT CCA TCC ATC CCA CA-3′ | 660 | 22 cycles | 58°C |
| Antisense 5′-CTT CAG AGG CAG GAA ACA GG-3′ | ||||
| TNF-α | Sense 5′-ACT GAA CTT CGG GGT GAT TG-3′ | 334 | 29 cycles | 57°C |
| Antisense 5′-GTG GGT GAG GAG CAG GTA GT-3′ | ||||
| MCP-1 | Sense 5′-TAT GCA GGT CTC TGT CAC GC-3′ | 225 | 28 cycles | 56°C |
| Antisense 5′-TTC CTT ATT GGG GTC AGC AC-3′ | ||||
| MIP-1β | Sense 5′-CGT GTC TGC CTT CTC TCT CC-3′ | 220 | 25 cycles | 58°C |
| Antisense 5′-CAC AGA TTT GCC TGC CTT TT-3′ | ||||
| 18S | Sense 5′-TGA GGC CAT GAT TAA GAG GG-3′ | 201 | 24 cycles | 57°C |
| Antisense 5′-AGT CGG CAT CGT TTA TGG TC-3′ |
ICAM-1, intercellular adhesion molecule-1; TNF-α, tumour necrosis factor-α; MCP-1, monocyte chemoattractant protein-1; MIP-1β, macrophage inflammatory protein-1β.
Enzyme-linked immunosorbent assay (ELISA) quantification of TNF-α and in bronchoalveolar lavage fluid
TNF-α was assessed in the BAL fluid using a standard sandwich ELISA purchased from PharMingen, San Diego, CA, UISA. The minimum detectable concentration of TNF-α was 15 pg/ml.
Western blot analysis of MIP-1β and surfactant protein-B in BAL
BAL fluids obtained from all animals were loaded and electrophoresed in a 7·5% sodium dodecyl sulphate (SDS)-polyacrylamide gel. After separation, the proteins were transblotted to a nitrocellulose membrane for 1 h at 150 V (Bio-Rad, Hercules, CA, USA). Equal loading of proteins was confirmed by Ponceau S staining. The blot was washed in PBS and blocked with PBS/5% low fat milk/0·1% Tween-20 for 1 h at room temperature, followed by an overnight incubation with a polyclonal rabbit anti-rat MIP-1β or a polyclonal goat anti-rat surfactant protein-B (SP-B) antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in blocking buffer. All washing steps were performed three times with PBS/0·1% Tween-20. A secondary horseradish peroxidase-labelled anti-rabbit or anti-goat IgG (1 : 5000) in blocking buffer was added for 1 h at room temperature. Signals were detected by enhanced chemiluminescence.
Albumin ELISA
For detection of albumin in BAL fluid, a sandwich ELISA was developed as described and modified previously [20,24]. Briefly, a coating carbonate buffer (0·1 M carbonate, pH 9·5) was used to dilute samples (1 : 1000) and a standard curve was created using recombinant rat albumin (RDI, Flanders, NJ, USA). A 96-well plate was coated with 100 µl/well and incubated overnight at 4°C. All washing steps (five times with 200 µl/well) were performed with PBS/0·05% Tween-20. To block non-specific binding, 3% dry milk in PBS was added for 1 h at 4°C. A first polyclonal rabbit anti-rat albumin antibody (RDI, Flanders, NJ, USA) was diluted in 3% dry milk/PBS to a concentration of 10 µg/ml and incubated for 1 h at 4°C (100 µl/well). A secondary horseradish peroxidase-labelled goat anti-rabbit antibody (Sigma, Buchs, Switzerland) was added to the wells for 1 h at 4°C (100 µl/well). To develop colour reaction, o-phenylenediamine dihydrochloride (OPD) (Sigma) was added to the wells (200 µl/well). The reaction was stopped with 3 M H2SO4 and optical density (OD) was determined at 492 nm (ELISA reader, Bioconcept, Allschwil, Switzerland).
Statistical analysis
All experiments were performed at least five times. For each data point, two groups of five animals each were treated. ELISA included three replicates with five different animals. Each data point in the graphs represents mean ± standard error of the mean (s.e.m.). Analysis of variance (anova) was performed to assess the statistical significance of differences. Differences were considered significant when P < 0·05. Δ expresses the difference at defined time points between LPS-normoxia and LPS-hypoxia animals.
Results
Hypoxia-, LPS- and LPS-hypoxia-induced lung injury
Hypoxia-induced lung injury data have been published previously [20]. Those results showed a mild inflammatory reaction regarding lung injury compared to LPS-induced lung injury. The relevant data comparing the three different groups are summarized in Table 2. In hypoxia animals, the increase of neutrophils in BALF as well as increase of MPO, TNF-α, MIP-1β, SP-B and albumin quantitatively was small compared to the group of LPS-animals. Therefore, we focused on a comparison between LPS and LPS-hypoxia animals.
Table 2.
Summary: hypoxia-, lipopolysaccharide (LPS)- and LPS-hypoxia-induced lung inflammation.
| Neutrophils in BALF (×106/ml) | MPO (arbitrary units) | TNF-α (pg/ml) | MIP-1β (% relative increase) | SP-B (% relative increase) | albumin (ng/ml) | |
|---|---|---|---|---|---|---|
| 2 h | ||||||
| Control | 0·2 ± 0·7 | 1·0 ± 0·0 | 26·3 ± 1·6 | |||
| Hypoxia | 0·5 ± 0·9 | 1·8 ± 0·2 | 1220·6 ± 45·6 | No increase | No increase | 43·1 ± 5·1 |
| LPS | 10·4 ± 2·3 | 2·3 ± 0·5 | 4224·7 ± 592 | 104·6 ± 12·3 | 123·4 ± 15·7 | 81·9 ± 4·8 |
| LPS-hypoxia | 31·8 ± 2·6** | 4·1 ± 0·5* | 9800·5 ± 130·8** | 104·0 ± 13·5 | 335·4 ± 12·9* | 95·8 ± 1·6 |
| 4 h | ||||||
| Control | 0·5 ± 0·5 | 1·0 ± 0·0 | 19·7 ± 5·6 | |||
| Hypoxia | 0·5 ± 0·0 | 1·53 ± 0·1 | 320·5 ± 15·4 | No increase | 63·1 ± 1·4 | 61·2 ± 2·7 |
| LPS | 33·5 ± 12·6 | 4·3 ± 0·6 | 1294·2 ± 269·5 | 214·7 ± 9·6 | 575·8 ± 35·4 | 114·6 ± 20·0 |
| LPS-hypoxia | 75·0 ± 14·4 | 7·8 ± 0·3** | 4124·5 ± 409·3* | 315·2 ± 15·3* | 789·9 ± 26·5* | 236·0 ± 17·0* |
| 6 h | ||||||
| Control | 0·8 ± 0·5 | 1·0 ± 0·0 | 36·6 ± 10·4 | |||
| Hypoxia | 0·7 ± 0·0 | 1·7 ± 0·1 | 220·6 ± 9·4 | No increase | 103·2 ± 9·4 | 39·3 ± 0·8 |
| LPS | 86·3 ± 8·0 | 5·1 ± 0·4 | 910·3 ± 106·1 | 380·8 ± 13·2 | 555·6 ± 25·4 | 218·3 ± 29·8 |
| LPS-hypoxia | 135·4 ± 5·4** | 8·1 ± 0·7** | 4622·2 ± 990·3* | 555·6 ± 45·2* | 840·4 ± 30·5* | 349·9 ± 7·2* |
| 8 h | ||||||
| Control | 0·8 ± 0·6 | 1·0 ± 0·0 | 16·3 ± 3·6 | |||
| Hypoxia | 0·3 ± 0·0 | 1·7 ± 0·1 | 220·6 ± 9·4 | No increase | 103·2 ± 9·4 | 39·3 ± 0·8 |
| LPS | 72·3 ± 8·0 | 4·2 ± 1·1 | 1016·3 ± 211·7 | 280·5 ± 10·5 | 865·5 ± 10·4 | 43·7 ± 2·9 |
| LPS-hypoxia | 89·5 ± 20·0 | 4·7 ± 1·4 | 1466·8 ± 1123·7 | 445·9 ± 22·2 | 989·5 ± 3·1* | 111·4 ± 12·6* |
LPS, lipopolysaccharide:
P < 0·05 LPS compared to LPS-hypoxia. BALF, brochoalveolar lavage fluid:
P < 0·01 LPS compared to LPS-hypoxia. MPO, myeloperoxidase. TNF-α, tumour necrosis factor-α. MIP-1β, macrophage inflammatory protein-1β. SP-B, surfactant protein B.
Interstitial neutrophil accumulation
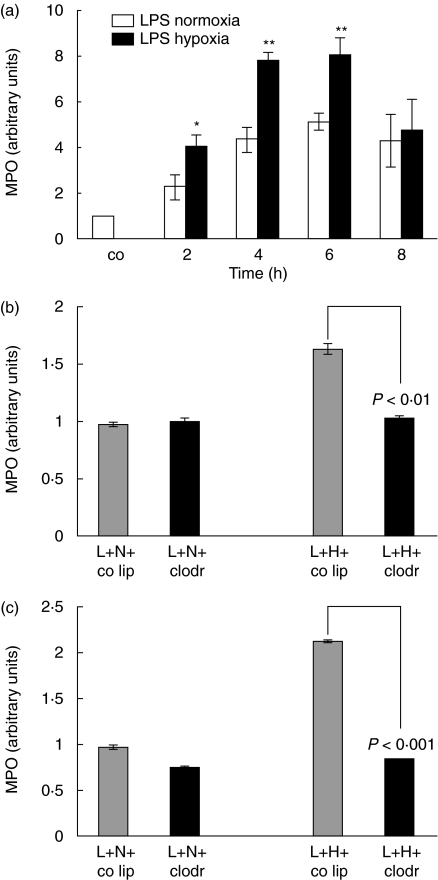
To test the hypothesis of a combined injury of LPS-hypoxia leading to enhanced neutrophil recruitment compared to LPS-normoxia, lung MPO was determined. An increase of MPO was seen at 2, 4, 6 and 8 h in LPS-normoxia and LPS-hypoxia animals compared to control animals (Fig. 1a). When comparing LPS-normoxia animals with LPS-hypoxia animals, a significant difference in MPO was seen at 2, 4 and 6 h with higher MPO values in the LPS-hypoxia group with a difference of 80% at 2 h (P< 0·05), 80% at 4 h (P< 0·01) and 78% at 6 h (P< 0·01).
Fig. 1.
(a) Determination of myeloperoxidase (MPO) activity as a measure of parenchymal neutrophil content. Lipopolysaccharide (LPS), 150 µg, was instilled intratracheally and animals were exposed to normoxia or hypoxia for 2, 4, 6 and 8 h. Lungs were lavaged; the right lung of each animal was removed and homogenized in sample buffer. Optical density was measured at 420 nm over 360 s. The value for control animals was defined as 1 and all other values were adapted. Values are mean ± s.e.m. from five animals. *P < 0·05, **P < 0·01 between LPS-normoxia and LPS-hypoxia animals. (b) Determination of MPO activity as a measure of parenchymal neutrophil content. Animals were pretreated with control liposomes (co lip) or clodronate-liposomes (clodr); 72 h later 150 µg lipopolysaccharide (L) was instilled intratracheally and animals were exposed to either normoxia (N) or hypoxia (H) for 2 h. Values are mean ± s.e.m. from five animals. (c) Determination of myeloperoxidase (MPO) activity as a measure of parenchymal neutrophil content. Animals were pretreated with control liposomes (co lip) or clodronate-liposomes (clodr); 72 h later 150 µg lipopolysaccharide (L) was instilled intratracheally and animals were exposed to either normoxia (N) or hypoxia (H) for 6 h. Values are mean ± s.e.m. from five animals.
The impact of the presence of alveolar macrophages on neutrophil accumulation in the very early injury was studied through macrophage depletion. Alveolar macrophages were eliminated with intratracheally applied clodronate-liposomes 72 h before LPS-injury. LPS-normoxia and LPS-hypoxia animals were evaluated after 2 and 6 h of injury (Fig. 1b,c). As described above, in LPS-hypoxia animals pretreated with control liposomes MPO increased compared to LPS-normoxia animals with control liposomes. In LPS-hypoxia animals without alveolar macrophages, however, this additional hypoxia-induced interstitial neutrophil influx was not observed (LPS-hypoxia control liposomes compared with LPS-hypoxia clodronate P < 0·01 for 2 h and P < 0·001 for 6 h). Thus, the presence of alveolar macrophages appears to be critical for enhanced neutrophil accumulation under LPS-hypoxia.
Neutrophil accumulation in the respiratory compartment
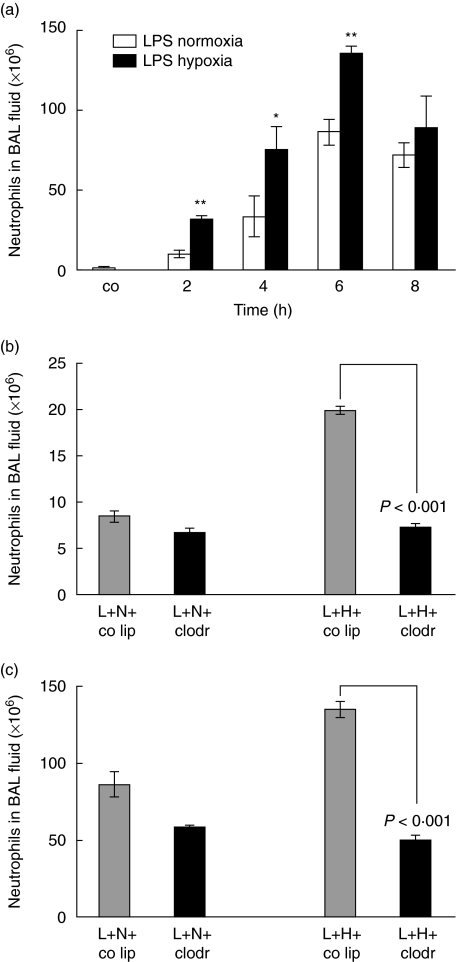
After airway instillation of 150 µg LPS into rats and exposure to normoxia or 10% hypoxia, cell accumulation in BAL fluid was evaluated from 2 h to 8 h after stimulation. As shown in Fig. 2a, the number of neutrophils rose from 1·6 × 106 cells/ml in control rats to a peak of 86·3 × 106 cells/ml at 6 h in LPS-normoxia rat lungs (P< 0·005 compared to control animals), and to 135·0 × 106 cells/ml at 6 h in LPS-hypoxia lungs, respectively (P< 0·0001 compared to control animals). The following difference was calculated between LPS-normoxia versus LPS-hypoxia animals: at 2 h, 22 × 106 cells/ml (120%) (P< 0·01), at 4 h, 41 × 106 cells/ml (164%) (P< 0·05) and at 6 h, 49 × 106 cells/ml (57%) (P< 0·01).
Fig. 2.
(a) Total cell count in bronchoalveolar lavage (BAL) fluid. Lipopolysaccharide (LPS), 150 µg, was instilled intratracheally and animals were exposed to normoxia or hypoxia for 2, 4, 6 and 8 h. Lungs were lavaged and cells from BAL fluid were analysed using cytospins and Diff-Quick staining. Values are mean ± s.e.m. from five animals. *P < 0·05 and **P < 0·01 between LPS-normoxia and LPS-hypoxia animals. (b) Total cell count in BAL fluid. Animals were pretreated with control liposomes (co lip) or clodronate-liposomes (clodr); 72 h later 150 µg LPS (L) was instilled intratracheally and animals were exposed to either normoxia (N) or hypoxia (H) for 2 h. Values are mean ± s.e.m. from five animals. (c) Total cell count in BAL fluid. Animals were pretreated with control liposomes (co lip) or clodronate-liposomes (clodr); 72 h later 150 µg lipopolysaccharide (L) was instilled intratracheally and animals were exposed to either normoxia (N) or hypoxia (H) for 6 h. Values are mean ± s.e.m. from five animals.
The kinetics of neutrophil recruitment observed in BAL fluid of macrophage-depleted animals were similar to interstitial neutrophils (Fig. 2b,c). Respectively, in 2 h, 6 h LPS-hypoxia animals with alveolar macrophages neutrophil count in BAL fluid was 20 × 106 cells/ml (135 × 106 cells/ml), while upon macrophage depletion neutrophil count remained in a range with 7·3 × 106 cells/ml (50 × 106 cells/ml) (P< 0·001 for 2 and 6 h): the same cell count was observed in LPS-hypoxia animals without alveolar macrophages as in LPS-normoxia animals with alveolar macrophages.
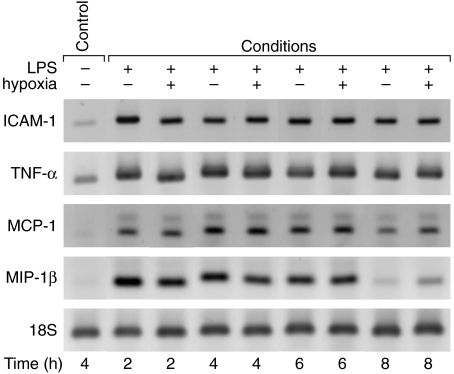
mRNA expression of inflammatory mediators in whole lung
Due to the apparent involvement of the respiratory compartment in the inflammatory process of this two-hit injury, we were interested, furthermore, to evaluate a potential role of the interstitial compartment. To reveal a difference of mRNA expression between the LPS-normoxia and LPS-hypoxia animals, lavaged lungs were homogenized at time-points 2, 4, 6 and 8 h. RT-PCR and gel electrophoresis were performed to determine mRNA for possibly active inflammatory mediators such as ICAM-1, TNF-α, MCP-1 and MIP-1β in whole lung (Fig. 3). mRNA was then quantified through densitometry (data not shown). Although there was an increase in mRNA expression within the LPS-normoxia and LPS-hypoxia group, no intergroup difference was detected during the time course.
Fig. 3.
Changes in expression of intercellular adhesion molecule-1 (ICAM-1), tumour necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1β (MIP-1β) mRNA in lipopolysaccharide (LPS)-normoxia and LPS-hypoxia animals. LPS, 150 µg, was instilled intratracheally and animals were exposed to normoxia or hypoxia for 2, 4, 6 and 8 h. Lungs were lavaged, and whole lung RNA was extracted. Reverse transcription-polymerase chain reaction (RT-PCR) was performed according to the primers and annealing thermocycle conditions shown in Table 1. Equal loading was shown with 18S bands.
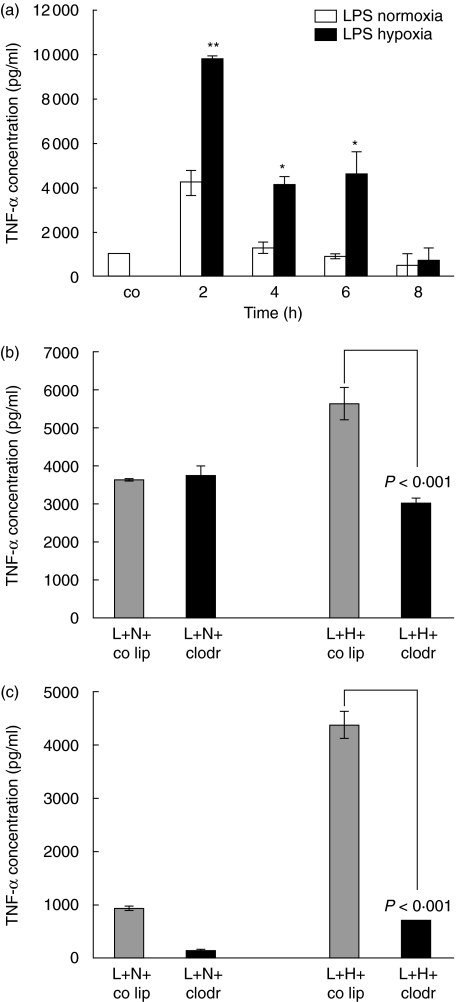
TNF-α content in bronchoalveolar lavage fluid
TNF-α is a known inflammatory mediator responsible for neutrophil recruitment. The concentration of this cytokine was determined in BAL fluid and related to the presence of neutrophils (Fig. 4a). No TNF-α was detectable in BAL fluid of control PBS animals. In LPS-normoxia animals TNF-α concentration peaked with 4224 pg/ml at 2 h (P< 0·05 compared to control animals). For the LPS-hypoxia group the concentrations at 2 h was 9800 pg/ml (P< 0·0001 compared to control animals). Therefore, additional exposure to hypoxia led to an increased concentration of TNF-α in BAL fluid at 2 h (Δ = 5576 pg/ml, P < 0·01), at 4 h (Δ = 2830 pg/ml, P < 0·05) and 6 h (Δ = 3712 pg/ml, P < 0·05).
Fig. 4.
(a) Tumour necrosis factor-α (TNF-α) protein concentration in bronchoalveolar lavage (BAL) fluid of lipopolysaccharide (LPS)-normoxia and LPS-hypoxia animals. LPS, 150 µg, was instilled intratracheally and animals were exposed to normoxia or hypoxia for 2, 4, 6 and 8 h. Lungs were lavaged, and TNF-α was determined using a standard enzyme-linked immunosorbent assay (ELISA). Values are mean ± s.e.m. from five animals. *P < 0·05 and **P < 0·01 between LPS-normoxia and LPS-hypoxia animals. (b) TNF-α protein concentration in BAL fluid of alveolar macrophage-competent and -depleted animals, exposed to lipopolysaccharide (LPS)-normoxia and LPS-hypoxia. Animals were pretreated with control liposomes (co lip) or clodronate-liposomes (clodr); 72 h later 150 µg LPS (L) was instilled intratracheally and animals were exposed to either normoxia (N) or hypoxia (H) for 2 h. TNF-α was determined using a standard ELISA. Values are mean ± s.e.m. from five animals. (c) TNF-α protein concentration in BAL fluid of alveolar macrophage-competent and -depleted animals, exposed to LPS-normoxia and LPS-hypoxia. Animals were pretreated with control liposomes (co lip) or clodronate-liposomes (clodr); 72 h later 150 µg LPS (L) was instilled intratracheally and animals were exposed to either normoxia (N) or hypoxia (H) for 6 h. TNF-α was determined using a standard ELISA. Values are mean ± s.e.m. from five animals.
To elucidate further the role of the presence of TNF-α secreted by alveolar macrophages in the respiratory compartment, lungs were evaluated after depletion of alveolar macrophages. As shown in Fig. 4b and c, TNF-α concentration in 2 h and 6 h LPS-hypoxia animals decreased significantly compared to LPS-hypoxia animals with alveolar macrophages (P< 0·001). Without alveolar macrophages the same amount of TNF-α was found in LPS-hypoxia animals than in LPS-normoxia animals with alveolar macrophages. These data strongly suggest that alveolar macrophages are the source of TNF-α secretion in LPS injury under additional stimulation by hypoxia.
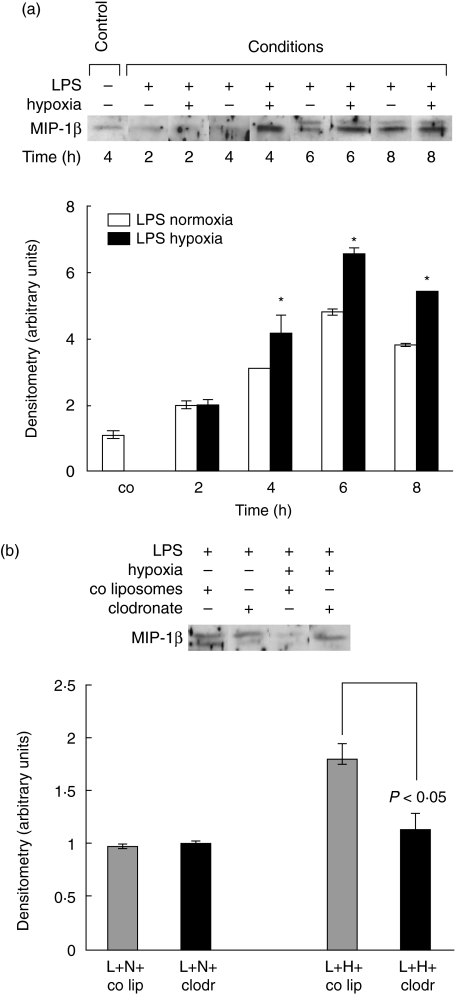
MIP-1β content in BALF
LPS-normoxia and LPS-hypoxia animals demonstrated enhanced increase with a peak at 6 h of injury (Fig. 5a). LPS-hypoxia animals, however, showed higher concentrations between 4 h and 8 h than LPS-normoxia animals (at 4 h Δ = 110%, at 6 h Δ = 180% and at 8 h Δ = 160%, all P < 0·05).
Fig. 5.
(a)Macrophage inflammatory protein-1β (MIP-1β) protein determination in bronchoalveolar lavage (BAL) fluid of lipopolysaccharide (LPS)-normoxia and LPS-hypoxia animals. LPS, 150 µg, was instilled intratracheally and animals were exposed to normoxia or hypoxia for 2, 4, 6 and 8 h. Lungs were lavaged, and proteins were electrophoresed on a sodium dodecyl sulphate-polyacrylamide gel and transblotted to a nitrocellulose membrane. The blot represents one of five experiments. Densitometry was performed. Value for control animals was defined as 1 and all other values were adapted. Values are mean ± s.e.m. from five animals. *P < 0·05 between LPS-normoxia and LPS-hypoxia animals. (b) MIP-1β protein concentration in BAL fluid of alveolar macrophage-competent and -depleted animals, exposed to LPS-normoxia and LPS-hypoxia. Animals were pretreated with control liposomes (co lip) or clodronate-liposomes (clodr); 72 h later 150 µg LPS (L) was instilled intratracheally and animals were exposed to either normoxia (N) or hypoxia (H) for 6 h. BAL fluid proteins were electrophoresed on a SDS-polyacrylamide gel and transblotted to a nitrocellulose membrane. The blot represents one of five experiments.
Elimination of alveolar macrophages did not affect the response in MIP-1β expression within and between the LPS-normoxia and LPS-hypoxia group at 2 h (figure not shown). At 6 h, MIP-1β expression in macrophage-depleted LPS-hypoxia animals was similar to the findings in LPS-normoxia animals with macrophages (P< 0·01 LPS-hypoxia animals without alveolar macrophages compared to LPS-hypoxia animals with alveolar macrophages) (Fig. 5b).
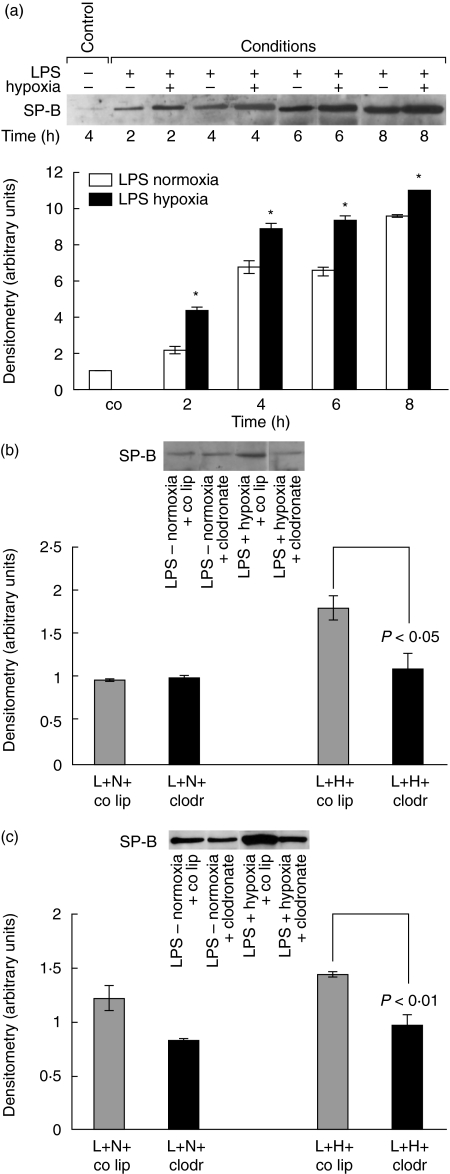
Effect of LPS and hypoxia on surfactant protein-B
Surfactant proteins are essential for full expansion of the alveoli. SP-B was determined to evaluate whether additional hypoxia might influence SP-B production. Western blot analysis for SP-B showed an increase in LPS-normoxia animals of 120% at 2 h up to 860% at 8 h, compared to LPS-hypoxia animals with an increase of 340% up to 1000% at the same time-points (Fig. 6a). LPS-hypoxia values were significantly higher than LPS-normoxia with Δ = 220% at 2 h, Δ = 210% at 4 h, Δ = 280% 6 h and Δ = 140% at 8 h (all P-values < 0·05).
Fig. 6.
(a) Surfactant protein-B (SP-B) protein determination in bronchoalveolar lavage (BAL) fluid of lipopolysaccharide (LPS)-normoxia and LPS-hypoxia animals. LPS, 150 µg, was instilled intratracheally and animals were exposed to normoxia or hypoxia for 2, 4, 6 and 8 h. Lungs were lavaged, and proteins were electrophoresed on a sodium dodecyl sulphate-polyacrylamide gel and transblotted to a nitrocellulose membrane. The blot represents one of five experiments. Densitometry was performed. Value for control animals was defined as 1 and all other values were adapted. Values are mean ± s.e.m. from five animals. *P < 0·05 between LPS-normoxia and LPS-hypoxia animals. (b) SP-B protein concentration in BAL fluid of alveolar macrophage-competent and -depleted animals, exposed to LPS-normoxia and LPS-hypoxia. Animals were pretreated with control liposomes (co lip) or clodronate-liposomes (clodr); 72 h later 150 µg LPS (L) was instilled intratracheally and animals were exposed to either normoxia (N) or hypoxia (H) for 2 h. BAL fluid proteins were electrophoresed on a sodium dodecyl sulphate-polyacrylamide gel and transblotted to a nitrocellulose membrane. The blot represents one of five experiments. (c) SP-B protein concentration in BAL fluid of alveolar macrophage-competent and -depleted animals, exposed to LPS-normoxia and LPS-hypoxia. Animals were pretreated with control liposomes (co lip) or clodronate-liposomes (clodr); 72 h later 150 µg LPS (L) was instilled intratracheally and animals were exposed to either normoxia (N) or hypoxia (H) for 6 h. BAL fluid proteins were electrophoresed on a sodium dodecyl sulphate-polyacrylamide gel and transblotted to a nitrocellulose membrane. The blot represents one of five experiments.
As shown in Fig. 6b and c, alveolar macrophages depletion affected SP-B concentrations in BAL fluid of LPS-hypoxia animals. The additional hypoxia-induced secretion of SP-B in LPS-hypoxia animals with alveolar macrophages was not observed in LPS-hypoxia animals without alveolar macrophages (for 2 h P < 0·05, for 6 h P < 0·01).
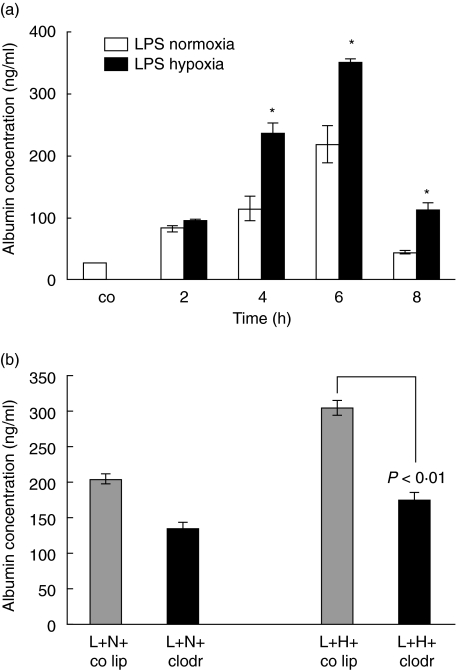
Effect of LPS and hypoxia on capillary leakage
Exposure of LPS-animals to hypoxia had also a significant effect on capillary permeability. Figure 7a shows albumin concentrations in BAL fluid. Control animals showed a concentration of 40·3 ng/ml. Capillary leakage peaked at 6 h with 218 ng/ml in the LPS-normoxia animals compared to 350 ng/ml in the LPS-hypoxia animals. The leakage was significantly higher in LPS-hypoxia compared to LPS-normoxia animals (4 h: Δ = 121 ng/ml, P < 0·05; 6 h: Δ = 132 ng/ml, P < 0·05; 8 h: Δ = 67 ng/ml, P < 0·05).
Fig. 7.
Enzyme-linked immunosorbent assay (ELISA) for albumin content in bronchoalveolar lavage (BAL) fluid. Lipopolysaccharide (LPS), 150 µg, was instilled intratracheally and animals were exposed to normoxia or hypoxia for 2, 4, 6 and 8 h. Lungs were lavaged and albumin was analysed in BAL fluid. Values are means ± s.e.m. from five animals. *P < 0·05 between LPS-normoxia and LPS-hypoxia animals. (b) ELISA for albumin content in BAL fluid of alveolar macrophage-competent and -depleted animals, exposed to LPS-normoxia and LPS-hypoxia. Animals were pretreated with control liposomes (co lip) or clodronate-liposomes (clodr); 72 h later 150 µg LPS (L) was instilled intratracheally and animals were exposed to either normoxia (N) or hypoxia (H) for 6 h. The blot represents one of five experiments.
Macrophage depletion did not affect capillary leakage at 2 h of injury (figure not shown). However, at 6 h after injury (Fig. 7b), albumin concentration in BAL fluid of LPS-hypoxia animals without alveolar macrophages was similar to LPS-normoxia animals with alveolar macrophages (P< 0·01 LPS-hypoxia animals without alveolar macrophages compared to LPS-hypoxia animals with alveolar macrophages).
In summary, the joint action of LPS and hypoxia results in a more than additive effect when comparing LPS and hypoxia with LPS-hypoxia values.
Discussion
Current knowledge about the role of hypoxia in LPS-induced lung inflammation is limited. The present study underlines the aggravating effect of hypoxia on acute endotoxin-induced lung injury in vivo. The damage in endotoxin-induced lung injury is characterized by increased expression of inflammatory mediators, extensive neutrophil accumulation and increased vascular permeability. The main findings of this study provide evidence that hypoxia has a substantial effect on LPS-induced inflammation, increasing the magnitude of lung injury. Macrophage depletion was performed in order to test the research hypothesis that alveolar macrophages might be key effector cells in this two-hit injury. Based on the present results, alveolar macrophages appear to play an important role in modulating the severity of injury.
Animal studies mimicking human ALI have been useful to provide valuable observations regarding the mechanisms underlying the pathogenesis of this injury. However, the endotoxin-induced lung injury model in animals has its limitations in reflecting a human clinical syndrome such as ALI or ARDS. The presence of multiple predisposing disorders in patients substantially increase the risk for ALI. Direct pulmonary disorders such as pneumonia, aspiration or pulmonary contusion as well as non-pulmonary risk factors such as sepsis or severe trauma with shock are well-known risk constellations [25]. It was suggested in a workshop from the NHLBI Working Group that two-hit models might be more appropriate to reflect common comorbidities and risk factors in patients [26]. Recent studies have supported the hypothesis of a ‘two-hit’ model in the pathogenesis of lung injury: an initial insult priming for subsequent organ damage in response to a second stimulus. This has been observed in haemorrhagic shock and also in a model of acid aspiration in combination with sepsis. In these models it was possible to show an enhanced inflammatory answer upon the two hits [24,27–29]. Most convincingly, our results demonstrated that hypoxia, in combination with another injury, has to be considered as an inductor of enhanced inflammatory reactions. In addition, there is evidence from earlier studies that alveolar pO2 is a potent immunomodulatory signal, whose reductions early after endotoxaemia enhance lung inflammation in ARDS, corroborating our data [30].
LPS-normoxia produced an increase of 400% in neutrophil accumulation compared to control PBS-normoxia animals, which is consistent with previous observations [4]. A combination of LPS and hypoxia, however, resulted in a 700% increase of lung neutrophils, which represents an almost twofold neutrophil influx in the presence of hypoxia. Activated PMNs are known to release neutral proteases, such as leucocyte elastase or myeloperoxidase, and they also produce oxygen metabolites. These mediators are mainly responsible for decomposition of alveolar matrix in association with several pulmonary diseases. It is known in the ARDS that neutrophil sequestration and migration within the lung remain histological hallmarks of this lung injury [31,32]. Agorreta et al. demonstrated enhanced nitric oxide synthase-2 expression in LPS-hypoxia-injured lungs [17]. Therefore, hypoxia might further contribute to the development of acute lung injury or ARDS. Although this two-hit injury model reflects a possible clinical situation much more effectively, it has its limitations. A wide variety of different factors causes ARDS, while our model deals only with locally applied LPS.
Macrophage depletion by intratracheal application of dichloromethylene diphosphonate-(Cl2MDP)-liposomes (clodronate liposomes) as phagolysozymes is a well-established method [33]. Phagocytosis of clodronate-liposomes has been shown to result in the selective elimination of macrophages at a rate of up to 80% However, it is known that a mild accumulation of neutrophils is induced directly by this procedure [23]. This observation was also confirmed in our study. In addition, it is obvious that the number of cells in BAL fluid is much higher in liposome studies. A potential contamination of liposomes with endotoxin was excluded using a limulus test. Application of liposomes in this model might slightly trigger an inflammation, although mechanisms are not clear.
LPS has been shown to stimulate increased production of TNF-α in the lung. Xing et al. found that increased levels of whole lung TNF-α were present after 30 min of LPS-induced lung injury and peaked within 6 h [34]. At an early time-point alveolar macrophages were the major source of TNF-α, while at later time-points neutrophils were the predominant cell, releasing TNF-α. In 2 h LPS-normoxia animals of this study, TNF-α in the respiratory compartment seems to be macrophage-independent. This is a surprising finding, as most of the depletion studies showed a response of TNF-α upon macrophage depletion [23,35,36]; but all these studies focused at later time-points of an injury, and therefore no information exists about this very early phase of the inflammation. In addition, different experimental models were used. At 6 h of LPS injury, however, alveolar macrophages seem to be the major source for TNF-α. To verify the role of alveolar macrophages in the two-hit injury, alveolar macrophage depletion was performed and TNF-α content in BAL fluid was analysed at 2 h and 6 h of inflammation. At both time-points, TNF-α was thoroughly secreted by these effector cells, underlining the pivotal role of these cells.
Previous studies have shown that the chemoattractant MIP-1β might be involved in neutrophil recruitment in acute lung injury [10]. At 2 h of LPS-hypoxia injury MIP-1β expression, however, was not congruent with neutrophil recruitment. There were significantly more neutrophils recruited in hypoxia-LPS than in normoxia-LPS animals, while MIP-1β concentrations in BAL fluid were similar in both groups. Interestingly, at 6 h after injury, MIP-1β could be a driving force for neutrophil accumulation secreted by alveolar macrophages as MIP-1β concentration was decreased in alveolar macrophages depleted LPS-hypoxia animals compared to LPS-normoxia animals.
In many injury models, ICAM-1 plays a key role in mediating neutrophil adhesion to endothelial cells and thereby triggering transmigration of neutrophils to the site of inflammation [37,38]. MCP-1 is an important chemoattractant, not only for mononuclear cells but also for neutrophils, as shown previously [39–41]. Interestingly, in our model expression of both inflammatory mediators in whole lung were not enhanced in LPS-hypoxia compared to LPS-normoxia, assuming that they were not involved in the additional neutrophil recruitment upon LPS-hypoxia injury.
An interesting observation was the fact that injury parameters decreased after 6 h of injury. Acute lung injury comprises a complex cascade of inflammatory reactions. Upon stimulation − as shown in this study − different inflammatory mediators are up-regulated. Beside activation of proinflammatory mediators, expression of mediators with anti-inflammatory characteristics such as IL-10 is also triggered [42]. The observed decrease of injury in the hypoxia, LPS and LPS-hypoxia model might reflect a role of the latter.
The results of these experiments suggest strongly that release of the inflammatory mediator TNF-α by alveolar macrophages in the respiratory compartment appears to be critical for additional neutrophil accumulation in LPS-hypoxia animals compared to LPS-normoxia animals. As shown previously in a model of acute hypoxia-induced lung inflammation, alveolar macrophages play a central role upon decreased levels of oxygen [20]. In vitro data from Hempel et al. have demonstrated that exposure of alveolar macrophages to hypoxia increased LPS-stimulated expression of the cytokines IL-1 and TNF-α[43]. Leeper-Woodford et al. also observed altered cytokine secretion in vitro in alveolar macrophages and indicated that acute exposure to hypoxia up-regulated LPS-induced TNF-α release from lung macrophages [44]. Our in vivo results not only support these previous in vitro observations that hypoxia induces increased TNF-α release, but also demonstrate alveolar macrophages to be the major source of this cytokine in this experimental system of two-hit injury.
With the depletion of alveolar macrophages it was possible to show that other cells than alveolar macrophages are involved in the inflammatory response upon stimulation with LPS or exposure to hypoxia as a significant production of mediators was observed even after macrophage depletion. An important contribution can be expected from alveolar epithelial cells as previously shown [19,45]. Although not defined as effector cells, they produce cytokines, chemokines and adhesion molecules. Therefore, it can be implied that respiratory epithelial cells also play an important role in the LPS-hypoxia-induced injury.
There is a complex interaction of cytokines and vascular permeability in acute lung injury. TNF-α has a wide range of biological pathological properties that include changing the integrity of endothelium [46,47]. In in vitro studies TNF-α increased permeability of endothelial and epithelial cell monolayers [48,49]. Our data at 6 h confirm that the observed changes in permeability in LPS-hypoxia compared to LPS-normoxia animals could be linked to macrophage-derived TNF-α.
The present studies demonstrate that inflammatory reactions in the lung might be compartmentalized events. No difference in cytokine and chemokine expression in the interstitial space was measured between LPS-normoxia and LPS-hypoxia animals. However, in the respiratory compartment, LPS-hypoxia animals had higher concentrations of inflammatory mediators than LPS-normoxia animals. Lung compartmentalization as evidenced by cell recruitment or cytokine production has also been hypothesized by other authors [13,50].
Alveolar stability is maintained by the formation and maintenance of a surfactant film at the air–liquid interface of the alveolus. Key components of this film are phospholipids associated with surfactant protein such as SP-B. The present findings show an enhanced SP-B accumulation in LPS-hypoxia animals compared to LPS-normoxia animals. Similar data of increased levels of SP-B upon injury were also shown in lungs exposed to silica and hyperoxia [51–54]. In endotoxin-induced lung injury, Sugahara et al. confirmed these results with enhanced mRNA expression for SP-B in alveolar epithelial type II cells [55]. On the other side, Ingenito et al. showed a clear decrease of SP-B protein concentration in BAL fluid of LPS-injured animals [56]. However, the two studies used very different LPS doses and modes of administration. In addition, different time-points were evaluated. Increases of SP-B in LPS-hypoxia compared to LPS-normoxia animals in our study may most probably reflect increased SP-B secretion in order to counteract the more accentuated injury with LPS and alveolar hypoxia [57].
In summary, parameters of lung injury such as inflammatory mediators, neutrophil recruitment and permeability increased in a model of ALI combining LPS-induced injury with exposure to hypoxia. We suggest that this model of a two-hit injury reflects more clearly the situation of human ALI with common comorbidities and risk factors. Although neutrophils predominate in this lung injury, alveolar macrophages seem to have a critical role.
Acknowledgments
The authors wish to thank Christian Gasser, artist, Institute of Physiology, University of Zurich, Switzerland, for development of the illustrations. This study was supported by the Swiss National Science Foundation grant no. 31–55702·98, Gebert Ruef Foundation, Switzerland, the Jubiläumsstiftung der Schweizerischen Lebensversicherung- und Rentenanstalt, the Hartmann-Müller Foundation, Switzerland and the Bonizzi-Theler Foundation, Switzerland.
References
- 1.Ulich TR, Yin S, Remick DG, Russell D, Eisenberg SP, Kohno T. Intratracheal administration of endotoxin and cytokines. IV. The soluble tumor necrosis factor receptor type I inhibits acute inflammation. Am J Pathol. 1993;142:1335–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Ulich TR, Howard SC, Remick DG, et al. Intratracheal administration of endotoxin and cytokines. VIII. LPS induces E-selectin expression; anti-E-selectin and soluble E-selectin inhibit acute inflammation. Inflammation. 1994;18:389–98. doi: 10.1007/BF01534436. [DOI] [PubMed] [Google Scholar]
- 3.Ulich TR, Howard SC, Remick DG, et al. Intratracheal administration of endotoxin and cytokines. VI. Antiserum to CINC inhibits acute inflammation. Am J Physiol. 1995;268:L245–50. doi: 10.1152/ajplung.1995.268.2.L245. [DOI] [PubMed] [Google Scholar]
- 4.Beck-Schimmer B, Schimmer RC, Warner RL, et al. Expression of lung vascular and airway ICAM-1 after exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 1997;17:344–52. doi: 10.1165/ajrcmb.17.3.2861. [DOI] [PubMed] [Google Scholar]
- 5.van Helden HP, Kuijpers WC, Steenvoorden D, et al. Intratracheal aerosolization of endotoxin (LPS) in the rat: a comprehensive animal model to study adult (acute) respiratory distress syndrome. Exp Lung Res. 1997;23:297–316. doi: 10.3109/01902149709039228. [DOI] [PubMed] [Google Scholar]
- 6.Matthay MA. Conference summary: acute lung injury. Chest. 1999;116:119S–26S. doi: 10.1378/chest.116.suppl_1.119s. [DOI] [PubMed] [Google Scholar]
- 7.Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999;281:354–60. doi: 10.1001/jama.281.4.354. [DOI] [PubMed] [Google Scholar]
- 8.Brieland JK, Flory CM, Jones ML, et al. Regulation of monocyte chemoattractant protein-1 gene expression and secretion in rat pulmonary alveolar macrophages by lipopolysaccharide, tumor necrosis factor-a, and interleukin-1b. Am J Respir Cell Mol Biol. 1995;12:104–9. doi: 10.1165/ajrcmb.12.1.7811465. [DOI] [PubMed] [Google Scholar]
- 9.Monton C, Torres A. Lung inflammatory response in pneumonia. Monaldi Arch Chest Dis. 1998;53:56–63. [PubMed] [Google Scholar]
- 10.Bless NM, Huber-Lang M, Guo RF, et al. Role of CC chemokines (macrophage inflammatory protein-1 beta, monocyte chemoattractant protein-1, RANTES) in acute lung injury in rats. J Immunol. 2000;164:2650–9. doi: 10.4049/jimmunol.164.5.2650. [DOI] [PubMed] [Google Scholar]
- 11.Wegner CD, Gundel RH, Reilly P, Haynes N, Letts LG, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science. 1990;247:456–9. doi: 10.1126/science.1967851. [DOI] [PubMed] [Google Scholar]
- 12.Horgan MJ, Ge M, Gu J, Rothlein R, Malik AB. Role of ICAM-1 in neutrophil-mediated lung vascular injury after occlusion and reperfusion. Am J Physiol. 1991;261:H1578–84. doi: 10.1152/ajpheart.1991.261.5.H1578. [DOI] [PubMed] [Google Scholar]
- 13.Mulligan MS, Vaporciyan AA, Warner RL, et al. Compartmentalized roles for leukocytic adhesion molecules in lung inflammatory injury. J Immunol. 1995;154:1350–63. [PubMed] [Google Scholar]
- 14.Hallman M, Spragg R, Harrell JH, Moser KM, Gluck L. Evidence of lung surfactant abnormality in respiratory failure. Study of bronchoalveolar lavage phospholipids, surface activity, phospholipase activity, and plasma myoinositol. J Clin Invest. 1982;70:673–83. doi: 10.1172/JCI110662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welty-Wolf KE, Carraway MS, Huang YC, Simonson SG, Kantrow SP, Piantadosi CA. Bacterial priming increases lung injury in Gram-negative sepsis. Am J Respir Crit Care Med. 1998;158:610–9. doi: 10.1164/ajrccm.158.2.9704064. [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Kapus A, Marsden PA, et al. Regulation of Toll-like receptor 4 expression in the lung following hemorrhagic shock and lipopolysaccharide. J Immunol. 2002;168:5252–9. doi: 10.4049/jimmunol.168.10.5252. [DOI] [PubMed] [Google Scholar]
- 17.Agorreta J, Garayoa M, Montuenga LM, Zulueta JJ. Effects of acute hypoxia and lipopolysaccharide on nitric oxide synthase-2 expression in acute lung injury. Am J Respir Crit Care Med. 2003;168:287–96. doi: 10.1164/rccm.200209-1027OC. [DOI] [PubMed] [Google Scholar]
- 18.Kisala JM, Ayala A, Stephan RN, Chaudry IH. A model of pulmonary atelectasis in rats: activation of alveolar macrophage and cytokine release. Am J Physiol. 1993;264:R610–4. doi: 10.1152/ajpregu.1993.264.3.R610. [DOI] [PubMed] [Google Scholar]
- 19.Beck-Schimmer B, Schimmer RC, Madjdpour C, Bonvini JM, Pasch T, Ward PA. Hypoxia mediates increased neutrophil and macrophage adhesiveness to alveolar epithelial cells. Am J Respir Cell Mol Biol. 2001;25:780–7. doi: 10.1165/ajrcmb.25.6.4433. [DOI] [PubMed] [Google Scholar]
- 20.Madjdpour C, Jewell UR, Kneller S, et al. Decreased alveolar oxygen induces lung inflammation. Am J Physiol. 2003;284:L360–7. doi: 10.1152/ajplung.00158.2002. [DOI] [PubMed] [Google Scholar]
- 21.Laurikka J, Wu ZK, Iisalo P, et al. Regional ischemic preconditioning enhances myocardial performance in off-pump coronary artery bypass grafting. Chest. 2002;121:1183–9. doi: 10.1378/chest.121.4.1183. [DOI] [PubMed] [Google Scholar]
- 22.Seiler P, Aichele P, Odermatt B, Hengartner H, Zinkernagel RM, Schwendener RA. Crucial role of marginal zone macrophages and marginal zone metallophils in the clearance of lymphocytic choriomeningitis virus infection. Eur J Immunol. 1997;27:2626–33. doi: 10.1002/eji.1830271023. [DOI] [PubMed] [Google Scholar]
- 23.Broug-Holub E, Toews GB, van Iwaarden JF, et al. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–46. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemzek JA, Call DR, Ebong SJ, Newcomb DE, Bolgos GL, Remick DG. Immunopathology of a two-hit murine model of acid aspiration lung injury. Am J Physiol. 2000;278:L512–20. doi: 10.1152/ajplung.2000.278.3.L512. [DOI] [PubMed] [Google Scholar]
- 25.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 26.Matthay MA, Zimmerman GA, Esmon C, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute Working Group. Am J Respir Crit Care Med. 2003;167:1027–35. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 27.Fan J, Marshall JC, Jimenez M, Shek PN, Zagorski J, Rotstein OD. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following. J Immunol. 1998;161:440–7. [PubMed] [Google Scholar]
- 28.Price SA, Spain DA, Wilson MA, Harris PD, Garrison RN. Altered vasoconstrictor and dilator responses after a ‘two-hit’ model of sequential hemorrhage and bacteremia. J Surg Res. 1999;81:59–64. doi: 10.1006/jsre.1998.5437. [DOI] [PubMed] [Google Scholar]
- 29.Spain DA, Kawabe T, Keelan PC, Wilson MA, Harris PD, Garrison RN. Decreased alpha-adrenergic response in the intestinal microcirculation after ‘two-hit’ hemorrhage/resuscitation and bacteremia. J Surg Res. 1999;84:180–5. doi: 10.1006/jsre.1999.5638. [DOI] [PubMed] [Google Scholar]
- 30.Matuschak GM, Munoz CF, Johanns CA, Rahman R, Lechner AJ. Upregulation of postbacteremic TNF-alpha and IL-1alpha gene expression by alveolar hypoxia/reoxygenation in perfused rat lungs. Am J Respir Crit Care Med. 1998;157:629–37. doi: 10.1164/ajrccm.157.2.9707120. [DOI] [PubMed] [Google Scholar]
- 31.Skerrett SJ, Martin TR, Chi EY, Peschon JJ, Mohler KM, Wilson CB. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am J Physiol. 1999;276:L715–27. doi: 10.1152/ajplung.1999.276.5.L715. [DOI] [PubMed] [Google Scholar]
- 32.Acute lung injury. Am J Respir Crit Care Med. 1998;158:675–9. doi: 10.1164/ajrccm.158.2.15823. Round Table Conference. [DOI] [PubMed] [Google Scholar]
- 33.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xing Z, Jordana M, Kirpalani H, Driscoll KE, Schall TJ, Gauldie J. Cytokine expression by neutrophils and macrophages in vivo. endotoxin induces tumor necrosis factor-alpha, macrophage inflammatory protein-2, interleukin-1 beta, and interleukin-6 but not RANTES or transforming growth factor-beta 1 mRNA expression in acute lung inflammation. Am J Respir Cell Mol Biol. 1994;10:148–53. doi: 10.1165/ajrcmb.10.2.8110470. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto S, Pittet JF, Hong K, et al. Depletion of alveolar macrophages decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am J Physiol. 1996;270:L819–28. doi: 10.1152/ajplung.1996.270.5.L819. [DOI] [PubMed] [Google Scholar]
- 36.Koay MA, Gao X, Washington MK, et al. Macrophages are necessary for maximal nuclear factor-kappa B activation in response to endotoxin. Am J Respir Cell Mol Biol. 2002;26:572–8. doi: 10.1165/ajrcmb.26.5.4748. [DOI] [PubMed] [Google Scholar]
- 37.Wawryk SO, Novotny JR, Wicks IP, et al. The role of the LFA-1/ICAM-1 interaction in human leukocyte homing and adhesion. Immunol Rev. 1989;108:135–61. doi: 10.1111/j.1600-065x.1989.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 38.Springer TA. Adhesion receptors in the immune system. Nature. 1990;346:425–34. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 39.Johnston B, Burns AR, Suematsu M, Issekutz TB, Woodman RC, Kubes P. Chronic inflammation upregulates chemokine receptors and induces neutrophil migration to monocyte chemoattractant protein-1. J Clin Invest. 1999;103:1269–76. doi: 10.1172/JCI5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe T, Higuchi K, Hamaguchi M, et al. Monocyte chemotactic protein-1 regulates leukocyte recruitment during gastric ulcer recurrence induced by tumor necrosis factor-alpha. Am J Physiol. 2004;287:G919–28. doi: 10.1152/ajpgi.00372.2003. [DOI] [PubMed] [Google Scholar]
- 41.Vozzelli MA, Mason SN, Whorton MH, Auten RL., Jr Antimacrophage chemokine treatment prevents neutrophil and macrophage influx in hyperoxia-exposed newborn rat lung. Am J Physiol. 2004;286:L488–93. doi: 10.1152/ajplung.00414.2002. [DOI] [PubMed] [Google Scholar]
- 42.Mulligan MS, Warner RL, Foreback JL, Shanley TP, Ward PA. Protective effects of IL-4, IL-10, IL-12, and IL-13 in IgG immune complex-induced lung injury: role of endogenous IL-12. J Immunol. 1997;159:3483–9. [PubMed] [Google Scholar]
- 43.Hempel SL, Monick MM, Hunninghake GW. Effect of hypoxia on release of IL-1 and TNF by human alveolar macrophages. Am J Respir Cell Mol Biol. 1996;14:170–6. doi: 10.1165/ajrcmb.14.2.8630267. [DOI] [PubMed] [Google Scholar]
- 44.Leeper-Woodford SK, Detmer K. Acute hypoxia increases alveolar macrophage tumor necrosis factor activity and alters NF-kappaB expression. Am J Physiol. 1999;276:L909–16. doi: 10.1152/ajplung.1999.276.6.L909. [DOI] [PubMed] [Google Scholar]
- 45.Beck-Schimmer B, Madjdpour C, Kneller S, et al. Role of alveolar epithelial ICAM-1 in lipopolysachharide-induced lung inflammation. Eur Resp J. 2002;19:1142–50. doi: 10.1183/09031936.02.00236602. [DOI] [PubMed] [Google Scholar]
- 46.Abe Y, Sekiya S, Yamasita T, Sendo F. Vascular hyperpermeability induced by tumor necrosis factor and its augmentation by IL-1 and IFN-gamma is inhibited by selective depletion of neutrophils with a monoclonal antibody. J Immunol. 1990;145:2902–7. [PubMed] [Google Scholar]
- 47.Horvath CJ, Ferro TJ, Jesmok G, Malik AB. Recombinant tumor necrosis factor increases pulmonary vascular permeability independent of neutrophils. Proc Natl Acad Sci USA. 1988;85:9219–23. doi: 10.1073/pnas.85.23.9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brett J, Gerlach H, Nawroth P, Steinberg S, Godman G, Stern D. Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J Exp Med. 1989;169:1977–91. doi: 10.1084/jem.169.6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burke-Gaffney A, Keenan AK. Does TNF-alpha directly increase endothelial cell monolayer permeability? Agents Actions. 1993;38:C83–5. doi: 10.1007/BF01991145. (Special Issue) [DOI] [PubMed] [Google Scholar]
- 50.Millo JL, Schultz MJ, Williams C, et al. Compartmentalisation of cytokines and cytokine inhibitors in ventilator-associated pneumonia. Intens Care Med. 2004;30:68–74. doi: 10.1007/s00134-003-2060-0. [DOI] [PubMed] [Google Scholar]
- 51.Miller BE, Bakewell WE, Katyal SL, Singh G, Hook GE. Induction of surfactant protein (SP-A) biosynthesis and SP-A mRNA in activated type II cells during acute silicosis in rats. Am J Respir Cell Mol Biol. 1990;3:217–26. doi: 10.1165/ajrcmb/3.3.217. [DOI] [PubMed] [Google Scholar]
- 52.Lesur O, Veldhuizen RA, Whitsett JA, et al. Surfactant-associated proteins (SP-A, SP-B) are increased proportionally to alveolar phospholipids in sheep silicosis. Lung. 1993;171:63–74. doi: 10.1007/BF00542334. [DOI] [PubMed] [Google Scholar]
- 53.Nogee LM, Wispe JR, Clark JC, Weaver TE, Whitsett JA. Increased expression of pulmonary surfactant proteins in oxygen-exposed rats. Am J Respir Cell Mol Biol. 1991;4:102–7. doi: 10.1165/ajrcmb/4.2.102. [DOI] [PubMed] [Google Scholar]
- 54.Wikenheiser KA, Wert SE, Wispe JR, et al. Distinct effects of oxygen on surfactant protein B expression in bronchiolar and alveolar epithelium. Am J Physiol. 1992;262:L32–9. doi: 10.1152/ajplung.1992.262.1.L32. [DOI] [PubMed] [Google Scholar]
- 55.Sugahara K, Iyama K, Sano K, Kuroki Y, Akino T, Matsumoto M. Overexpression of surfactant protein SP-A, SP-B, and SP-C mRNA in rat lungs with lipopolysaccharide-induced injury. Lab Invest. 1996;74:209–20. [PubMed] [Google Scholar]
- 56.Ingenito EP, Mora R, Cullivan M, et al. Decreased surfactant protein-B expression and surfactant dysfunction in a murine model of acute lung injury. Am J Respir Cell Mol Biol. 2001;25:35–44. doi: 10.1165/ajrcmb.25.1.4021. [DOI] [PubMed] [Google Scholar]
- 57.Tokieda K, Iwamoto HS, Bachurski C, et al. Surfactant protein-B-deficient mice are susceptible to hyperoxic lung injury. Am J Respir Cell Mol Biol. 1999;21:463–72. doi: 10.1165/ajrcmb.21.4.3436. [DOI] [PubMed] [Google Scholar]