Abstract
Up to 20% of Crohn's disease (CD) patients respond poorly to glucocorticoids (GC). A product of an alternative splicing of the glucocorticoid receptor (GR) premRNA, GRβ, may play a role as a dominant inhibitor of the glucocorticoid response. Increasing evidence suggests that inflammatory cytokines such as interleukin (IL)-18 alternate the splicing of the primary transcript between the two isoforms GRβ and GRα in hGR gene of CD patients. The aim of this study is to assess the expression of GRα and GRβ in patients with CD and to look for a possible correlation between these receptors and the response to glucocorticoid treatment. Forty-two CD patients and 17 healthy volunteers were studied. Quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed using real-time PCR techniques. Serum IL-18 protein levels were measured by enzyme-linked immunosorbent assay (ELISA). The amount of hGRα-mRNA in patients in remission was significantly lower than in controls (P < 0·05). The amount of hGRβ-mRNA was significantly higher in GC-resistant patients in the active stage of disease compared with all other groups (P < 0·05). Patients in the active stage of the disease had higher levels of IL-18 than patients in remission and both had higher levels than controls (P < 0·05). The amounts of IL-18 were directly correlated with the amount of hGRβ mRNA in GC-resistant patients with an active disease. High levels of hGRβ might be connected to GC resistance. IL-18 might participate in the alternative splicing of the hGR preliminary mRNA of CD patients. The results support the theory that augmented hGRβ mRNA expression level in PBMC is connected with GC-resistance of CD patients.
Keywords: Crohn's disease, glucocorticoid receptor alpha, glucocorticoid receptor beta, glucocorticoid receptor mRNA, IL-18
Introduction
Glucocorticoids (GC) have a known immunosuppressive effect and are used widely in the treatment of chronic inflammatory disease such as Crohn's disease (CD) [1,2]. However, up to 20% of CD patients are resistant to glucocorticoid from the onset of treatment [3]. Because the administration of glucocorticoid can cause a wide range of side effects such as Cushingoid features, osteoporosis, cataract and other toxicities [4,5], it is important to avoid treatment in those who will not benefit from its use.
GC anti-inflammatory response is mediated through glucocorticoid receptor α (GRα), which is a ligand-dependent transcription factor [6]. Binding of the GRα to the steroid causes translocation of the hormone-receptor complex from the cytoplasm to the nucleus and the activated complex binds to GC response element (GRE) on the DNA.
Two human glucocorticoid receptor isoforms have been described, GRα and GRβ[7]. Both isoforms are produced by alternative splicing of the same gene and share the first 8 exons but differ in exon 9. Due to this difference they do not share the same carboxy terminus, and GRβ is unable to bind the hormone and to transactivate the glucocorticoid responsive promoter [8].
The molecular mechanism underlying steroid resistance is still unknown. Recently it was suggested that hGRβ might serve as a dominant negative inhibitor of the GRα activity. A relatively high endogenous expression of the GRβ isoform in a cell-type-specific pattern might be responsible for the GRβ-mediated anti-glucocorticoid activity [8–11]. Honda et al. [12] have reported that hGRβ mRNA expression in peripheral lymphocytes in ulcerative colitis (UC) patients have a close relationship to their GC response.
Increasing evidence suggests that cytokines and/or growth factors might participate in the control of GRβ expression. Combination of the cytokines interleukin (IL)-2 and IL-4 in peripheral blood of glucocorticoid-resistant asthma patients was found to enhance the expression of GRβ[13]. IL-18, an early signal in the development of T lymphocyte helper type 1 (Th-1) response, was seen to be increased in CD patients [14,15]. Moreover, in in-vitro culture experiments of human peripheral blood mononuclear cells (PBMC) in inflammatory bowel disease (IBD) patients, IL-18 was found to induce the expression of hGRβ mRNA [16].
The aim of the study was to determine if levels of hGRα and hGRβ can predict a patient's response to glucocorticoid therapy. IL-18 levels in the serum of CD patients were also measured in order to investigate a possible connection between IL-18 and alternative splicing of the GR gene. We also attempted to develop a reliable, reproductive and rapid technique for the detection and quantification of hGRα and hGRβ expression.
Materials and methods
Patients and materials
Blood from 42 CD patients and 17 healthy volunteers was examined. All patients with CD had been diagnosed by accepted criteria, i.e. typical clinical symptoms, histological manifestation, colonoscopy, computerized tomography or small bowel radiology [17,18]. Patients were divided into subgroups according to their Crohn's disease activity index (CDAI) at the time of blood donation: active stage of disease (CDAI ≥150) and inactive stage of disease (CDAI < 150); and according to their previous GC-response. Patients who went into remission on 40 mg/day dose of prednisone (or an equivalent medication) within 4 weeks were considered GC-responsive. Patients who did not respond to GC therapy received either azathioprine or anti-tumour necrosis factor (TNF). GC-resistant patients who were in remission were maintained in remission by either azathioprine (four patients) or methotrexate (two patients); one patient entered remission spontaneously. Patients whose CDAI did not decrease below 150 or who required surgery were considered GC-resistant. Characteristics of patients in each subgroup and healthy volunteers are summarized in Table 1.
Table 1.
Characteristics of patients with Crohn's disease (CD)
| No. of cases | Gender (F/M) | Age (years) | CDAIa (at blood donation) | |
|---|---|---|---|---|
| Inactive disease | ||||
| GC-responsive | 6 | 4/2 | 40 ± 21 | 79 ± 37 |
| GC-resistant | 7 | 2/5 | 40 ± 17 | 80 ± 46 |
| Active disease | ||||
| GC-responsive | 14 | 5/9 | 31 ± 14 | 243 ± 64 |
| GC-resistant | 15 | 10/5 | 36 ± 15 | 259 ± 80 |
| Control | 17 | 13/4 | 46 ± 18 | |
Data are presented as mean ± s.d.
CDAI, Crohn's disease activity index. GC: glucocorticoid receptor.
Isolation of peripheral blood mononuclear cells
Ten ml of heparinized blood was donated by the CD patients and by the healthy volunteers. PBMC were isolated by Ficoll gradient centrifugation using Histopaque 1077 (Sigma, St. Louis, MO, USA). Informed consent was obtained from each patient included in the study. The protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
Detection and analysis of hGRα and hGRβ mRNA
Expression of hGRα and hGRβ mRNA in PBMC was determined by reverse transcription of total RNA followed by quantitative reverse transcription-polymerase chain reaction (RT-PCR). Total RNA from PBMC was isolated by EZ-RNA total RNA isolation kit (Biological Industries, Beit Haemek, Israel). cDNA was synthesized by extension of random hexamer primer with mouse mammary leukaemia virus (MMLV) reverse transcriptase (EZ-first strand cDNA synthesis kit for RT-PCR, Biological Industries). The transcript of human porphobilinogen deaminase (hPBGD) was amplified from each of the cDNA samples to serve as an internal standard and for normalizing the results as described by Wang et al. (19) (LightCycler-hPBGD housekeeping gene set; Roche Diagnostics GmbH, Mannheim, Germany). This method allows simultaneous reverse transcription and real-time PCR amplification of two mRNAs of specific genes of interest (analyte genes) and mRNA of constantly transcribed genes (housekeeping genes) in a single-tube reaction. Serial dilutions of hGRα and hGRβ RT-PCR products were used as external standards in each run.
Amplification of the target transcript was monitored using labelled hybridization probes that hybridize to an internal sequence of the amplified target. Data analysis of the LightCycler results was performed with the second derivative maximum method (LightCycler software version 3·5, Roche Diagnostics GmbH, Mannheim, Germany).
PCR conditions and reagents
Quantitative real-time PCR was performed with the LightCycler instrument (Roche Applied Science, Penzberg, Germany) using fluorescent resonance energy transfer (FRET) chemistry. Each 10 µl of PCR reaction contained 1 µl cDNA and final concentrations of: 0·5 µ M each primer, 0·2 µ M of each probe and 2 m M Mg+2 and 1 µl ‘FastStart’ PCR reaction mix (LightCycler-FastStart DNA Master Hybridization Probes kit; Roche Diagnostics GmbH). Primers and hybridization probes are summarized in Table 2. Each of the PCR programmes consisted of 10 min at 95°C for activation of the FastStart Taq DNA polymerase followed by 45 PCR cycles of denaturing at 95°C for 10 s, annealing at 60°C for 10 s (hGRα) or 55°C for 10 s followed by 50°C for 10 s from the 11th cycle (for hGRβ) and elongation at 72°C for 20 s. For hPBGD the transcript was amplified according to the manufacturer's instructions (Roche Diagnostics GmbH).
Table 2.
Primers and hybridization probes used to detect hGRα and hGRβ transcripts
| Oligonucleotidea | Position in message | |
|---|---|---|
| hGRα | ||
| Forward primer12 | CCTAAGGACGGTCTGAAGAGC | 2158–2178 |
| Reverse primer12 | GCCAAGTCTTGGCCCTCTAT | 2616–2635 |
| Donor probe | TTGTCAGTTGATAAAACCGCTGCCAGTTCT-flu | 2290–2261 |
| Acceptor probe | LC Red640-GCTGGAGTTTCCTTCCCTCTTGACAATG | 2259–2230 |
| hGRβ | ||
| Forward primer19 | ACACAGGCTTCAGGTATCTT | 2091–2110 |
| Reverse primer12 | CCACGTATCCTAAAAGGGCAC | 2503–2523 |
| Donor probe | TGTGCACTTCGTTGTCAATAATAAGTCAACT-flu | 2421–2451 |
| Acceptor probe | LC Red640-ATGCTCATCGACAACTATAGGAGGCTTT | 2453–2480 |
Oligonucleotide sequences are 5′−3′.
Detection of IL-18 in serum
IL-18 concentration in patients' serum as well as healthy volunteers were measured by enzyme-linked immunosorbent assay (ELISA) (MBL, Naka-Ku Nagoya, Japan) in only 31 CD patients and 10 healthy volunteers, due to technical problems with the serum specimens.
Statistical analysis
Values are given as mean ± s.e.m. unless otherwise stated. The data were analysed using SPSS statistical software (SPSS Inc. Chicago, IL, USA). Statistical differences in the amounts of hGRα and hGRβ mRNA between the different groups were analysed using the non-parametric Mann–Whitney two-sample test and the Kruskall–Wallis test. Correlation between GR mRNA values and IL-18 content were analysed with Pearson's correlation coefficient. Statistical significance was based on a two-sided P-value of less than 0·05.
Results
The quantitative real-time PCR assay we used proved to be extremely sensitive in comparison to previously reported assays, which used conventional PCR products that were run on an agarose gel [7] or used Syber green fluorescence [8]. We used hybridization probes that are separated by one nucleotide and are complementary to the sequence within the central region of exon 9α for hGRα or exon 9β for hGRβ. HGR expression was normalized using specific primers and hybridization probes for the PBGD gene. PBGD was used in our assay because it belongs to the low abundance class of mRNAs. To achieve absolute quantification of hGR mRNA we used serial dilutions of in-vitro transcribed hGRα or hGRβ RNA as an external standard.
The amounts measured for hGRα mRNA and hGRβ mRNA in PBMC of CD patients and healthy volunteers are summarized in Table 3. The mean amount of hGRα mRNA expression in PBMC was lower in CD patients compared with healthy volunteers. The amounts were significantly lower in patients in remission (P < 0·05) compared to patients with active disease.
Table 3.
Mean expression of hGRα mRNA, hRGβ mRNA in Crohn's disease (CD) patients and controls
| No. of cases | hGRα/PBGD (× 10−3) | hGRβ/PBGD (× 10−4)* | |
|---|---|---|---|
| Controls | 17 | 81·1 ± 21 | 6·18 ± 1·0 |
| CD patients | 42 | 44·4 ± 7·2 | 13 ± 5.3 |
| Inactive disease | 13 | 8·2 ± 2·2 | 3·8 ± 5·3 |
| GC-responsive | 6 | 9·12 ± 3·7 | 6·14 ± 3·7 |
| GC-resistant | 7 | 7·47 ± 2·7 | 1·88 ± 0·5 |
| Active disease | 29 | 63·6 ± 17 | 15·8 ± 7·0 |
| GC-responsive | 14 | 77·1 ± 25 | 5·69 ± 2·2 |
| GC-resistant | 15 | 51·0 ± 23 | 23·8 ± 13 |
The amounts of hGRα mRNA and hGRβ mRNA in peripheral blood mononuclear cells (PBMC) of CD patients and controls were normalized using the result of quantitative reverse transcription-polymerase chain reaction of human porphobilinogen deaminase (hPBGD) mRNA in each individual case. GC: glucocorticoid receptor.
The mean amount of hGRβ mRNA in GC-resistant active patients was significantly higher than in all other groups (patients as well as healthy volunteers) (P < 0·05) hGRβ was significantly decreased in GC resistant compared to GC responsive patients with inactive disease. Our assay allowed us to measure hGRβ mRNA expression in all samples (patients and controls).
IL-18 protein levels in CD patients and healthy volunteers
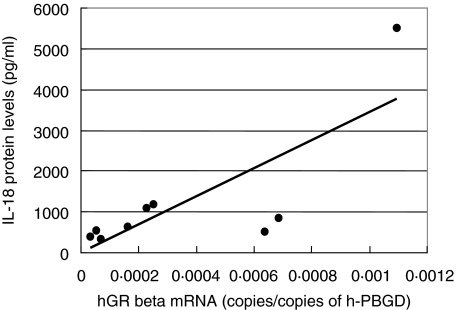
The levels in CD patients and healthy volunteers are summarized in Table 4. ELISA of patients' serum showed that CD patients, whether active or inactive, had up to sixfold higher amounts of IL-18 protein levels compared to healthy volunteers. Patients in the active stage of disease (CDAI ≥150) had up to twofold higher amounts of IL-18 protein than CD patients in remission (CDAI < 150). That serum IL-18 levels rise together with the CDAI was also shown in an as yet unreported study of ours in 120 patients with CD. The serum concentrations of IL-18 showed a significant correlation to the amounts of hGRβ mRNA in GC-resistant patients in an active stage of disease (r = 0·774, P < 0·05) (Fig. 1). These results indicate that IL-18 might play a role in the alternative splicing of the hGR preliminary mRNA of CD patients.
Table 4.
Mean levels of cytokine interleukin (IL)-18 in Crohn's disease (CD) patients and controls
| No. of cases | IL-18 (pg/ml)* | |
|---|---|---|
| Inactive disease | ||
| 567 ± 100 | 6 | GC-responsive |
| 652 ± 193 | 6 | GC-resistant |
| Active disease | ||
| 963 ± 188 | 10 | GC-responsive |
| 1227 ± 542 | 9 | GC-resistant |
| 218 ± 46 | 10 | Controls |
Results are reported as mean ± s.e. GC: glucocorticoid receptor.
Fig. 1.
Positive correlation between the amounts of hGRb mRNA in glucocorticoid (GC)-resistant patients in an active stage of disease and their interleukin (IL)-18 protein levels (n = 9) (r = 0·774, P < 0·05).
Discussion
In the present study we have analysed the expression and regulation of hGRα and hGRβ isoforms in human PBMC, and we have presented a fast and reproducible method for quantification of GR mRNA expression levels. In contrast to current end-point quantification methods, such as the competitive PCR assay, the fluorometric method for cycle-to-cycle quantification of PCR product proved to be a fast and reliable quantification method.
We found that the expression of hGRα mRNA in PBMC of CD patients is significantly higher in patients with active disease and in control subjects than in patients with inactive disease, whether steroid-resistant or steroid-responsive. This is an interesting and intriguing finding, for which we as yet have no satisfactory explanation. Other authors have also described low levels of hGR in autoimmune diseases [20]. Hori et al. [21] found a lower amount of GRα mRNA in PBMC of CD patients compared with healthy volunteers. Most of the patients in their study were in remission, therefore their finding of lower levels of GRα in patients compared to controls are in agreement with our group of patients in remission and our control group. Andreae et al. [22] found that children with autoimmune disease had lower amounts of GR on PBMC than healthy children. Schlaghecke et al. [23] have also described a reduced number of GR in mononuclear leucocytes of rheumatoid arthritis patients compared with healthy volunteers. All these studies point to down-regulation of the hGRα in patients with autoimmune disease during remission. In contrast, Raddatz et al.[32] did not find a difference in GR levels between patients with inflammatory bowel disease and healthy subjects; they did, however, find that GR expression was decreased in biopsies from ulcerative colitis patients. The significance of this observation is not yet clear and further studies of GC receptor regulation are required.
We chose to measure IL-18 levels because IL-18 was found to be a part of hGR regulation [16,30]. Levels of hGRβ showed a significant correlation with IL-18. However, the discrepancy we found between IL-18 levels, which rise in all CD patients, whether in remission or during an exacerbation of disease, and hGRα levels, which are lower in CD patients during remission than in controls, suggests that other mechanisms and inflammatory mediators such as TGFα may also play a role in the regulation of GRα levels.
The other significant finding of our study was that the level of hGRβ was significantly higher in steroid resistant CD patients during an exacerbation but not during a remission. That the augmented level of hGRβ could not be due to GC treatment has been shown in other studies [25,26]. Korn et al. showed that in bronchial cells in vivo, exposure to GC did not cause augmented levels of hGRβ mRNA [27]. Honda et al. [12] showed that high doses of GC are not the inducers for the alternative splicing of the hGR gene in PBMC of UC patients.
The precise role of hGRβ is not known. The level of this form of the GC receptor is 200–500 times lower than the level of hGRα[28,29], so it could not directly block hGRα activity by competitively binding to the same ligand [25]. On the other hand, higher levels of hGRβ were found to be connected to GC resistance in ulcerative colitis patients [12] and in asthma patients [13]. It was also found that within tissues, hGRβ is expressed at high levels in a cell type-specific manner [10], therefore it may inhibit hGRα by another, still unknown, mechanism. Metabolism of hGRβ in patients resistant to glucocorticoids was different than in those who were glucocortocoid-sensitive both during exacerbation of disease, where it was significantly higher, and during remission, when it was significantly lower. Our findings support the possible role for hGRβ as a predictor of steroid resistance. Further study is needed to confirm the precise role of the hGRβ molecule.
It is conceivable that IL-18 and other proinflammatory cytokines contribute to the alternative splicing of the hGR gene by inducing an imbalanced expression of the hGRβ isoform in PBMC of CD patients. Indeed, our results support previous hypotheses that high hGRβ isoform expression in PBMC can be used to predict glucocorticoid resistance during the active phase of the disease [20,21]. Further studies are, however, required as to the function and regulation of steroid receptors.
Acknowledgments
This study was supported by a grant from the Hermann and Dan Mayer Foundation.
References
- 1.Summers RW, Switz DM, Sessions JT, Jr, et al. National cooperative Crohn's disease study: results of drug treatment. Gastroenterology. 1979;77:847–69. [PubMed] [Google Scholar]
- 2.Malchow H, Ewe K, Brandes JW, et al. European cooperative Crohn's disease study (ECCDS): results of drug treatment. Gastroenterology. 1984;86:249–66. [PubMed] [Google Scholar]
- 3.Munkholm P, Langholz E, Davidsen M, Binder V. Frequency of glucocorticoid resistance and dependency in Crohn's disease. Gut. 1994;35:360–2. doi: 10.1136/gut.35.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singleton JW, Law DH, Kelley ML, Jr, Mekhjian HS, Sturdevant RA. National cooperative Crohn's disease study: adverse reaction to study drugs. Gastroenterology. 1979;77:70–82. [PubMed] [Google Scholar]
- 5.Baldassano RN. Anti-TNF therapies have eliminated the need for steroids in pediatric Crohn's disease: pro. Why use steroids if safer therapies are available? Inflamm Bowel Dis. 2001;7:338–41. doi: 10.1097/00054725-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ, Adfcock I. Anti-inflammatory actions of steroids: molecular mechanism. Trends Pharmacol Sci. 1993;14:436–41. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- 7.Hollenberg SM, Weinberger C, Ong ES, et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–41. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oakley RH, Madhabananda S, Cidlowski JA. The human glucocorticoid receptor β isoform. expression, biochemical properties, and putative function. J Biol Chen. 1996;2:9550–9. doi: 10.1074/jbc.271.16.9550. [DOI] [PubMed] [Google Scholar]
- 9.Bamberger CM, Bamberger AM, deCastro M. Glucocorticoid receptor β, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest. 1995;95:2435–41. doi: 10.1172/JCI117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oakley RH, Webster JC, Sar M, Parker CR, Jr, Cidlowski JA. Expression and subcellular distribution of the β-isoform of the human glucocorticoid receptor. Endocrinology. 1997;138:5028–38. doi: 10.1210/endo.138.11.5501. [DOI] [PubMed] [Google Scholar]
- 11.Bamberger CM, Else T, Bamberger AM, Beil FU, Schulte HM. Regulation of the human interleukin-2 gene by the α and β isoforms of the glucocorticoid receptor. Mol Cell Endocrinol. 1997;36:23–8. doi: 10.1016/s0303-7207(97)00209-8. [DOI] [PubMed] [Google Scholar]
- 12.Honda M, Orii F, Ayabe T, et al. Expression of glucocorticoid receptor beta in lymphocytes of patients with glucocorticoid-resistant ulcerative colitis. Gastroenterology. 2000;118:859–66. doi: 10.1016/s0016-5085(00)70172-7. [DOI] [PubMed] [Google Scholar]
- 13.Leung DY, Hamid Q, Vottero A, et al. Association of glucocorticoid insensitivity with increased expression of glucocorticoid receptor beta. J Exp Med. 1997;186:1567–74. doi: 10.1084/jem.186.9.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pizarro TT, Michie MH, Bentz M, et al. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829–35. [PubMed] [Google Scholar]
- 15.Monteleone G, Trapasso F, Parrello T, et al. Bioactive IL-18 expression is up-regulated in Crohn's disease. J Immunol. 1999;163:143–7. [PubMed] [Google Scholar]
- 16.Orii F, Ashida T, Nomura M, et al. Quantitative analysis for human glucocorticoid receptor α/β mRNA in IBD. Biochem Biophys Res Commun. 2002;296:1286–94. doi: 10.1016/s0006-291x(02)02030-2. [DOI] [PubMed] [Google Scholar]
- 17.Best WR, Becktelw JM, Singleton JW. Rederived values of the eight coefficients of the Crohn's disease activity index (CDAI) Gastroenterology. 1979;77:843–6. [PubMed] [Google Scholar]
- 18.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. Gastroenterology. 1976;70:438–44. [PubMed] [Google Scholar]
- 19.Wang C, Gao D, Vaglenov A, Kaltenboeck B. One-step real-time duplex reverse transcription PCRs simultaneously quantify analyte and housekeeping gene mRNAs. Biotechniques. 2004;36:518–19. doi: 10.2144/04363RN06. [DOI] [PubMed] [Google Scholar]
- 20.Schlechte JA, Ginsberg BH, Shermann BM. Regulation of the glucocorticoid receptor in human lymphocytes. J Steroid Bioch. 1982;16:69–74. doi: 10.1016/0022-4731(82)90145-5. [DOI] [PubMed] [Google Scholar]
- 21.Hori T, Watanabe K, Miyaoka M, et al. Glucocorticoid receptors and Crohn's disease: expression of mRNA for glucocorticoid receptors in peripheral blood mononuclear cells of patients with Crohn's disease. J Gasteroenterol Hepatol. 2002;17:1070–7. doi: 10.1046/j.1440-1746.2002.02841.x. [DOI] [PubMed] [Google Scholar]
- 22.Andreae J, Tripmacher R, Weltrich R, et al. Effects of glucocorticoid therapy on glucocorticoid receptors in children with autoimmune diseases. Pediatr Res. 2001;49:130–5. doi: 10.1203/00006450-200101000-00025. [DOI] [PubMed] [Google Scholar]
- 23.Schlaghecke R, Kornely E, Wollenhaupt J, Specker C. Glucocorticoid receptors in rheumatoid arthritis. Arthritis Rheum. 1992;35:740–4. doi: 10.1002/art.1780350704. [DOI] [PubMed] [Google Scholar]
- 24.Raddatz D, Middel P, Bockemuhl M, et al. Glucocorticoid receptor expression in inflammatory bowel disease: evidence for a mucosal down regulation in steroid-unresponsive ulcerative colitis. Alim Pharmacol Therap. 19:1365–2036. doi: 10.1046/j.1365-2036.2003.01802.x. [DOI] [PubMed] [Google Scholar]
- 25.Shipman GF, Bloomfield CD, Gajl-Peczalska KJ, Munck AU, Smith KA. Glucocorticoids and lymphocytes. III. Effects of glucocorticoid administration on lymphocyte glucocorticoid receptor. Blood. 1983;61:1086–90. [PubMed] [Google Scholar]
- 26.Pujols L, Mullol J, Perez M, et al. Expression of the human glucocorticoid receptor α and β isoforms in human respiratory epithelial cells and their regulation by dexamethason. Am J Respir Cell Mol Biol. 2001;24:49–57. doi: 10.1165/ajrcmb.24.1.4024. [DOI] [PubMed] [Google Scholar]
- 27.Korn SH, Wouters EF, Wesseling G, Arends JW, Thunnissen FB. In vitro and in vivo modulation of α and β-glucocorticoid receptor mRNA in human bronchial epithelium. Am J Respir Crit Care Med. 1977;155:1117–22. doi: 10.1164/ajrccm.155.3.9116996. [DOI] [PubMed] [Google Scholar]
- 28.Oakley RH, Jewell CM, Yudt MR, Bofetiado DM, Cidlowski JA. The dominant negative activity of the human glucocorticoid receptor β isoform. J Biol Chem. 1999;274:27857–66. doi: 10.1074/jbc.274.39.27857. [DOI] [PubMed] [Google Scholar]
- 29.Carlstedt-Duke J. Glucocorticoid receptor β, View II. Trends Endocrinol Metab. 1999;10(8):339–42. doi: 10.1016/s1043-2760(99)00178-2. [DOI] [PubMed] [Google Scholar]
- 30.Dong Y, Poellinger L, Gustafsson JA, Okret S. Regulation of glucocorticoid receptor expression: evidence for transcriptional and posttranscriptional mechanisms. Mol Endocrinol. 1988;2:1256–64. doi: 10.1210/mend-2-12-1256. [DOI] [PubMed] [Google Scholar]