Abstract
Capsular polysaccharide from Actinobacillus actinomycetemcomitans Y4 (Y4 CP) induces bone resorption in a mouse organ culture system and osteoclast formation in mouse bone marrow cultures, as reported in previous studies. We also found that Y4 CP inhibits the release of interleukin (IL)-6 and IL-8 from human gingival fibroblast (HGF). Thus Y4 CP induces various responses in localized tissue and leads to the secretion of several cytokines. However, the effects of Y4 CP on human monocytes/macrophages are still unclear. In this study, THP-1 cells, which are a human monocytic cell line, were stimulated with Y4 CP, and we measured gene expression in inflammatory cytokine and signal transduction pathways. IL-1β and tumour necrosis factor (TNF)-α mRNA were induced from Y4 CP-treated THP-1 cells. IL-1β mRNA expression was increased according to the dose of Y4 CP, and in a time-dependent manner. IL-1β mRNA expression induced by Y4 CP (100 µg/ml) was approximately 7- to 10-fold greater than that in the control by real-time PCR analysis. Furthermore, neither PD98059, a specific inhibitor of extracellular signal-regulated kinase nor SB203580, a specific inhibitor of p38 kinase prevented the IL-1β expression induced by Y4 CP. However, JNK Inhibitor II, a specific inhibitor of c-Jun N-terminal kinase (JNK) prevented the IL-1β mRNA expression induced by Y4 CP in a concentration-dependent manner. These results indicate that Y4 CP-mediated JNK pathways play an important role in the regulation of IL-1β mRNA. Therefore, Y4 CP-transduced signals for IL-1β induction in the antibacterial action of macrophages may provide a therapeutic strategy for periodontitis.
Keywords: Actinobacillus actinomycetemcomitans, IL-1, macrophage, JNK
Introduction
Periodontitis is a chronic inflammatory disease characterized by gingival inflammation and alveolar bone resorption. Periodontitis is often caused by infections with Gram-negative bacteria including Actinobacillus actinomycetemcomitans[1,2] and Porphyromonas gingivalis[3,4].
A. actinomycetemcomitans is a gram-negative, capnophilic, fermentative coccobacillus that has been implicated in the aetiology and pathogenesis of several forms of periodontal disease [1]. Clinical, microbiological, and immunological studies have explored the correlation between A. actinomycetemcomitans and several types of periodontitis [5,6]. A. actinomycetemcomitans produces several tissue-damaging products such as leukotoxin [7,8], lipopolysaccharide (LPS), capsular polysaccharide [9–11], alkaline and acid phosphatases, an epitheliotoxin, a fibroblast inhibitory factor, and a bone resorption-inducing toxin [5].
Amano et al. [12] extracted a serotype-specific capsular polysaccharide-like antigen from whole cells of A. actinomycetemcomitans Y4 (serotype b) by autoclaving, purified it by ion-exchange chromatography and gel filtration, and showed that it is a polymer that consists of a repeating disaccharide unit −3)–d-fucopyranosyl-(1,2)-l-rhamnopyranosyl-(1—. Previous studies have shown that A. actinomycetemcomitans Y4 capsular polysaccharide (Y4 CP) induces IL-1 from a mouse macrophage cell line [13], bone resorption in a mouse organ culture system and osteoclast formation in mouse bone marrow cultures [14,15], and inhibits the release of IL-6 and IL-8 from human gingival fibroblasts [16]. On the other hand, the fact that LPS is a bacterial component of gram-negative bacteria was revealed in studies on the details of innate immune responses through gene expression and signal transduction pathways [17,18]. LPS induces mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK), c-jun NH2-terminal protein kinase (JNK), and p38 mitogen-activated protein kinase (p38). These play key roles in LPS-mediated signal transduction between extracellular membrane stimulation and the cytoplasmic response and nuclear activity in the activation of the gene [19,20].
However, there has still been no report on the effect of Y4 CP on human immunocytes. In this study, we found that Y4 CP affected the gene expression of inflammatory cytokine in macrophages, which play an important role in host defense and inflammation, and examined which signal transduction pathways are used in this gene expression.
Materials and methods
Cell culture protocol
THP-1 cells were differentiated to macrophage as follows. THP-1 cells (Dainippon Pharmaceutical Co., Ltd. Japan) were grown in RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine and 2 × 10−5 M 2-ME in 5% CO2-air humidified atmosphere at 37°C. THP-1 cells were treated with 50 nM 1,25-dihydroxy-vitamin D3 (Calcitriol, Wako, Japan) for 72 h, washed three times with PBS and allowed to rest overnight in RPMI 1640 with 5% FCS [21].
Microorganisms
A. actinomycetemcomitans Y4 (serotype b) were grown in Todd-Hewitt broth (Difco Laboratories, Detroit, MI, USA) supplemented with 1% (wt/vol) yeast extract at 37°C for 3 days in an atmosphere of 5% CO2[22]. The organisms were harvested by centrifugation, washed three times with distilled water, and lyophilized.
Extraction of Y4 CP
The lyophilized cell suspension (300 mg/ml) in saline was autoclaved at 121°C for 15 min [23]. After being autoclaved, the suspension was cooled and centrifuged at 10 000 × g for 20 min, and the supernatant was collected. Extraction was repeated on residual whole cells. The supernatants were combined, dialysed extensively with distilled water, and lyophilized.
Purification of Y4 CP
Serotype antigens were purified according to the method of Amano et al. [12]. The autoclaved extracts of A. actinomycetemcomitans Y4 were solubilized with 0·01 M Tris hydrochloride, pH 8·2, to give a final concentration of 100 mg (dry weight) of bacterial extract per ml and dialysed against the buffer at 4°C for 2 days. A 5 ml portion of the antigen suspension was applied to a column of DEAE-Sephadex A-25 (2 × 30 cm; Pharmacia Fine Chemicals, Piscataway, NJ, USA) that had been equilibrated with the buffer and eluted with 200 ml of the buffer followed by a linear gradient of 0 to 1 M NaCl in the buffer at 4°C. Fractions (10 ml each) were monitored for total sugar, protein, and phosphorus. Fractions that showed a positive reaction with anti-A. actinomycetemcomitans Y4 serum by immunodiffusion were combined and concentrated in a rotary evaporator. These preparations were dialysed with distilled water at 4°C for 3 days, applied to a column of Sephacryl S-300 (1·5 × 100 cm; Pharmacia), and eluted with distilled water. Fractions that contained the CP with serotype-specific antigens were pooled and lyophilized.
RT-PCR assay and real-time PCR analysis
For RT-PCR, total cellular RNA was prepared using TRIzol reagent. cDNA was synthesized from total RNA by the extension of random primers with 200 U of Superscript II. PCR of the cDNA was performed using AccuPower PCR PreMix (BIONEER, Daejeon, Korea), which contains specific primers at 20 pmol. The following primers were showed at Table 1. The synthesized PCR products were separated by electrophoresis on 1·5% agarose gel and visualized by ethidium bromide staining. To quantify IL-1β mRNA, real-time PCR was performed using an ABI Prism 7000 (Applied Biosystems, Foster City, CA, USA) with TaqMan Universal PCR master mixture (Applied Biosystems). For PCR, 5 µl of sample was directly added to 45 µl of a RT-PCR mixture prepared from 2× RT-PCR TaqMan Universal PCR master mixture containing each primer at a concentration of 1 µM, 2 mM MgCl, and 100 µM probe. The cycle parameters were as follows: 3 min at 95°C and 40 cycles of 1 min at 95°C, 1 min at 52°C, and 1 min 30 s at 72°C. Cycling was preceded by incubation for 10 min at 95°C to activate AmpliTaq Gold. For reverse transcription, all of these steps were preceded by 30 min of incubation at 48°C. Amplification mixtures were analysed using the ABI Prism detection system. Changes in gene expression were assessed using the comparative Ct method. (http://docs.appliedbiosystems.com/pebiodocs/04303859.pdf)
Table 1.
Primer sequences used for RT-PCR.
| Human gene | Sequence | Product size | |
|---|---|---|---|
| IL-1β | sense | AAA CAG ATG AAG TGC TCC TTC AGG | 390 bp |
| antisense | TGG AGA ACA CCA CTT GTT GCT CCA | ||
| IL-6 | sense | GTG TTG CCT GCT GCC TTC CCT G | 320 bp |
| antisense | CTC TAG GTA TAC CTC AAA CTC CAA | ||
| TNF-α | sense | CAG AGG GAA GAG TTC CCC AG | 324 bp |
| antisense | TGG AGA ACA CCA CTT GTT GCT CCA | ||
| IL-12p35 | sense | CAC TCC AGA CCC AGG AAT GT | 293 bp |
| antisense | TAC TAA GGC ACA GGG CCATC | ||
| IL-12p40 | sense | AAG GAG GCG AGG TTC TAA GC | 414 bp |
| antisense | TGA TGA AGA AGC TGC TGG TG | ||
| IL-18 | sense | GCT TGA ATC TAA ATT ATTATC AGT C | 334 bp |
| antisense | CAA ATT GCA TCT TAT TAT CAT G | ||
| GAPDH | sense | GTC TTC ACC ACC ATG GAG AAG GCT | 393 bp |
| antisense | CAT GCC AGT GAG CTT CCC GTT CA |
Inhibitors
PD98059, a specific inhibitor of extracellular signal-regulated kinase (ERK), SB203580, a specific inhibitor of p38 kinase, and JNK inhibitor II, a specific inhibitor of c-Jun N-terminal kinase (JNK), were purchased from Calbiochem-Novabiochem (La Jolla, CA, USA).
Statistical analysis
Data were analysed using the SPSS II software package (SPSS Japan Inc., Tokyo, Japan). The experimental groups were compared by one-way anova with the Tukey HSD-test.
Results
Y4 CP-induced IL-1β mRNA expression in undifferentiated and differentiated THP-1 cells
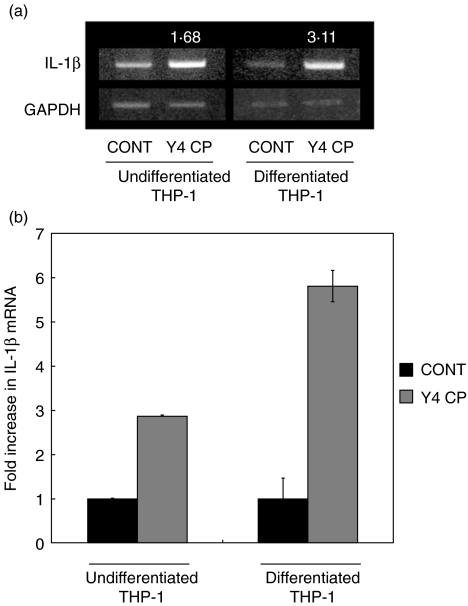
The reactivity of Y4 CP in undifferentiated and differentiated THP-1 cells was evaluated by the induction of IL-1β mRNA which is secreted from monocyte/macrophage. Both cell groups were treated with or without Y4 CP (100 µg/ml) for 4 h. After treatment, total RNA was harvested, and IL-1β mRNA expression was analysed by RT-PCR. The densities of PCR products were expressed numerically using NIH image (N.I.H. USA). The densities of undifferentiated and differentiated THP-1 cells were standardized by GAPDH of each group. The ratio of undifferentiated or differentiated versus control was calculated by the density of each. The ratios of undifferentiated and differentiated THP-1 cells were 1·68 and 3·11, respectively (Fig. 1a). Furthermore, quantitative analysis was performed by real-time PCR. While IL-1β mRNA expression of undifferentiated THP-1 cells was approximately 3-fold that in the control, IL-1β mRNA expression in differentiated THP-1 cells was approximately 6-fold that in the control upon Y4 CP stimulation (Fig. 1b).
Fig. 1.
Y4 CP–induced IL-1β mRNA expression in undifferentiated and differentiated THP-1 cells. To confirm the reactivity of Y4 CP, undifferentiated and differentiated THP-1 cells were examined with regard to IL-1β mRNA expression. Both cell groups were cultured with Y4 CP (100 µg/ml) or medium alone (control) for 4 h. After treatment, total RNA was prepared. IL-1β mRNA expression was analysed by RT-PCR. The densities of PCR products were expressed numerically using NIH image (N.I.H., USA). The densities of undifferentiated and differentiated THP-1 cells were standardized by GAPDH of each group. (a) The ratio of undifferentiated or differentiated versus control was calculated by the density of each. (b) IL-1β mRNA expression was analysed by real-time PCR.
Inflammatory cytokine mRNA expression compared to that of IL-1β
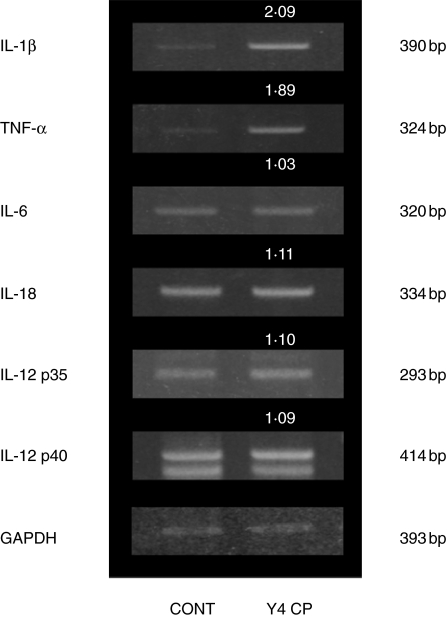
Monocyte and macrophage are well known to secrete not only IL-1β but also other inflammatory cytokines. Therefore, mRNA expression of TNF-α, IL-6, IL-12 and IL-18 from differentiated THP-1 cells that had been stimulated with Y4 CP was examined by RT-PCR. As shown in Fig. 2, the densities of PCR products were calculated using the same formula as in Fig. 1: the ratios of IL-1β, TNF-α, IL-6, IL-12 p35, IL-12 p40 and IL-18 were 2·09, 1·89, 1·03, 1·10, 1·09, and 1·11, respectively. IL-1β and TNF-α mRNA are both strongly expressed by Y4 CP.
Fig. 2.
Expression of cytokine mRNAs in differentiated THP-1 cells stimulated with Y4 CP. Differentiated THP-1 cells were treated with Y4 CP (100 µg/ml) or medium alone (control) for 4 h. After treatment, total RNA was prepared and inflammatory cytokine mRNA expression was examined by RT-PCR. The densities of PCR products were calculated using the same formula as in Fig. 1.
Quantitative analysis of IL-1β mRNA expression in differentiated THP-1 cells after Y4 CP stimulation
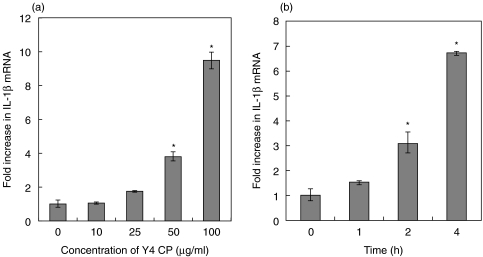
The optimal dose and duration of culture are very important for measuring mRNA expression. We treated differentiated THP-1 cells with various concentrations (0, 10, 25, 50, 100 µg/ml) of Y4 CP for various durations (0, 1, 2, 4 h). After stimulation, the expression of IL-1β mRNA in differentiated THP-1 cells was evaluated by real-time PCR. Treatment of differentiated THP-1 cells with Y4 CP caused an increase in the expression of IL-1β mRNA in a dose and time-dependent manner (Fig. 3a). When differentiated THP-1 cells were stimulated with 100 µg/ml Y4 CP for 4 h, Y4 CP induced approximately 7- to 10-fold greater IL-1β mRNA expression than that in the control.
Fig. 3.
(a) Effect of Y4 CP on IL-1β mRNA expression in differentiated THP-1 cells. Differentiated THP-1 cells were cultured in the presence of different concentrations of Y4 CP (10–100 µg/ml) or without Y4 CP as a control. (b) Effect of the duration of culture (1–4 h) with Y4 CP (100 µg/ml) on IL-1β mRNA expression in differentiated THP-1 cells. IL-1β mRNA levels were determined by real-time PCR. The results are expressed as ratios of the levels in the Control. Values shown are means+-standard deviations of triplicate assays. *P < 0·01 versus control.
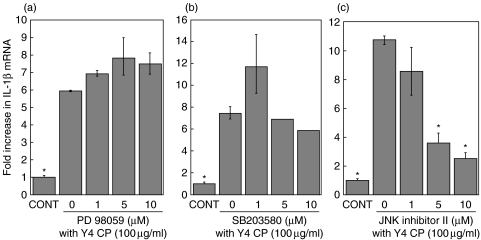
Signal pathways in IL-1β mRNA expression
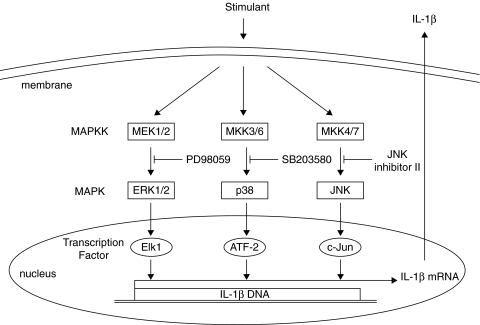
Various members of the MAPK family may modulate the expression of IL-1β in stimulated monocytes/macrophages. To investigate which MAPK pathway is involved in the expression of IL-1β mRNA when differentiated THP-1 cells are stimulated with Y4 CP, we used inhibitors of several MAPKs (Fig. 4). Differentiated THP-1 cells were treated with PD98059 (1–10 µM), SB203580 (1–10 µM), JNK Inhibitor II (1–10 µM), or vehicle (DMSO) for 30 min, respectively, and then stimulated with Y4 CP (100 µg/ml) for 4 h.
Fig. 4.
Proposed model for signal transduction pathways of MAP kinase in the regulation of IL-1β expression.
Pretreatment of THP-1 with SB203580 led to a slight decrease in the expression of IL-1β mRNA. However, significant differences were not observed upon pretreatment with SB203580 or PD98059. On the other hand, JNK Inhibitor II prevented the up-regulation of IL-1β mRNA expression in Y4 CP-stimulated THP-1; indeed, the expression of IL-1β mRNA was inhibited by 76% with 10 µM JNK Inhibitor II. These findings suggest that the JNK pathway is probably involved in mediating the response to Y4 CP (Fig. 5).
Fig. 5.
Effects of protein kinase inhibitors, i.e. inhibitors of ERK (PD98059)(a), p38 (SB203580)(b) and JNK (SP600125)(c), on Y4 CP-induced IL-1β mRNA expression. Differentiated THP-1 cells were pretreated with PD98059, SB203580, or JNK Inhibitor II (1, 5, 1 0 µM) for 30min. Cells were then cultured with Y4 CP (100 µg/ml) for an additional 4 h. Values shown are means ± standard deviations of triplicate assays. *P < 0·01 versus Y4 CP stimulation without inhibitor.
Discussion
Periodontitis is initiated by oral microbacteria such as P. gingivalis or A. actinomycetemcomitans that induce an inflammatory cascade, which stimulates host-mediated tissue destruction. Recent advances in the understanding of inflammation have provided insight into the mechanisms involved in periodontal tissue destruction. Several mediators appear to be involved, including a variety of cytokines produced by several different cell types. The primary mediators, such as IL-1 and TNF, have been shown to contribute to several events that are essential for the initiation of an inflammatory response and, ultimately, tissue destruction [24–26]. They can induce the up-regulation of adhesion molecules on leucocytes and endothelial cells and stimulate the production of chemokines, which are needed to recruit circulating leucocytes. IL-1 also induces the expression of other mediators that amplify or sustain the inflammatory response, such as prostaglandins, and the production of lytic enzymes, such as matrix metalloproteinases; they also can enhance bacterial killing and phagocytic activity [27]. Furthermore, IL-1 is synergistic in its capacity to enhance bone resorption [28]. Although the periodontium has a high capacity for repair following injury, in some situations cytokines may limit repair by inducing apoptosis of matrix-producing cells [29]. Moreover, many studies have reported that gingival crevicular fluid (GCF) IL-1 levels are significantly elevated in all forms of periodontitis, compared to health or gingivitis[30–39]. Ishihara et al. [30] reported that the degree of periodontitis, classified according to alveolar bone resorption, was correlated with the total amounts of IL-1α and IL-1β in GCF and the level of an IL-1 activity index. Thus, IL-1 is a significant and integral component of the host response to periodontal infection.
A. actinomycetemcomitans is a major pathogenic bacterium that is responsible for aggressive periodontitis (localized juvenile periodontitis). A. actinomycetemcomitans LPS has been shown to play a role in cellular and humoral immunity and inflammatory bone resorption in vitro. For example, LPS from A. actinomycetemcomitans induced IL-1 and prostaglandin E2 production from calvarial organ cultures, and IL-1 is responsible for the induction of bone resorption [40] and osteoclast formation in mouse bone marrow cultures [41]. LPS from A. actinomycetemcomitans also induces IL-6, which is related to the proliferation and maturation of plasma cells, and is produced by monocytes [42] and human gingival fibroblasts [43]. However, the biological activities of A. actinomycetemcomitans CP are completely different from those of A. actinomycetemcomitans LPS. For instance, while A. actinomycetemcomitans LPS induces IL-6 production, its CP reduces the production of IL-6 by gingival fibroblasts. Therefore, it seems there are different signalling pathways among A. actinomycetemcomitans LPS and CP in immunocytes.
Y4 CP induced IL-1β mRNA expression in both undifferentiated and differentiated THP-1 cells. Differentiated THP-1 cells showed significantly increased IL-1β mRNA expression compared to undifferentiated THP-1 cells (Fig. 1). The cell markers CD14 and CD11a, which are involved in cell signalling in response to a range of bacterial pathogen-associated molecular patterns, are both increased upon treatment of these cells with vitamin D3[44]. In our experiments, increased IL-1β expression might help explain the enhanced sensitivity of cells to Y4 CP. We previously reported that Y4 CP could increase osteoclast formation in mouse bone marrow culture systems, and concluded that IL-1 secreted by bone marrow cells after Y4 CP stimulation might induce osteoclast formation. This previous study supports the notion that Y4 CP affects immunocytes such as monocyte/macrophage in bone marrow cells. However, IL-1β production was not able to detected though mRNA expression was observed in this experiment. We speculate that Y4 CP might induce the IL-1β mRNA expression only and other Y4 component such as leukotoxin will activate the caspase-1 activity, and it will help to produce the mature IL-1 production [45].
A recent study has shown that the induction of the IL-1β gene in mouse calvarial bone cells stimulated with P. gingivalis fimbria is regulated by transcriptional factor activation protein-1 [46]. In contrast, in A. actinomycetemcomitans, IL-1β expression was only measured upon LPS stimulation [47,48], and not with other A. actinomycetemcomitans components. Our previous studies have only considered cytokine production in vitro. Therefore, there is no evidence concerning signalling molecules. The MAPK pathway is one of the major modulators of cytokine mRNA expression; consequently MAPK pathways were examined under our experimental conditions. JNK inhibitor II, a specific inhibitor of JNK, significantly and additively suppressed IL-1β mRNA expression along with Y4 CP. On the other hand, PD98059 and SB203580, specific inhibitors of ERK1/2 and p38 kinase, had no effect on IL-1β expression by Y4 CP. These results suggest that at least a JNK pathway is essential for IL-1β expression after the stimulation of THP-1 cells with Y4 CP.
Many reports deal with IL-1β expression induced by LPS in monocyte/macrophage and related signalling pathways. LPS induces MAPKs, including ERK, JNK and p38. These molecules play key roles in LPS-mediated signal transduction between extracellular membrane stimulation and the cytoplasmic response and nuclear activity in the activation of the gene [19,20]. Specifically, ERK activation involves cytokine induction and regulation during responses to bacterial products [49–51]. In addition to responding to numerous physiological and stress stimuli [52–55], JNK is considered to play roles in regulating the expression of various stress-induced proteins and inflammatory cytokines [55,56]. p38 is activated in response to stress signals such as LPS, osmotic stress, and pro-inflammatory cytokines [51,57–59]. Previous studies have shown that the p38 pathway plays a critical role in LPS-stimulated cytokine release [49,59], including IL-1 and TNF induction in monocytes [20]. In this study, the JNK pathway was shown to be important not only for LPS- but also for Y4 CP-stimulated IL-1β expression. Many of the downstream targets of the JNK pathway are transcription factors, including c-Jun, ATF-2, and Elk-1 [60]. These transcription factors regulate various genes that encode inflammatory mediators. In addition, a JNK pathway inhibitor also blocked gene transcription and reduced protein production. For example, the immunosuppressant dexamethasone reduced the LPS-induced translation of TNF-α mRNA by selectively inhibiting the JNK pathway [61]. The JNK-to-c-jun pathway is important for cell apoptosis and the cell cycle, and Y4 CP also induced osteoblast apoptosis [62]. Therefore, further studies are warranted to examine Y4 CP-induced apoptosis in macrophage through a JNK pathway. Recently, it is find that TLRs are important for the recognition of various bacterial components. Previous our group indicated that Y4 CP induces to make osteoclast formation in C3H/HeJ mouse bone marrow cells [15]. It is thought that the recognition of Y4 CP does not need TLR4. However, the rest of TLRs necessity for Y4 CP recognition of THP-1cells is still unknown. Therefore, TLRs and Y4 CP relationship will discover for future project.
In conclusion, the present results showed that A. actinomycetemcomitans Y4 CP induces IL-1β gene expression in macrophage. Our results also suggest that the bioactivity of Y4 CP is at least mediated by the activation of JNK. The current data provide new insight into the induction of immune responses by Y4 CP, and we believe that these findings may be useful for further research into infectious diseases such as periodontitis.
Acknowledgments
This work was supported in part by Grants-in-Aid (16592082, 16791325) and a ‘AGU High-Tech Research Center’ Project for private Universities: matching fund subsidy from MEXT (Ministry of Education, Culture, Sports, Science and Technology), 2003–07 of Japan.
References
- 1.Zambon JJ, Christersson LA, Slots J. Actinobacillus actinomycetemcomitans in human periodontal disease. Prevalence in patient groups and distribution of biotypes and serotype within families. J Periodontol. 1983;54:707–11. doi: 10.1902/jop.1983.54.12.707. [DOI] [PubMed] [Google Scholar]
- 2.Zambon JJ, Christersson LA, Genco RJ. Diagnosis and treatment of localized juvenile periodontitis. J Am Dent Assoc. 1986;113:295–9. doi: 10.14219/jada.archive.1986.0152. [DOI] [PubMed] [Google Scholar]
- 3.Haapasalo M, Ranta H, Ranta K, Shah H. Black-pigmented Bacteroides spp. in human apical periodontitis. Infect Immun. 1986;53:149–53. doi: 10.1128/iai.53.1.149-153.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slots J. Bacterial specificity in adult periodontitis: a summary of recent work. J Clin Periodontol. 1986;13:912–7. doi: 10.1111/j.1600-051x.1986.tb01426.x. [DOI] [PubMed] [Google Scholar]
- 5.Slots J, Genco RJ. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease. virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984;63:412–21. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- 6.Zambon JJ. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985;12:1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 7.Taichman NS, Dean RT, Sanderson CJ. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect Immun. 1980;28:258–68. doi: 10.1128/iai.28.1.258-268.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai CC, Shenker BJ, DiRienzo JM, Malamud D, Taichman NS. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect Immun. 1984;43:700–5. doi: 10.1128/iai.43.2.700-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iino Y, Hopps RM. The bone-resorbing activities in tissue culture of lipopolysaccharides from the bacteria Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Capnocytophaga ochracea isolated from human mouths. Arch Oral Biol. 1984;29:59–63. doi: 10.1016/0003-9969(84)90043-8. [DOI] [PubMed] [Google Scholar]
- 10.Kiley P, Holt SC. Characterization of the lipopolysaccharide from Actinobacillus actinomycetemcomitans Y4 and N27. Infect Immun. 1980;30:862–73. doi: 10.1128/iai.30.3.862-873.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson M, Kamin S, Harvey W. Bone resorbing activity of purified capsular material from Actinobacillus actinomycetemcomitans. J Periodontal Res. 1985;20:484–91. doi: 10.1111/j.1600-0765.1985.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 12.Amano K, Nishihara T, Shibuya N, Noguchi T, Koga T. Immunochemical and structural characterization of a serotype-specific polysaccharide antigen from Actinobacillus actinomycetemcomitans Y4 (serotype b) Infect Immun. 1989;57:2942–6. doi: 10.1128/iai.57.10.2942-2946.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi T, Nishihara T, Ishihara Y, et al. Murine macrophage interleukin-1 release by capsular-like serotype-specific polysaccharide antigens of Actinobacillus actinomycetemcomitans. Infect Immun. 1991;59:18–23. doi: 10.1128/iai.59.1.18-23.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueda N, Nishihara T, Ishihara Y, Amano K, Kuroyanagi T, Noguchi T. Role of prostaglandin in the formation of osteoclasts induced by capsular-like polysaccharide antigen of Actinobacillus actinomycetemcomitans strain Y4. Oral Microbiol Immunol. 1995;10:69–75. doi: 10.1111/j.1399-302x.1995.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 15.Nishihara T, Ueda N, Amano K, et al. Actinobacillus actinomycetemcomitans Y4 capsular-polysaccharide-like polysaccharide promotes osteoclast-like cell formation by interleukin-1 alpha production in mouse marrow cultures. Infect Immun. 1995;63:1893–8. doi: 10.1128/iai.63.5.1893-1898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuko O, Yuichi I, Masahiro O. Capsular polysaccharide from Actinobacillus actinomycetemcomitans inhibits IL-6 and IL-8 production in human gingival fibroblast. J Periodontal Res. 2003;38:191–7. doi: 10.1034/j.1600-0765.2003.00656.x. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein SL, Gold MR, DeFranco AL. Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc Natl Acad Sci USA. 1991;88:4148–52. doi: 10.1073/pnas.88.10.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenton MJ, Golenbock DT. LPS-binding proteins and receptors. J Leukoc Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25. [DOI] [PubMed] [Google Scholar]
- 19.Geppert TD, Whitehurst CE, Thompson P, Beutler B. Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol Med. 1994;1:93–103. [PMC free article] [PubMed] [Google Scholar]
- 20.Gray JG, Chandra G, Clay WC, et al. A CRE/ATF-like site in the upstream regulatory sequence of the human interleukin 1 beta gene is necessary for induction in U937 and THP-1 monocytic cell lines. Mol Cell Biol. 1993;13:6678–89. doi: 10.1128/mcb.13.11.6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vey E, Zhang JH, Dayer JM. INF-γ and 1,25 (OH) 2D3 induce on THP−1 cells distinct patterns of cell surface antigen expression, cytokine production, and responsiveness to contact with activated T cells. J Immunol. 1992;149:2040–6. [PubMed] [Google Scholar]
- 22.Nishihara T, Koga T, Hamada S. Suppression of murine macrophage interleukin-1 release by the polysaccharide portion of Haemophilus actinomycetemcomitans lipopolysaccharide. Infect Immun. 1988;56:619–25. doi: 10.1128/iai.56.3.619-625.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rantz LA, Randall E. Use of autoclaved extracts of hemolytic streptococci for serological grouping. Stanford Med Bull. 1955;13:290–1. [PubMed] [Google Scholar]
- 24.Feghali C, Wright T. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs J, Roebuck K, Archibeck M, Hallab N, Glant T. Osteolysis: Basic science. Clin Orthop. 2001;393:71–7. doi: 10.1097/00003086-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Ridderstad A, Abedi-Valugerdi M, Moller E. Cytokines in rheumatoid arthritis. Ann Med. 1991;23:219–23. doi: 10.3109/07853899109148051. [DOI] [PubMed] [Google Scholar]
- 27.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 28.Stashenko P, Dewhirst F, Peros W, Kent RL, Ago J. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987;138:1464–8. [PubMed] [Google Scholar]
- 29.Amin A, Dave M, Attur M, Abramson S. COX-2, NO, and cartilage damage and repair. Curr Rheumatol Rep. 2000;2:447–53. doi: 10.1007/s11926-000-0019-5. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara Y, Nishihara T, Kuroyanagi T, et al. Gingival crevicular interleukin-1 and interleukin-1 receptor antagonist levels in periodontally healthy and diseased sites. J Periodont Res. 1997;32:524–9. doi: 10.1111/j.1600-0765.1997.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 31.Charon JA, Luger TA, Mergenhagen SE, Oppenheim JJ. Increased thymocyte-activating factor in human gingival fluid during gingival inflammation. Infect Immun. 1982;38:1190–5. doi: 10.1128/iai.38.3.1190-1195.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mergenhagen SE. Thymocyte activating factor(s) in human gingival fluids. J Dent Res. 1984;63:461–4. doi: 10.1177/00220345840630031901. [DOI] [PubMed] [Google Scholar]
- 33.Kobashima H, Maeda K, Iwamoto Y, et al. Partial characterization of an interleukin-1-like factor in human gingival crevicular fluid from patients with chronic inflammatory periodontal disease. Infect Immun. 1990;58:2621–7. doi: 10.1128/iai.58.8.2621-2627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuki Y, Yamamoto T, Hara K. Localization of interleukin-1 (IL-1) mRNA-expressing macrophages in human inflamed gingival and IL-1 activity in gingival crevicular fluid. J Periodont Res. 1993;28:35–42. doi: 10.1111/j.1600-0765.1993.tb01048.x. [DOI] [PubMed] [Google Scholar]
- 35.Curtis DA, Kao R, Plesh O, Finzen F, Franz L. Crevicular fluid analysis around two failing dental implants: a clinical report. J Prosthodont. 1997;6:210–4. doi: 10.1111/j.1532-849x.1997.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 36.Tsai C-C, Ho Y-P, Chen C-C. Levels of interleukin-1 and interleukin-8 in gingival crevicular fluids in adult periodontitis. J Periodontol. 1995;66:852–9. doi: 10.1902/jop.1995.66.10.852. [DOI] [PubMed] [Google Scholar]
- 37.Salvi GE, Yalda B, Collins JG, et al. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontol. 1997;68:127–5. doi: 10.1902/jop.1997.68.2.127. [DOI] [PubMed] [Google Scholar]
- 38.Gonzales JR, Herrmann JM, Boedeker RH, Francz PI, Biesalski H, Meyle J. Concentration of interleukin-1beta and neutrophil elastase activity in gingival crevicular fluid during experimental gingivitis. J Clin Periodontol. 2001;28:544–9. doi: 10.1034/j.1600-051x.2001.028006544.x. [DOI] [PubMed] [Google Scholar]
- 39.Kinane DF, Winstanley FP, Adonogianaki E, Moughal NA. Bioassay of interleukin 1 (IL-1) in human gingival crevicular fluid during experimental gingivitis. Arch Oral Biol. 1992;37:153–6. doi: 10.1016/0003-9969(92)90011-v. [DOI] [PubMed] [Google Scholar]
- 40.Ishihara Y, Nishihara T, Maki E, Noguchi T, Koga T. Role of interleukin-1 and prostaglandin in in vitro bone resorption induced by Actinobacillus actinomycetemcomitans lipopolysaccharide. J Periodont Res. 1991;26:155–60. doi: 10.1111/j.1600-0765.1991.tb01639.x. [DOI] [PubMed] [Google Scholar]
- 41.Ueda N, Koide M, Ohguchi M, et al. Involvement of prostaglandin E2 and interleukin-1 alpha in the differentiation and survival of osteoclasts induced by lipopolysaccharide from Actinobacillus actinomycetemcomitans Y4. J Periodont Res. 1998;33:509–16. doi: 10.1111/j.1600-0765.1998.tb02351.x. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal S, Piesco NP, Johns LP, Riccelli AE. Differential expression of IL-1 beta, TNF-alpha, IL-6, and IL-8 in human monocytes in response to lipopolysaccharides from different microbes. J Dent Res. 1995;74:1057–65. doi: 10.1177/00220345950740040501. [DOI] [PubMed] [Google Scholar]
- 43.Kent LW, Rahemtulla F, Hockett RD, Jr, Gilleland RC, Michalek SM. Effect of lipopolysaccharide and inflammatory cytokines on interleukin-6 production by healthy human gingival fibroblasts. Infect Immun. 1998;66:608–14. doi: 10.1128/iai.66.2.608-614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galdiero M, Isonto MD, Vitello M, Finamore E, Peluso L, Galdiero M. Porins from Salmonella enterica serovar Typhimurium induce TNF-α, IL-6 and IL-8 release by CD14-independent and CD11a/CD18-dependent mechanisms. Microbiology. 2001;147:2697–704. doi: 10.1099/00221287-147-10-2697. [DOI] [PubMed] [Google Scholar]
- 45.Kelk P, Claesson R, Hanstrom L, Lerner UH, Kalfas S, Johansson A. Abundant secretion of bioactive interleukin-1β by human macrophages induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun. 2005;73:453–8. doi: 10.1128/IAI.73.1.453-458.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naganuma K, Amano S, Takeda H, Kitano S, Hanazawa S. Role of transcriptional factor activation protein-1 in endogenous expression of the interleukin-1 beta gene involved in Porphyromonas gingivalis fimbria-stimulated bone resorption in the mouse calvarial system. Oral Microbiol Immunol. 2000;15:53–7. doi: 10.1034/j.1399-302x.2000.150109.x. [DOI] [PubMed] [Google Scholar]
- 47.Nishida E, Hara Y, Kaneko T, Ikeda Y, Ukai T, Kato I. Bone resorption and local interleukin-1alpha and interleukin-1beta synthesis induced by Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis lipopolysaccharide. J Periodontal Res. 2001;36:1–8. doi: 10.1034/j.1600-0765.2001.00637.x. [DOI] [PubMed] [Google Scholar]
- 48.Schytte Blix IJ, Helgeland K, Hvattum E, Lyberg T. Lipopolysaccharide from Actinobacillus actinomycetemcomitans stimulates production of interleukin-1beta, tumor necrosis factor-alpha, interleukin-6 and interleukin-1 receptor antagonist in human whole blood. J Periodontal Res. 1999;34:34–40. doi: 10.1111/j.1600-0765.1999.tb02219.x. [DOI] [PubMed] [Google Scholar]
- 49.Carter AB, Monick MM, Hunninghake GW. Both Erk and p38 kinases are necessary for cytokine gene transcription. Cell Mol Biol. 1999;20:751–8. doi: 10.1165/ajrcmb.20.4.3420. [DOI] [PubMed] [Google Scholar]
- 50.Scherle PA, Jones EA, Favata MF, Daulerio AJ, Covington MB, Nurnberg SA, Magolda RL, Tracks JM. Inhibition of MAP kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J Immunol. 1998;161:5681–6. [PubMed] [Google Scholar]
- 51.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–6. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 52.Pombo CM, Bonventre JV, Avruch J, Woodgett JR, Kyriakis JM, Force T. The stress-activated protein kinases are major c-Jun amino-terminal kinases activated by ischemia and reperfusion. J Biol Chem. 1994;269:26546–51. [PubMed] [Google Scholar]
- 53.Devary Y, Gottlieb RA, Lau LF, Karin M. The stress-activated protein kinases are major c-Jun amino-terminal kinases activated by ischemia and reperfusion. Mol Cell Biol. 1991;11:2804–11. [Google Scholar]
- 54.Binetruy B, Smeal T, Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991;351:122–7. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- 55.Hambleton J, Weinstein SL, Lem L, DeFranco AL. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA. 1996;93:2774–8. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reimann T, Buscher D, Hipskind RA, Krautwald S, Lohmann-Matthes ML, Baccarini M. Lipopolysaccharide induces activation of the Raf-1/MAP kinase pathway. A putative role for Raf-1 in the induction of the IL-1 beta and the TNF-alpha genes. J Immunol. 1994;153:5740–9. [PubMed] [Google Scholar]
- 57.Geng Y, Valbracht J, Lotz M. Selective activation of the mitogen-activated protein kinase subgroups c-Jun NH2 terminal kinase and p38 by IL-1 and TNF in human articular chondrocytes. J Clin Invest. 1996;98:2425–30. doi: 10.1172/JCI119056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–11. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 59.Lee JC, Laydon JT, McDonnell PC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–46. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 60.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–6. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 61.Swantek JL, Cobb MH, Geppert TD. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation: glucocorticoids inhibit TNF-alpha translation by blocking JNK/SAPK. Mol Cell Biol. 1997;17:6274–82. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamamoto S, Mogi M, Kinpara K, et al. Anti-proliferative capsular-like polysaccharide antigen from Actinobacillus actinomycetemcomitans induces apoptotic cell death in mouse osteoblastic MC3T3–E1 cells. J Dent Res. 1999;78:1230–7. doi: 10.1177/00220345990780060601. [DOI] [PubMed] [Google Scholar]