Abstract
Rapid overproduction of proinflammatory cytokines are characteristic of sepsis. CD14dimCD16+ monocytes are thought to be major producers of cytokine and have been shown to be elevated in septic patients. Toll-like receptors (TLR) are pattern recognition receptors important in mediating the innate immune response and their activation can lead to production of cytokines. Using whole blood culture and flow cytometry we have investigated TLR2 and TLR4 regulation after stimulation with sepsis-relevant antigens [lipopolysaccharide (LPS), Staphylococcal enterotoxin B (SEB) and peptidoglycan (PGN)]. The percentage of CD14dimCD16+ monocyte population expanded at 20 h post-stimulation, after a rise in tumour necrosis factor (TNF)-α and interleukin (IL)-6 at 2 h. A strong positive correlation between the percentage of CD14dimCD16+ monocytes and secreted TNF-α was demonstrated (r= 0·72). Furthermore, we were able to induce expansion of the CD14dimCD16+ population to approximately 35% of all monocytes with the addition of recombinant TNF-α to the whole blood culture. TLR4 was found to be expressed 2·5 times higher on CD14dimCD16+ compared to CD14+ CD16– monocytes, while TLR2 expression was similar in both subpopulations. The CD14dimCD16+ and CD14+ CD16– monocyte populations were different in their response to various antigens. LPS down-regulated TLR4 by 4·9 times in CD16+ monocytes compared to only 2·3 times in CD16– monocytes at 2 h. LPS was able to up-regulate TLR2 by 6·2 times after 2 h, with no difference between the subpopulations. LPS further up-regulated TLR2 by 18·4 times after 20 h only in the CD14+ CD16– population. PGN and SEB induced no significant changes in TLR2 or TLR4 expression. We hypothesize that following exposure to bacterial antigens, subsequent TNF-α drives a differentiation of monocytes into a CD14dimCD16+ subpopulation.
Keywords: Fc receptor, human, monocytes, Toll-like receptor
Introduction
The immunology of severe sepsis and septic shock is poorly defined, despite many studies investigating the pathogenesis of this syndrome. With mortality rates of up to 50%[1,2] greater understanding of the interactions between host and microbe is necessary to improve patient outcome. Given the rapid progression of sepsis and immediate recruitment of the inflammatory cytokine cascade, the early innate response of the immune system to the pathogen is likely to play a critical role.
Pattern recognition receptors such as Toll-like receptors (TLRs) are important mediators of the innate immune response. TLR4, in conjunction with CD14, is well characterized as the receptor for lipopolysaccharide (LPS), a cell wall component of Gram-negative bacteria. TLR2 has been demonstrated to act as a receptor for components of Gram-positive bacteria such as peptidoglycan (PGN) and lipoteichoic acid [3]. Activation of both these receptors by their ligands can lead to production of proinflammatory cytokines [including tumour necrosis factor (TNF)-α and interleukin (IL)-6] that are characteristic of sepsis.
There is increasing evidence that both Gram-positive organisms as well as Gram-negative organisms play pathogenic roles in sepsis. While LPS is a major contributor to Gram-negative sepsis, staphylococcal and streptococcal exotoxins (superantigens) and peptidoglycan are believed to be important in Gram-positive sepsis [4]. Superantigens have the specific ability to activate a large proportion of T cells, bridging antigen-presenting cells and T lymphocytes through binding to HLA-DR and the Vβ region of T-cell receptors, respectively. This activation results in production of large amounts of cytokines. In turn, cytokine generation has been shown to regulate TLR signalling pathways. Synergistic interactions are known to occur between superantigens and other bacterial components such as LPS leading to increased mortality in animal models of septic shock [5,6].
CD16, the low affinity receptor for immunoglobulin G (IgG:FcγRIII), is expressed mainly on natural killer (NK) lymphocytes and neutrophils; however, a subpopulation of monocytes (CD14dimCD16+), first described by Zeigler-Heitbrock and colleagues [7], expresses CD16. This population represents about 10% of monocytes in healthy adults, expanding in various inflammatory conditions such as sepsis [8,9], rheumatoid arthritis [10] and HIV [11].
The aim of this study is to mimic the differentiation of CD14dimCD16+ monocytes ex vivo and determine the interaction of clinically relevant bacterial antigens on monocyte subpopulations, including the regulation of TLR2 and TLR4 on these cells. Although some research has been undertaken exploring the relationship between LPS and TLR4 regulation and components of Gram-positive organisms and TLR2 [12–14], regulation of these receptors after stimulation with bacterial antigens has not been explored previously in relation to monocyte subpopulations. We investigated the differentiation of monocytes as their phenotype changed from CD14+ CD16– to CD14dimCD16+, resulting in an expansion of this population. While others have shown these CD14dimCD16+ monocytes are proinflammatory in nature, we have shown that TNF-α in fact plays an important role in CD14+ CD16– monocyte differentiation into CD14dimCD16+ monocytes. Further to phenotypic changes we have demonstrated these two subpopulations to respond differently to stimulation with LPS, Staphylococcus aureus enterotoxin B (SEB) and PGN.
Materials and methods
Whole blood assay
Whole blood, 500 µl, was diluted with 500 µl RPMI-1640 supplemented with antibiotics and 5% heat-inactivated fetal bovine serum and incubated at 37°C with gentle rotation in tightly capped 5 ml polystyrene tubes (Becton Dickinson, San Jose, CA, USA). Cells were stimulated with 100 ng/ml of SEB (Sigma, St Louis, MO, USA), LPS (Escherichia coli O55:B5; Calbiochem, La Jolla, CA, USA) or PGN (Staphylococcus aureus; Sigma). After 2 and 20 h, culture supernatants were collected and stored at −20°C until cytokine analysis. The remaining cells were stained for flow cytometry. Coulter counts were collected on each donor specimen using the Ac.T diff Hematology Analyser (Beckman Coulter, Fullerton, CA, USA). LPS contamination of SEB and PGN was lower than 0·02 EU/ml, as determined by Limulus amoebocyte lysate chromogenic end-point assay (HyCult Biotechnology, the Netherlands).
Cytokine enzyme-linked immunosorbent assays (ELISAs)
TNF-α and IL-6 were measured by capture ELISA using OptEIA sets (Becton Dickinson) according to the manufacturer's specifications. Sensitivity of the ELISAs were 8 pg/ml and 5 pg/ml, respectively.
Flow cytometry
Cell surface staining was performed on whole blood using CD14-PerCP (MφP9; Becton Dickinson), CD16-allophycocyanin (APC) (3G8; Caltag, Burlingame, CA, USA), TLR2-FITC (TL2·1; eBioscience, San Diego, CA, USA) and TLR4-PE (HTA125; eBioscience). Appropriate isotype controls were used. Based on their scatter profile, monocytes were gated picking up the lymphocyte tail on a FACSCalibur flow cytometer (Becton Dickinson). A total of 8000 CD14+ monocytes were acquired for each sample. The percentage of CD14dimCD16+ monocytes was calculated from two-colour dot-plot analysis (see Fig. 1). Isotype-matched control antibodies were used to determine the cut-off between negative and positive CD16 populations. Data were analysed using FlowJo software (Tree Star Inc., Ashland, OR, USA). Relative fluorescence intensity was determined by subtracting the geometric mean fluorescence intensity of the isotype control from the sample.
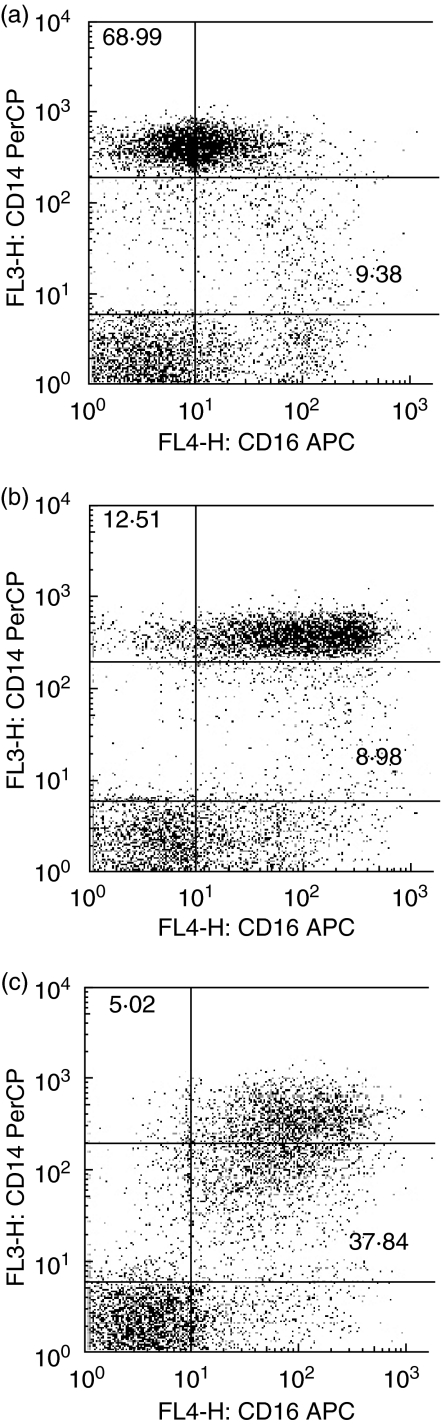
Fig. 1.
Effect of Staphylococcus aureus enterotoxin B (SEB) on CD14 and CD16 expression on human monocytes. Whole blood was stimulated with 100 ng/ml of SEB for (a) zero, (b) two and (c) 20 hours. Cells were stained with CD14 PerCP and CD16 (APC) for flow cytometry. Dot plots are gated on monocytes based on their light-scatter properties. Bold figures represent percentages of CD14+ CD16– cells and CD14dimCD16+ cells. Data shown are representative of five individual experiments.
Cytokine-induced CD14dimCD16+ monocytes
Whole blood, 500 µl, was diluted with 500 µl RPMI-1640 supplemented with antibiotics and 5% heat-inactivated fetal bovine serum and incubated at 37°C with gentle rotation in tightly capped 5 ml polystyrene tubes (Becton Dickinson). Cells were stimulated with 2 ng/ml or 20 ng/ml recombinant human TNF-α (R&D Systems, Minneapolis, MN, USA) or 100 ng/ml of Staphylococcus aureus enterotoxin B (SEB; Sigma). After 20 h, cells were stained for flow cytometric analysis.
Patients and blood sampling
Sepsis patients
After informed consent was obtained, blood samples were collected into EDTA tubes from 10 critically ill patients with sepsis from the intensive care unit (ICU) of the Royal Melbourne Hospital. Sepsis was defined by using the criteria of the American College of Chest Physicians and Society of Critical Care Medicine consensus conference [15]. Demographic and routine clinical data, including illness severity scores, were collected (Table 1). Blood samples from five non-septic ICU patients with intracerebral haemorrhages were used as negative controls. All blood samples were collected within 24 h of ICU admission and staining for flow cytometry as described above was commenced within 2 h of collection. The Human Research and Ethics Committee of the Royal Melbourne Hospital approved this study.
Table 1.
Clinical details of patients sampled
| Control (n = 5) | Sepsis (n = 10) | |
|---|---|---|
| Age, years, median (IQR) | 72·0 (47·0, 83·0) | 44·0 (29·8, 66·0) |
| Sex, no. of males (%) | 3 (60) | 5 (50) |
| SOFA† score, median (IQR) | 8·0 (3·5, 10·5) | 6·5 (3·5, 16·0) |
| APACHE II‡ score, median (IQR) | 19·0 (14·0, 24·0) | 15·5 (11·8, 22·3) |
| Deaths, n (%) | 0 (0) | 2 (20) |
| ICU LOS*, days, median (IQR) | 5·5 (2·6, 6·6) | 2·5 (1·3, 10·7) |
| Microbiology of sepsis patients | ||
| Gram-negative | 0 | 3 |
| Gram-positive | 0 | 4 |
| No organism cultured | 0 | 2 |
| Viral | 0 | 1 |
Healthy volunteers
Peripheral blood was collected in Li-heparin tubes from five healthy volunteers, two females and three males aged between 20 and 45 years.
Statistical analyses
Non-parametric statistical analyses were performed using the Mann–Whitney U rank sum test and Spearman's correlation coefficient because of the low numbers of experiments performed (Prism, San Diego, CA, USA). The probability level of P = 0·05 was set for statistical significance.
Results
Change in monocyte subpopulations with time in whole blood cultures
In fresh whole blood from healthy volunteers the Coulter counts were 2·54 ± 0·19 × 106/ml lymphocytes, 0·38 ± 0·08 × 106/ml monocytes and 4·16 ± 0·47 × 106/ml granulocytes.
Freshly stained blood from healthy adults showed two distinct monocyte subpopulations (Fig. 1a). One population showed high expression of CD14 (CD14+ CD16–) and the other showed strong expression of CD16 with diminished CD14 expression (CD14dimCD16+).
In unstimulated whole blood cultures, 5·7 ± 2·0% of all CD14+ monocytes were CD14dimCD16+(Fig. 2). Compared with the initial percentage, over culture time, the CD14dimCD16+ monocyte population almost doubled to 10·2 ± 1·4% at 2 h and remained unchanged at 11·5 ± 2·9% of all monocytes at 20 h.
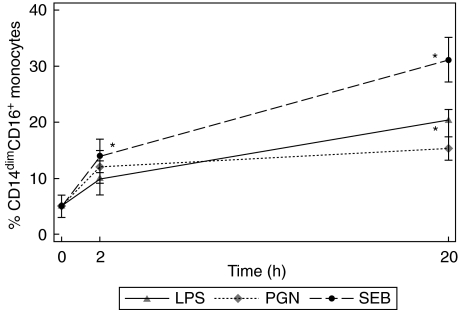
Fig. 2.
Change in the percentage of the CD14dimCD16+ monocyte subpopulation with culture and stimulation. Whole blood cultures were stimulated with 100 ng/ml lipopolysaccharide (LPS), peptidoglycan (PGN) or Staphylococcus aureus enterotoxin B (SEB) for 0, 2 and 20 h. The CD14dimCD16+ monocyte subpopulation was measured by flow cytometry. Experiments represent the mean and standard error for five individual experiments. Statistical significance was compared with the unstimulated cells (*P < 0·05).
Change in monocyte subpopulations with stimulation in whole blood cultures
Following stimulation with various bacterial components, there was a small increase in the percentage of CD14dimCD16+ monocytes at 2 h after SEB stimulation. However, after 20 h of stimulation the CD14dimCD16+ population differentiated significantly to 31·1 ± 5·3% with SEB stimulation (P< 0·05). (Figs 1b,c and 2). In comparison, at 20 h the CD14dimCD16+ population differentiated significantly to 20·3 ± 3·9% with LPS (P< 0·05). (Fig. 2). The degree of expansion after SEB stimulation was significantly greater than with LPS and PGN.
Proinflammatory cytokine response in stimulated whole blood cultures
LPS and SEB stimulation produced different kinetics of TNF-α and IL-6 production (Fig. 3). LPS elicited an early rise in TNF-α at 2 h post-stimulation (722 ± 110 pg/ml; P < 0·05), which remained high at 20 h (655 ± 302 pg/ml; P < 0·05). SEB stimulated TNF-α production to a lesser degree (46 ± 15 pg/ml) above the unstimulated control (16 ± 6 pg/ml) at 2 h, but increased significantly at 20 h post-stimulation (722 ± 185 pg/ml; P < 0·05). PGN did not induce cytokine in this whole blood model.
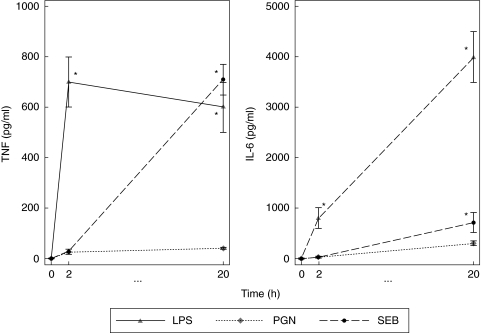
Fig. 3.
Stimulation of whole blood cultures with lipopolysaccharide (LPS) and Staphylococcus aureus enterotoxin B (SEB) induces cytokine. Whole blood cultures were stimulated with 100 ng/ml of LPS, peptidoglycan (PGN) or SEB for 0, 2 and 20 h. Tumour necrosis factor (TNF)-α and interleukin (IL)-6 enzyme-linked immunosorbent assays (ELISAs) were performed on culture supernatants. The results represent the mean and standard error for five individual experiments. Statistical significance was compared with the unstimulated cells (*P < 0·05).
LPS was also a potent inducer of IL-6. Early stimulation of whole blood produced 797 ± 52 pg/ml IL-6 at 2 h before increasing further to 4000 ± 775 pg/ml at 20 h post-stimulation. SEB induced low levels of IL-6 at 2 h (41 ± 4 pg/ml) but this increased dramatically at 20 h post-stimulation (705 ± 267 pg/ml). PGN showed the least induction of cytokine for both TNF-α and IL-6.
Correlation between the percentage of CD14dimCD16+ monocytes and TNF-α
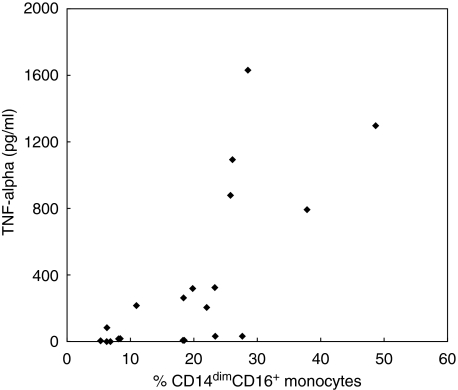
A strong positive correlation (r= 0·72) was found between the amount of TNF-α produced and the percentage of CD14dimCD16+ monocytes at 20 h post-stimulation (Fig. 4). This was a specific effect with no correlation found between IL-6 induction and the percentage of CD14dimCD16+ monocytes at 20 h (r= 0·24).
Fig. 4.
Correlation between tumour necrosis factor (TNF)-α and the percentage of CD14dimCD16+ monocytes. Whole blood cultures were stimulated for 20 h with or without 100 ng/ml of lipopolysaccharide (LPS), peptidoglycan (PGN) or Staphylococcus aureus enterotoxin B (SEB). Each dot represents data from an individual experiment. The percentage of CD14dimCD16+ monocytes were determined by flow cytometry and TNF-α was determined by enzyme-linked immunosorbent assay (ELISA) on the supernatants from the same cells. Correlation coefficient r = 0·72.
TLR2 surface expression on monocyte subpopulations in whole blood culture
TLR2 expression on different monocyte subgroups stimulated with bacterial components is shown in Fig. 5. We found no difference in TLR2 surface expression between the two monocyte subpopulations in freshly stained whole blood.
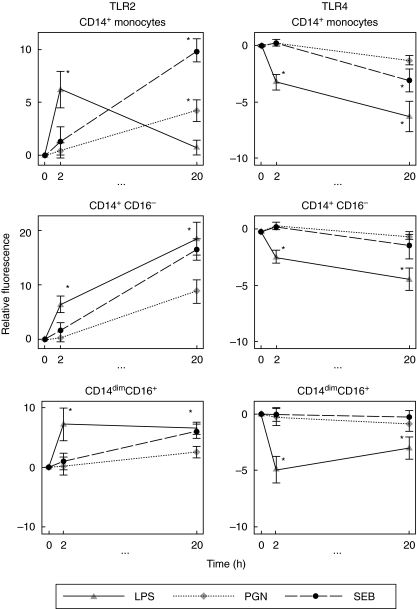
Fig. 5.
Toll-like receptor (TLR)2 and TLR4 on monocyte subpopulations in whole blood culture. Whole blood cultures were stimulated for 0, 2 and 20 h with 100 ng/ml lipopolysaccharide (LPS), peptidoglycan (PGN) or Staphylococcus aureus enterotoxin B (SEB). Using four-colour flow cytometry, TLR2 and TLR4 expression was measured on the total CD14+ population and the two subpopulations. Relative fluorescence was calculated by subtracting the mean fluorescence of the unstimulated cells from the mean fluorescence of the stimulated cells. Data represent the mean and standard error of five separate experiments. Statistical significance was compared with the unstimulated cells (*, P < 0·05)
With respect to the total CD14+ monocyte population, upon stimulation for 2 h, LPS up-regulated TLR2 (6·21 ± 1·74) compared to the unstimulated control (P< 0·05), whereas PGN and SEB stimulation had little effect (0·42 ± 0·73 and 1·31 ± 1·32, respectively). At 20 h, TLR2 expression was up-regulated by PGN (6·21 ± 1·47) and SEB (4·56 ± 1·40) (both P < 0·05).
At 2 h post-stimulation, there was little difference in the extent of up-regulation of TLR2 between the CD14+ CD16– and CD14dimCD16+ monocyte populations (Fig. 5, second and third panels). In both cases LPS stimulation up-regulated TLR2 expression on CD14+ CD16– (6·36 ± 1·49) and on CD14dimCD16+ monocytes (7·25 ± 2·78) (both P < 0·05). At 20 h, TLR2 was further up-regulated after LPS stimulation on CD14+ CD16– monocytes (18·36 ± 6·70) (P< 0·05). In comparison, TLR2 was not further up-regulated on CD14dimCD16+ monocytes.
TLR4 surface expression on monocyte subpopulations in whole blood culture
TLR4 expression on different monocyte subgroups stimulated with bacterial components is shown in Fig. 5. On monocytes from uncultured whole blood, TLR4 was found to be higher (2·48 ± 0·90) on CD14dimCD16+ monocytes than CD14+ CD16– monocytes.
In terms of the total CD14+ monocyte population, upon LPS stimulation TLR4 expression was down-regulated (3·25 ± 0·70) at 2 h in comparison to the unstimulated cells (P< 0·05). At 20 h post-stimulation all stimulants down-regulated TLR4. The greatest down-regulation in TLR4 expression was due to stimulation with LPS (6·29 ± 1·33) (P< 0·05), followed by a down-regulation by SEB (3·12 ± 2·2) (P< 0·05) and by PGN (1·30 ± 0·42) (not significant).
The pattern of TLR4 regulation at 2 h post-stimulation on both monocyte subpopulations followed that seen in the total CD14+ population. After 2 h of LPS stimulation, TLR4 was down-regulated on CD14dimCD16+ monocytes (4·92 ± 1·18) (P< 0·05) and on CD14+ CD16– monocytes (2·32 ± 0·59) (P< 0·05). At 20 h post-stimulation, down-regulation of TLR4 was greatest on CD14+ CD16– cells (4·34 ± 1·00) and to a lesser extent on CD14dimCD16+ monocytes (3·02 ± 2·22) (both P < 0·05). There was no significant change in TLR4 after PGN or SEB stimulation.
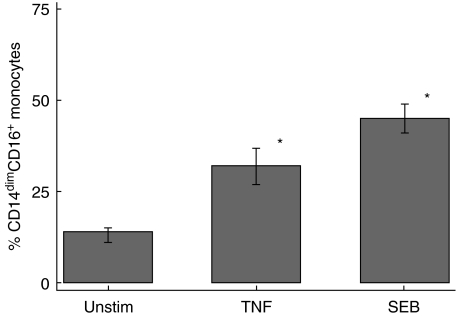
Cytokine induced CD14dimCD16+ monocytes
Stimulation of whole blood from five normal individuals with both TNF-α (34·4 ± 4·1%) and SEB (46·3 ± 4·5%) demonstrated a marked expansion of CD14dimCD16+ monocytes compared to unstimulated cells (13·5 ± 1·5%) (P< 0·05,Fig. 6).
Fig. 6.
Stimulation of whole blood cultures with 20 ng/ml of recombinant tumour necrosis factor (TNF)-α and 100 ng/ml of Staphylococcus aureus enterotoxin B (SEB) for 20 h. The results represent the mean and standard error for five individual experiments. The statistical significance is in comparison with unstimulated cells (*P < 0·05).
Sepsis patients, CD14dimCD16+ and TLR2 and TLR4
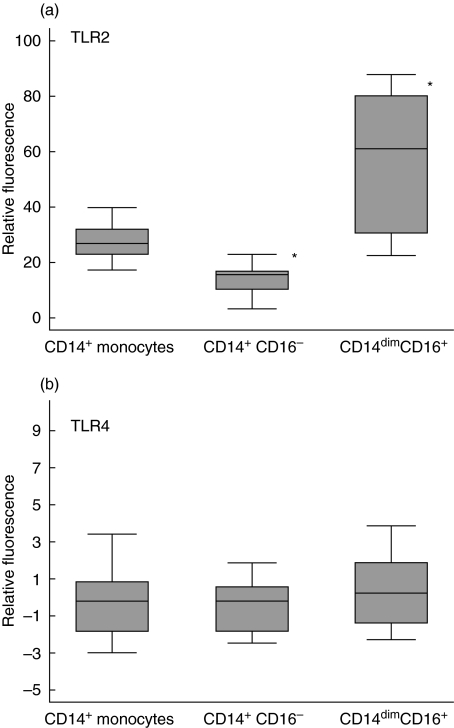
All 10 sepsis patients had elevated levels of CD14dimCD16+ monocytes (54·4 ± 9·5%). TLR2 levels were raised in sepsis patients in comparison to ICU non-sepsis control patients (data not shown). The average levels of TLR2 on CD14dimCD16+ monocytes were higher than on CD14+ CD16– monocytes (P< 0·01); these CD14dimCD16+ monocytes also demonstrated a larger variation in TLR2 levels (Fig. 7).
Fig. 7.
Differential expression of Toll-like receptors (TLRs) on monocyte subpopulations of sepsis patients. Expression of (a) TLR2 and (b) TLR4 was measured on individual patient monocyte subpopulations by four-colour flow cytometry. Relative fluorescence was calculated by subtracting the mean fluorescence of the isotype matched control from the mean fluorescence of the specific TLR stain. Lines represent the mean of 10 sepsis patients (*P < 0·05).
TLR4 levels were suppressed relative to ICU non-sepsis controls (data not shown) and did not show marked variation in either of the monocyte subpopulations (Fig. 7).
Discussion
CD14 positive monocytes have been recently divided into two subpopulations, namely one with CD16 surface expression but with diminished CD14 expression (CD14dimCD16+) and one without any CD16 expression (CD14+ CD16–). The CD14dimCD16+ monocytes demonstrate features of differentiated monocytes or tissue macrophages such as increased migration into tissues [16–19]. They have also been described as ‘proinflammatory’ in nature [20], producing high levels of proinflammatory cytokines, increased HLA-DR expression and little to no anti-inflammatory cytokines [21]. These features of CD14dimCD16+ monocytes suggest that they may play an important role in proinflammatory syndromes including sepsis and septic shock.
Our results demonstrate that CD14dimCD16+ monocytes are a distinct monocyte subpopulation that responds differently to bacterial components compared to CD14+ CD16– monocytes. We have shown that this subgroup of cells has both different phenotypic and functional characteristics. Previous studies by a number of authors [10,16,17] indicate that maturation of the monocyte is reflected in increased surface expression of CD16 and diminished expression of CD14. Our results, which demonstrate that it is possible to induce CD16 monocytes ex vivo with SEB, LPS and TNF-α, suggest that CD14dimCD16– cells may mature from CD14+ CD16– monocytes. However, maturation of circulating monocytes is likely to be only one contributing source. Some data by other authors support the theory that CD14dimCD16+ monocytes are more mature cells entering the circulation from tissue in extreme inflammatory conditions [17].
Ex vivo we found that the CD14dimCD16+ monocyte population was not significantly expanded until 20 h post-stimulation. SEB, a staphylococcal superantigen, known to be a potent inducer of TNF-α, stimulated the greatest expansion of CD14dimCD16+ monocytes. PGN gave the smallest rise in CD14dimCD16+ monocytes of all the stimulants used and was shown to also induce the least amount of TNF-α. With each of the stimulants used the proinflammatory cytokine production was already increased at 2 h. This strong relationship between TNF-α and CD16 expression was present with all the stimulants and may indicate that the cytokine plays an important role in up-regulating CD16 production on monocytes during maturation. We were able to support these data further by demonstrating that the expansion of CD14dimCD16+ monocytes could be induced by recombinant TNF-αex vivo. This hypothesis is also supported by previous clinical studies. Blumenstein et al. [22] reported TNF-α production prior to CD14dimCD16+ monocyte population expansion in a sepsis patient. Increased CD16 on macrophages in rheumatoid arthritis patients [23] has also been shown to correlate with higher circulating TNF-α levels. Furthermore, glucocorticoid-treated multiple sclerosis patients [24] showed a subsequent depletion of CD14dimCD16+ monocytes after suppression of proinflammatory cytokine production.
This is the first study examining the ex vivo effect of sepsis relevant antigens on monocyte subpopulations, including their TLR2/TLR4 expression.
Toll-like receptors belong to the family of pattern recognition receptors, vital to the regulation of the innate immune response. Activation of TLR2 and TLR4 through binding of their ligands leads to NFκB activation and transcription of many genes, including those involved in cell proliferation and inflammation.
Monocytes stimulated with LPS showed marked up-regulation of TLR2 surface expression at 20 h. This is consistent with Flo et al. [25], who also showed that TLR2 on monocytes did not increase markedly until long exposure (20 h) to LPS. This observed up-regulation of TLR2 is greatest on CD14+ CD16– monocytes. In comparison, CD14dimCD16+ monocytes demonstrated a much smaller response. This blunted response of the mature (CD16+) monocytes to express TLR2 may occur because they are more sensitive to negative feedback from TNF-α than their CD16– counterparts.
Binding of LPS to TLR4 has been shown to down-regulate TLR4 surface expression [26] on monocytes even though the mRNA transcript was up-regulated. Over 20 h, TLR4 was down-regulated on both monocyte subpopulations. On freshly stained blood, however, TLR4 expression is highest on CD14dimCD16+ monocytes, supporting their description as proinflammatory monocytes. At 20 h, the down-regulation from baseline was greatest on these CD14dimCD16+ monocytes. Hence, perhaps as monocytes mature and become CD16 positive, CD14 expression diminishes, as does TLR4, reflecting their close relationship as the endotoxin receptor.
Furthermore, Williams et al. [27] observed early up-regulation of TLR4 correlated with mortality in mice with polymicrobial sepsis. In sepsis it could be that CD16 is initially up-regulated along with TLR4 leading to TLR activation and subsequent over-expression of the cytokines that are characteristic of sepsis. The TLR4 down-regulation at 20 h in our ex vivo data suggests a possible negative feedback mechanism. If this negative feedback mechanism fails, this may add to septic mortality.
Our clinical data from 10 patients with severe sepsis and a high percentage of CD14dimCD16+ monocytes demonstrate, in a pattern similar to our ex vivo data, that TLR2 is markedly increased on CD16+ monocytes. This supports results we have shown previously in another inflammatory disease [28], where up-regulated TLR2 expression on monocytes correlated with the severity of hepatic cirrhosis and was likely to contribute to the increased circulating TNF-α seen in those patients. The TLR2 levels on CD14dimCD16+ cells in our sepsis patients, although highly variable, are markedly elevated compared to the CD14+ CD16– cells. TLR4 levels were suppressed overall and in individual subsets of monocytes. This is consistent with our longer (20 h) culture results with LPS ex vivo. Over-production of proinflammatory cytokines is a major feature of sepsis and contributes to the poor outcome of the patient [29–31]. The expansion of CD14dimCD16+ monocytes in sepsis may be an important contributor to this cytokine response.
With whole blood culture conditions, we have demonstrated that sepsis specific antigens have the ability to cause a similar marked expansion of CD14dimCD16+ monocytes as seen in sepsis. We have also demonstrated that the CD16+ and CD16– populations are different in their responses to various antigens. Coupled with the reported proinflammatory nature of CD14dimCD16+ monocytes and the strong positive correlation we found between the expansion of these cells and TNF-α, this monocyte population is likely to be of major importance in sepsis.
Acknowledgments
Christopher MacIsaac holds a medical postgraduate research scholarship from the NHMRC, Australia.
References
- 1.Clermont G, Angus DC, Kalassian KG, et al. Reassessing the value of short-term mortality in sepsis: comparing conventional approaches to modeling. Crit Care Med. 2003;31:2627–33. doi: 10.1097/01.CCM.0000094233.35059.81. [DOI] [PubMed] [Google Scholar]
- 2.Schoenberg MH, Weiss M, Radermacher P. Outcome of patients with sepsis and septic shock after ICU treatment. Langenbecks Arch Surg. 1998;383:44–8. doi: 10.1007/s004230050090. [DOI] [PubMed] [Google Scholar]
- 3.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–9. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 4.Bannan J, Visvanathan K, Zabriskie JB. Structure and function of streptococcal and staphylococcal superantigens in septic shock. Infect Dis Clin North Am. 1999;13:387–96. doi: 10.1016/s0891-5520(05)70081-7. ix. [DOI] [PubMed] [Google Scholar]
- 5.Dalpke AH, Heeg K. Synergistic and antagonistic interactions between LPS and superantigens. J Endotoxin Res. 2003;9:51–4. doi: 10.1179/096805103125001342. [DOI] [PubMed] [Google Scholar]
- 6.Visvanathan K, Charles A, Bannan J, et al. Inhibition of bacterial superantigens by peptides and antibodies. Infect Immun. 2001;69:875–84. doi: 10.1128/IAI.69.2.875-884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–34. [PubMed] [Google Scholar]
- 8.Fingerle G, Pforte A, Passlick B, et al. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–6. [PubMed] [Google Scholar]
- 9.Skrzeczynska J, Kobylarz K, Hartwich Z, Zembala M, Pryjma J. CD14+CD16+ monocytes in the course of sepsis in neonates and small children: monitoring and functional studies. Scand J Immunol. 2002;55:629–38. doi: 10.1046/j.1365-3083.2002.01092.x. [DOI] [PubMed] [Google Scholar]
- 10.Kawanaka N, Yamamura M, Aita T, et al. CD14+CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2002;46:2578–86. doi: 10.1002/art.10545. [DOI] [PubMed] [Google Scholar]
- 11.Zembala M, Bach S, Szczepanek A, Mancino G, Colizzi V. Phenotypic changes of monocytes induced by HIV-1 gp120 molecule and its fragments. Immunobiology. 1997;197:110–21. doi: 10.1016/S0171-2985(97)80061-7. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 13.Sato S, Nomura F, Kawai T, et al. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol. 2000;165:7096–101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura A, Lien E, Ingalls RR, et al. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 15.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler-Heitbrock HW, Fingerle G, Strobel M, et al. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993;23:2053–8. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- 17.Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schakel K. The CD16(+) (FcgammaRIII(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J Exp Med. 2002;196:517–27. doi: 10.1084/jem.20011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giordano D, Magaletti DM, Clark EA, Beavo JA. Cyclic nucleotides promote monocyte differentiation toward a DC-SIGN(+) (CD209) intermediate cell and impair differentiation into dendritic cells. J Immunol. 2003;171:6421–30. doi: 10.4049/jimmunol.171.12.6421. [DOI] [PubMed] [Google Scholar]
- 19.Ancuta P, Weiss L, Haeffner-Cavaillon N. CD14+CD16++ cells derived in vitro from peripheral blood monocytes exhibit phenotypic and functional dendritic cell-like characteristics. Eur J Immunol. 2000;30:1872–83. doi: 10.1002/1521-4141(200007)30:7<1872::AID-IMMU1872>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Belge KU, Dayyani F, Horelt A, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 21.Frankenberger M, Sternsdorf T, Pechumer H, Pforte A, Ziegler-Heitbrock HW. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood. 1996;87:373–7. [PubMed] [Google Scholar]
- 22.Blumenstein M, Boekstegers P, Fraunberger P, et al. Cytokine production precedes the expansion of CD14+CD16+ monocytes in human sepsis: a case report of a patient with self-induced septicemia. Shock. 1997;8:73–5. doi: 10.1097/00024382-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Blom AB, Radstake TR, Holthuysen AE, et al. Increased expression of Fcgamma receptors II and III on macrophages of rheumatoid arthritis patients results in higher production of tumor necrosis factor alpha and matrix metalloproteinase. Arthritis Rheum. 2003;48:1002–14. doi: 10.1002/art.10871. [DOI] [PubMed] [Google Scholar]
- 24.Fingerle-Rowson G, Angstwurm M, Andreesen R, Ziegler-Heitbrock HW. Selective depletion of CD14+CD16+ monocytes by glucocorticoid therapy. Clin Exp Immunol. 1998;112:501–6. doi: 10.1046/j.1365-2249.1998.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flo TH, Halaas O, Torp S, et al. Differential expression of Toll-like receptor 2 in human cells. J Leukoc Biol. 2001;69:474–81. [PubMed] [Google Scholar]
- 26.Muzio M, Bosisio D, Polentarutti N, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 27.Williams DL, Ha T, Li C, et al. Modulation of tissue Toll-like receptor 2 and 4 during the early phases of polymicrobial sepsis correlates with mortality. Crit Care Med. 2003;31:1808–18. doi: 10.1097/01.CCM.0000069343.27691.F3. [DOI] [PubMed] [Google Scholar]
- 28.Riordan SM, Skinner N, Nagree A, et al. Peripheral blood mononuclear cell expression of toll-like receptors and relation to cytokine levels in cirrhosis. Hepatology. 2003;37:1154–64. doi: 10.1053/jhep.2003.50180. [DOI] [PubMed] [Google Scholar]
- 29.Kox WJ, Volk T, Kox SN, Volk HD. Immunomodulatory therapies in sepsis. Intensive Care Med. 2000;26(Suppl. 1):S124–8. doi: 10.1007/s001340051129. [DOI] [PubMed] [Google Scholar]
- 30.Takala A, Nupponen I, Kylanpaa-Back ML, Repo H. Markers of inflammation in sepsis. Ann Med. 2002;34:614–23. doi: 10.1080/078538902321117841. [DOI] [PubMed] [Google Scholar]
- 31.Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25:1813–19. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on ‘sepsis-related problems’ of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]