Abstract
Heat shock proteins (HSP) are released by cells in response to stress signals. It is hypothesized that pathogenic bacteria stimulate the cells in the periodontium to up-regulate the expression of HSP60, which would stimulate macrophages, and possibly other cells, to produce proinflammatory cytokines. We sought to determine whether oral keratinocytes responded to recombinant human HSP60 and to identify the signalling pathways involved. In addition, whether oral keratinocytes are a source of endogenous HSP60 was also investigated. RT-PCR revealed that rhHSP60 induced expression of the IL-1β gene in the Human Oral Keratinocyte (HOK-16B) cell line and it was highest at the lowest concentration used (0·1 µg/ml). These responses were mediated via activation of p44/42 MAP-kinases and to a lesser extend the MAP-kinase SAP/JNK. Similar data was obtained from analysis of intracellular signalling pathways in HOK-16B cells by rhHSP70 and LPS (from both E. coli and the oral pathogen Porphyromonas gingivalis). However, there was little activation of p38 by rhHSP60. Blocking of the p44/42 pathway decreased HSP60-induced IL-1β gene expression and protein secretion. In addition, we discovered that self-HSP60 proteins were constitutively secreted by HOK-16B cells. Secretion of self-HSP60 was up-regulated in cells treated with LPS from P. gingivalis, but down-regulated with LPS from E. coli. To summarize, oral keratinocytes respond to exogenous HSP60 by triggering expression of the inflammatory cytokine IL-1β through activation of p44/42 MAP kinase. Oral keratinocytes are also a source for self-HSP60 and the secretion of this protein may be differentially modified by LPS from different bacterial species. These results highlight the importance of oral keratinocytes and HSPs in the development of an immune response against bacterial infection.
Keywords: IL-1β, inflammation, HSP60, oral keratinocytes
Introduction
The gingiva is constantly being challenged by bacteria within the oral environment. It is increasingly appreciated that epithelial tissues such as the gingival epithelia are not merely passive barriers to infection but have a proactive role in immune responses and the development of localized inflammatory conditions such as periodontitis [1–3]. The keratinocyte is the main cell type in gingival epithelial tissues. Histological studies reveal that oral keratinocytes express a variety of pro-inflammatory cytokines and chemokines including interleukin (IL)-1α, IL-1β, IL-6, IL-8 and tumour necrosis factor (TNF)-α [3–8].
HSPs are a highly conserved group of proteins found in a wide variety of organisms from bacteria to mammalian species. HSPs are abundant within cells and their levels increase rapidly in response to stress signals and local infection [9]. The main function of HSPs is to act as chaperones, thus helping protein folding, protecting proteins from denaturation or aggregation, and facilitating protein transport through membrane channels [10]. It is now widely accepted that HSPs have a direct role both in the adaptive and the innate immune responses [11]. HSPs were originally found to bind tumour-specific peptides and mediate antigen-specific tumour immunity [12] and also bind a range of virus and bacteria-specific antigens inducing activation of CD8+ and CD4+ T-cells, respectively [11,13,14]. HSPs are also capable of eliciting innate immune responses in a variety of target cells including monocytes, macrophages, dendritic cells and endothelial cells in a peptide-independent manner [15]. Thus HSP60 stimulates pro-inflammatory cytokine production in macrophages, endothelial cells and smooth muscle cells [16–19] and adhesion molecule expression (E-selectin, ICAM-1 and VAM-1) in vascular endothelial cells [20].
Immune responses to bacterial HSPs may generate cross-reacting immunity to self-HSP and precipitate damaging inflammatory responses [21,22]. However, in contrast to human HSP60, HSPs from the periodontal pathogens Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans failed to stimulate TNF-α production in monocytes suggesting that endogenous rather than bacterial HSPs are more likely to be involved in signalling innate responses in periodontal disease [23]. Significantly, immunohistochemical analysis showed high HSP60 expression in periodontitis lesions [23,24]. It has recently been demonstrated that human HSP60 protect keratinocytes from stress-induced cell death and stimulates keratinocyte migration [25,26]. These findings may be relevant to epithelial cell changes and tissue repair during chronic inflammatory conditions such as periodontal disease where HSPs may be released by local cells [25]. However, there is a paucity of information about the direct effect of endogenous HSPs on keratinocyte immune responses.
In the present study we sought to investigate how oral keratinocytes respond to recombinant human (rh) HSP60 identifying possible signalling pathways and to determine whether oral keratinocytes might be a source of HSP60. We demonstrate that self-HSP60 is involved in the regulation of expression of inflammatory cytokines in HOK-16B cells via activation of p44/42 MAP kinases and that secretion of self-HSP60 is highly increased by LPS from P. gingivalis. Results from this study suggest that HSP60 plays an important role in the development of an immune response against bacterial infection in oral keratinocytes.
Materials and methods
Cell culture
The immortalized human oral keratinocyte HOK-16B cell line [22] was cultured at 37°C, 5% CO2 in the serum free medium KBM® (Cambrex, Wokingham, UK) supplemented with 0·1 ng/ml EGF, 5 µg/ml insulin, 30 µg/ml bovine pituitary extract, 0·5 µg/ml hydrocortisone, 50 µg/ml gentamicin, 50 µg/ml amphoterecin (BulletKit®, Cambrex). In all experiments cell passages from 4 to 10 were used. Twenty-four hours after seeding the cells the medium was changed to remove any molecules secreted during cell adhesion. When cultures reached 80% confluence the medium was removed and cells were incubated in KBM without supplements for a further 24 h before adding treatment.
Analysis of cell signalling pathways
The concentration of rhHSP60 (Stressgen Biotechnologies, Bioquote Ltd, York, UK) which gave maximal IL-1β stimulation was determined in pilot experiments (not shown). Thus triplicate cultures of HOK-16B cells in 6-well plates were exposed to 0·1 µg/ml rhHSP60 for 5, 10, 20, 30 and 60 min. Parallel cultures containing 0·1 µg/ml rhHSP70 (Stressgen), 10 µg/ml E. coli LPS (Strain O126:B8, Sigma) and 10 µg/ml P, gingivalis LPS (strain W50) were also set up. In some experiments HOK cells were preincubated for 2 h with 10 µM of UO126 (MEK1/2 inhibitor, Cell Signalling Technologies, Hitchin, UK) to block activation of the MAP kinase p44/42 (ERK1/2) signalling pathway. After the incubation period the cell layer was washed with PBS and lysed with 200 µl 1xSDS sample buffer (0·5 M Tris-HCl, glycerol, 10% SDS, beta-mercaptoethanol, 0·05% (w/v) bromophenol blue) and 20 µl of proteinase inhibitor cocktail (Sigma, Poole, UK). Intracellular proteins were separated by SDS-PAGE in 10% gels and activation of signalling pathways determined by Western blotting.
Analysis of intracellular and secreted HSP60 protein
HOK-16B cells at 80% confluence were incubated in 6-well plates and treated for 18 h with PBS or with 10 µg/ml E. coli LPS or 10 µg/ml P. gingivalis LPS. After the incubation period the supernatants were collected, centrifuged at 600 g for 5 min to discard any cells that detached during the incubation period. Equal volumes of the cell supernatants were concentrated by using Microcon centrifugal filter devices (30 kD cut off, Amicon Bioseparations, Millipore, Watford, UK). The entire concentrated sample from each culture supernatant was used to detect secreted HSP60. To extract intracellular HSPs, the cell layer was washed with PBS and lysed with 1×SDS-sample buffer. prior to analysis by Western blotting (see below). Intracellular and secreted HSP60 were analysed by SDS-Page using 7% gels followed by Western blotting.
Western blotting
Samples were heated at 95°C for 5min, separated by SDS-PAGE and transferred to a nitrocellulose membrane (Optitran BA-S83, Schleicher & Schuell, London, UK) using a semidry electro-blotter (BDH, NELS, Newton Aycliffe, UK). A prestained protein marker (Bio-Rad, Hemel Hempstead, UK) was used to monitor the molecular weight of separated proteins. Ponceau's staining was performed to assess the quality of the transfer and to confirm equal protein loading. The membrane was blocked for 1 h at room temperature in TBS-T (137 mM NaCl, 2·7 mM KCl, 25 mM Tris-HCl pH 7·4, 0·1% Tween20) containing 5% w/v of dried skimmed milk (TBS-TM).
To analyse intracellular signalling pathways, the membranes containing blotted cell lysates were incubated overnight with primary antibodies against the phosphorylated forms of p44/42 (ERK1/2), SAPK/JNK and p38 MAP kinases and against the nonphosphorylated form of p44/42 (Cell Signalling Technologies) at 1 : 1000 dilution and mouse monoclonal antiactin antibodies (Oncogene Research Products, Nottingham, UK) used as a loading control, followed by secondary antibody diluted 1 : 2000 in TBS-TM.
To detect human HSP60 a mouse monoclonal antihuman HSP60 (Stressgen Biotechnologies) and mouse monoclonal antiactin (Oncogene Research Products) were used at a 1 : 1000 dilution. (HRP)-linked antimouse IgG (Cell Signalling Technologies) was used as a secondary antibody at a 1 : 2000 dilution.
Hybridizing proteins were detected using an ECL reaction which was performed following the manufacturer's instructions (ECL + plus Western blotting detection system, Amersham-Pharmacia Biotech, UK) and blots were developed by exposure to X-ray film (Super Rx Medical X-ray film, Fujifilm, London, UK).
IL-1β RT-PCR
HOK-16B cells at 80% confluence were treated in triplicates with 0·1 µg/ml rhHSP60 for 4 h in 24-well plates. Similar cultures containing 0·1 µg/ml rhHSP70, 10 µg/ml E. coli LPS and 10 µg/ml P. gingivalis LPS were also included. Cultures containing PBS only were included as a control. A standard reverse transcription PCR (RT-PCR) was used to determine the levels of IL-1β RNA in the cultured cells. Briefly, the RNA was extracted using an RNA extraction kit (Qiagen, Crawley, UK). 1 µg of RNA was reverse transcribed to cDNA using a TaqMan® RT-PCR kit (Applied Biosystems, Warrington, UK) and 1 µl of cDNA used as template for PCR (Reddymix™ PCR Master Mix, 1·5 mM MgCl2, ABgene, Surrey, UK) to amplify fragments of the IL-1β gene. IL-1β gene expression was expressed as a ratio of GAPDH gene expression determined simultaneously. Preliminary experiments varying PCR cycle number were carried out to determine the exponential phase for each reaction and according to this 18 and 25 cycles were used for GAPDH and IL-1β, respectively. Primers were designed using the web-based program Primer3 and obtained from Helena Biosciences (Sunderland, UK). Their sequences were as follows: IL-1βF 5′-gcaccttctttc ccttcatc-3′, IL-1βR 5′-cgcttttccatcttcttctttg-3′, GAPDHF 5′-atctctgccccctctgct-3′, GAPDHR 5′-cctgcttcaccaccttcttg-3′ and the expected sizes were 370 and 433 bp, respectively. PCR products were separated on a 0·8% agarose gel and visualized under the UV light. The intensity of the bands was quantified using Kodak 1D v3·5.2 Scientific Imaging System (Anachem).
IL-1β expression and protein secretion
This was followed by treatment with 0·1 µg/ml of rhHSP60 for 4 h for RT-PCR studies (see IL-1β RT-PCR) and for 6, 24 and 48 h for quantification of protein secretion. Cell supernatants were collected and IL-1β protein concentration was measured with DuoSet ELISA following manufacturer's instructions (R & D Systems, Abingdon, UK). Treatments were performed in triplicates.
Cell proliferation assays
103 cells per well were incubated in 96-well plates with 0·1 µg/ml of rhHSP60, 0·1 µg/ml rhHSP70, 10 µg/ml E. coli LPS, 10 µg/ml P. gingivalis LPS or PBS. After various incubation periods, the cell supernatant was removed and the plates with the cell layer still attached were stored at −80°C. The number of cells in each well was determined using the CyQuant® Cell Proliferation Assay kit (Molecular Probes, Cambridge Bioscience, Cambridge, UK). The fluorescent CyQuant GR dye binds to cellular nucleic acid directly measuring the number of cells rather than proliferation. Treatments were performed in triplicates.
Measurement of endotoxin levels
The levels of endotoxin in the commercial stocks of rhHSP60 and E. coli LPS was measured using the Limulus Amebocyte Lysate Pyrogent®5000 (Cambrex).
Statistics
Data were analysed using Student's t-test (two-tailed) and a P-value of < 0·05 considered statistically significant.
Results
Analysis of cell signalling pathways activated by HSP60
The levels of endotoxin present in 0·1 µg/ml solutions of rhHSP60 and rhHSP70 were negligible (0·0056 IU/ml and 0·0087 IU/ml, respectively), whereas the 10 µg/ml solutions of E. coli and P. gingivalis LPS contained 1042 and 2744 IU/ml, respectively. This confirms that the effects observed when cells were treated with rhHSP60 and rhHSP70 were unlikely to be the result of endotoxin contamination.
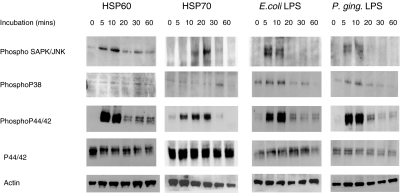
HOK-16B cells were incubated with 0·1 µg/ml rhHSP60 for 5, 10, 20, 30 and 60 min to assess the activation of known intracellular signalling pathways. Western blots were performed against the phosphorylated (active) forms of the MAP kinases p38, p44/42 (ERK1/2), SAPK/JNK, the nonphosphorylated p44/42 (ERK1/2) and actin. There was a basal level of phosphorylated SAPK/JNK present in control cells which increased after 5 and 10mins of treatment with rhHSP60. Levels of the phosphorylated form of p38 were not influenced by coincubation of HOK-16B cells with rhHSP60 (Fig. 1). There was a remarkable increase in activated p44/42 (ERK1/2) which peaked after 5–10min of incubation with rhHSP60. The levels of the control proteins (nonphosphorylated p44/42 and actin) remained constant over the full time course of exposure to rhHSP60 (Fig. 1). A similar pattern of activation was observed in cells treated with rhHSP70 although phosphorylated SAPK/JNK was not detected until after 10 min in these cultures. Co-incubation of HOK cells with both E. coli and P. gingivalis LPS produced pattern of activation of intracellular signalling pathways distinct from that elicited by rhHSP60 (Fig. 1). Thus, although both phosphorylated p44/42 (ERK1/2) and SAPK/JNK were also detected, phosphorylated p38 was detected in LPS stimulated cells but not in cells incubated with rhHSP60 (Fig. 1).
Fig. 1.
Western blots of the phosphorylated form of SAPK/JNK (P- SAPK/JNK), phosphorylated p38 (P-p38) and phosphorylated p44/42 (P-p44/42) and nonphosphorylated p44/42 (p44/42) and actin after treatment of HOK-16B cells with 0·1 µg/ml of rhHSP60, 0·1 µg/ml rhHSP70, 10 µg/ml E. coli LPS and 10 µg/ml P, gingivalis LPS for 0, 5, 10, 20, 30 or 60 min.
Effects of rhHSP60 on IL-1β mRNA and protein expression
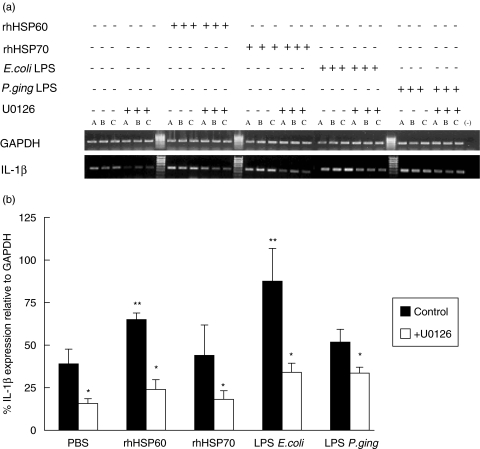
Unstimulated HOK-16B cells expressed IL-1β mRNA and this was significantly up-regulated by coincubation with 0·1 µg/ml rhHSP60 (Fig. 2). When activation of p44/42 was inhibited by pretreating HOK-16B cells with the MEK1/2 inhibitor U0126 prior to HSP60 treatment baseline and rhHSP60 stimulated IL-1β mRNA were both decreased (Fig. 2). Similar effects were observed in HOK cells stimulated with E. coli LPS (Fig. 2).
Fig. 2.
IL-1β mRNA expression in HOK-16B cells incubated with 0·1 µg/ml rhHSP60, 0·1 µg/ml rhHSP70, 10 µg/ml E. coli LPS and 10 µg/ml P. gingivalis LPS for 4 h in the absence (▪) or presence (□) of MEK1/2 inhibitor U0126 (10 mM). (a) Gel electrophoresis. A, B, C are triplicate experiments. (b) Densitometric analysis of results. Values are means ± SD, n = 3. *P < 0·05, **P < 0·01 compared to PBS.
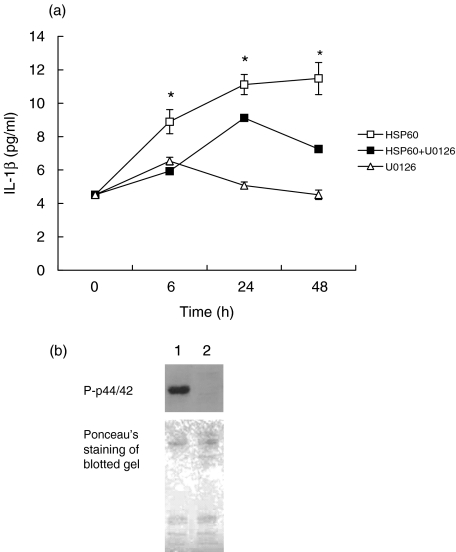
Similarly, HSP60 induced secretion of IL-1β protein and this was also reduced by pretreating cells with U0126 (Fig. 3). Western blotting of cell lysates from HSP60-treated cells, incubated in the presence and absence of U0126 confirmed the complete inhibition of p44/42 activation by this reagent (Fig. 3).
Fig. 3.
(a) Analysis of IL-1β protein secretion in HOK-16B cells incubated with rhHSP60 (0·1 µg/ml) for 6, 24 and 48 h in the absence (▪) or presence (□) of MEK1/2 inhibitor U0126 (10 mM) or with U0126 alone (Δ). Values are means ± SD, n = 3. *P < 0·05 compared to U0126 treatment. (b) Representative Western blot of the phosphorylated form of p44/42 (P-p44/42) in lysates from HOK-16B cells treated for 10min with HSP60 (0·1 mg/ml) in the absence (1) or presence (2) of MEK1/2 inhibitor U0126. Ponceau's staining was performed to assess the quality of the transfer and to confirm equal protein loading.
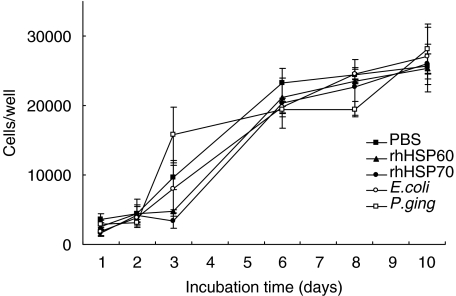
Cell proliferation rhHSP60 (0·1 µg/ml) had no statistically significant effect on the proliferation of HOK-16B cells compared to PBS controls over the 10 days the treatment lasted (Fig. 4). Furthermore, the growth characteristics of HOK-16B cells were also unaffected by 0·1 µg/ml rhHSP70, 10 µg/ml E. coli LPS and 10 µg/ml P. gingivalis LPS (Fig. 4). The growth characteristics of HOK-16B cells were unaffected by the presence of the MEK1/2 inhibitor U0126 indicating that this reagent is not cytotoxic at the concentrations used to inhibit MEK1/2 signalling (Fig. 3).
Fig. 4.
The effect of rhHSP60 on cell proliferation. HOK-16B cells were incubated in the presence of 0·1 µg/ml rhHSP60 (▴), 0·1 µg/ml rhHSP70 (•), 10 µg/ml E. coli LPS (○), 10 µg/ml P. gingivalis LPS (□) and PBS (▪). Cell number determined over a period of 10 days. Values are means ± SD, n= 3.
Intracellular and secreted HSP60 in HOK-16B cells
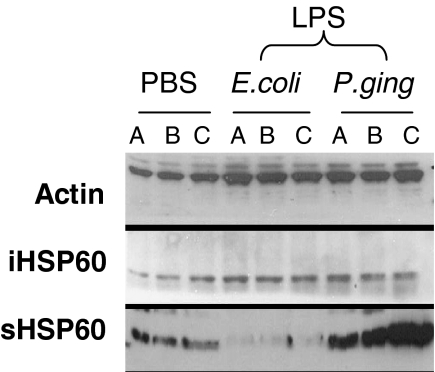
The intracellular and secreted levels of self-HSP60 were studied in HOK-16B cells after 18 h incubation with LPS from E. coli or from P. gingivalis. Western blots revealed that there was constitutive expression of intracellular HSP60 in untreated cells and this remained unchanged in cells exposed to either E. coli or P. gingivalis LPS (Fig. 5).
Fig. 5.
The effect of LPS on self-HSP60 expression. Upper panel, Western blot showing the effect of PBS or 10 µg/ml LPS from E. coli or P. gingivalis on intracellular (iHSP60) and secreted (sHSP60) self-HSP60 from HOK-16B cells after incubation for 18 h. Equal protein loading was confirmed using an actin antibody. A, B, C represent cell lysates from 3 independent cell cultures.
HOK-16B cells were also found to secrete HSP60 into the culture medium. However, treatment with LPS from E. coli induced a down-regulation in HSP60 protein secretion whereas LPS from P. gingivalis induced up-regulation of HSP60 secretion (Fig. 5).
Discussion
This study describes the initiation of an inflammatory response in the oral keratinocyte cell line HOK-16B triggered by rhHSP60. Furthermore, HOK-16B themselves are a source of HSP60 which may act in an autocrine or paracrine manner to regulate local inflammatory responses.
RhHSP60-induced activation of MAP-kinase signalling pathways in oral keratinocytes were investigated. Little or no activation was observed for SAPK/JNK or the p38 MAP-kinase pathways. However, strong activation of the p44/42 MAP-kinase pathway was observed after only 5min of treatment with rhHSP60 which then returned to control levels after 20min. Studies in the human skin keratinocyte cell line HaCaT revealed that whereas these cells respond to A. actinomycetemcomitans HSP60 by activation of the p44/42 (ERK1/2) and p38 MAPK signalling pathways these effects were not observed when rhHSP60 was used in this system [27]. The reasons for these differences are not clear. It is possible that keratinocytes from the oral epithelium and the skin may respond differently to rhHSP60 or that these findings reflect unique properties of the cell lines themselves. Clearly there is a need for further research involving primary cell lines derived from these tissues.
Treatment of HOK-16B cells with rhHSP60 also resulted in increased gene expression of the inflammatory cytokine IL-1β and increased IL-1β protein secretion. Pre-incubation with U0126 to inhibit p44/42 activation [28] resulted in a decrease in IL-1β gene and protein expression. This indicates that HSP60-induced IL-1β expression is stimulated via p44/42 activation. This suggests that keratinocytes in oral epithelial tissues are responsive to local extra-cellular stress signals and provides further evidence to support the principle that oral epithelial cells are active participants in host immune responses. To our knowledge there have been no other reported studies of the effects of human HSPs on oral keratinocytes. HSP60, HSP70 and LPS failed to activate NF-κB in human keratinocytes isolated from neonatal skin [29].
The interpretation of experiments involving recombinant proteins produced in bacteria, such as HSPs, may be confounded by the possible biological effect of LPS contamination of these preparations or by LPS bound to HSPs acting as molecular chaperones [30]. However we demonstrated that the amount of endotoxin, as measured by Limulus assay, in biologically active solutions of rhHSPs was of the order of 2000-fold lower than biologically active solutions of E. coli LPS in this particular cell line indicating that the effects of rhHSPs on HOK-16B cells observed in our experiments were not the result of LPS in the HSP preparations. Furthermore, there were differences in the pattern of activation of intracellular signalling pathways in HOK 16B cells by LPS and rhHSP60 in our experiments.
It is possible that some of the biochemical changes in the HOK-16B cultures could be explained by an indirect effect of the various treatments on cell division. To address this we tested rhHSP60 for its ability to induce cell proliferation. At the concentration used (0·1 mg/ml) rhHSP60 treatment and controls showed no difference in cell number over time when compared to control cells. This is consistent with previous findings demonstrating the lack of proliferative effect of exogenous human HSP on various endothelial and epithelial cell lines [27,31]. These findings also confirm this agent is not cytotoxic.
HSPs are released from a number of cell types and are present in significant concentrations in the circulation of healthy individuals and increase in certain vascular diseases [32]. The sources of extracellular HSPs in stress responses and infections and their mechanism of secretion remain unclear [11,32]. It is possible that cells will actively export HSPs in stress responses. We have presented data which suggest that oral keratinocytes secrete HSPs in culture and that the secretion of self-HSP60 is influenced by LPS from both P. gingivalis and E. coli. Thus, secretion of self-HSP60 was partially inhibited by LPS from E. coli but was increased by LPS from P. gingivalis. These responses may reflect a degree of difference in the response of oral keratinocytes to oral pathogens (e.g. P. gingivalis) or enteric bacteria (e.g. E. coli) and may also relate to the different molecular composition of both types of LPS [33]. Both types of LPS activate dendritic cells and macrophages to produce different cytokines and induce different types of adaptive immunity in vivo[34,35].
An alternative explanation for the appearance of HSPs in the culture medium is that they represent the release of cytoplasmic contents from dead and dying cells [36]. The cells cultured in the present experiments were actively dividing and the treatments which influenced HSP in the medium did not influence cell division. There was no morphological evidence of cell death in these cultures. Furthermore, the finding that E. coli LPS down-regulated HSP secretion from HOK-16B cells is consistent with HSP secretion being the result of an active biological effect rather than a coincidental finding associated with cell death. This is supported by studies using amino acid analogues suggesting that specific mechanisms exist for HSP secretion [37].
We have demonstrated that one potential target for HSP60 is the oral keratinocytes themselves and that this may lead to the up-regulation of pro-inflammatory cytokines. This may be an important initiating event in gingivitis and periodontitis, however, there are other cells in the proximity which may respond to HSPs as part of the host's response to subgingival plaque bacteria. There is accumulating evidence that HSPs may have a wide-ranging role in the regulation of acquired immunity over and above any role in early signalling of proinflammatory pathways [11,38]. Significantly, another heat shock protein, HSP70 has been shown to promote the differentiation of immature dendritic cells and has other direct immunoregulatory effects including promoting the accumulation of T-cells, macrophages and dendritic cells into the microenvironment of tumour cells expressing HSP70 [13,39,40]. It is noteworthy that dendritic cells are present in the gingival tissues and are activated in periodontal diseases [41]. It is tempting to suggest that HSPs may therefore contribute to local immunoregulatory circuits in the periodontium and that this process may be relevant to the pathogenesis of periodontal disease. It would be of great interest to investigate the effect of these mediators on dendritic cell maturation and function in the periodontium.
In summary, oral keratinocytes may play a role in the development of the immune response in the gingiva. They respond to LPS from oral pathogens by increasing the secretion of self-HSP60 which may in turn affect other keratinocytes or other cell types in the surrounding area. Exogenous HSP60 is capable of initiating an inflammatory response in oral keratinocytes by increasing the expression of pro-inflammatory cytokines such as IL-1β. Further work needs to be done looking at the effect of HSP60 in different oral keratinocyte cell lines or even primary keratinocytes.
Acknowledgments
We thank Professor No-Hee Park (UCLA School of Dentistry) for supplying HOK-16B cells, Dr M. Rangarajan (MRC Molecular Pathogenesis Group, Barts & the London School of Medicine & Dentistry, Queen Mary University of London) for supplying P. gingivalis LPS and Professor J Mark Thomason (School of Dental Sciences, University of Newcastle) for helpful advice throughout this project and in the preparation of this manuscript. This work was supported by a grant from the Newcastle Universities Hospitals Special Trustees and the Royal London Charitable Foundation (RAB).
References
- 1.Bartold PM, Walsh LJ, Narayanan AS. Molecular and cell biology of the gingiva. Periodontol. 2000;24:28–55. doi: 10.1034/j.1600-0757.2000.2240103.x. [DOI] [PubMed] [Google Scholar]
- 2.Dale BA. Periodontal epithelium. a newly recognized role in health and disease. Periodontol. 2002;30:70–8. doi: 10.1034/j.1600-0757.2002.03007.x. [DOI] [PubMed] [Google Scholar]
- 3.Suchett-Kaye G, Morrier JJ, Barsotti O. Interactions between non-immune host cells and the immune system during periodontal disease: role of the gingival keratinocyte. Crit Rev Oral Biol Medical. 1998;9:292–305. doi: 10.1177/10454411980090030301. [DOI] [PubMed] [Google Scholar]
- 4.Asai Y, Ohyama Y, General K, Ogawa T. Bacterial fimbriae and their peptides activate human gingival epithelial cells through Toll-like receptor 2. Infect Immun. 2001;69:7387–95. doi: 10.1128/IAI.69.12.7387-7395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang GT, Haake SK, Kim JW, Park NH. Differential expression of interleukin-8 and intercellular adhesion molecule-1 by human gingival epithelial cells in response to Actinobacillus actinomycetemcomitans or Porphyromonas gingivalis infection. Oral Microbiol Immunol. 1998;13:301–9. doi: 10.1111/j.1399-302x.1998.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 6.Miyauchi M, Sato S, Kitagawa S, Hiraoka M, Kudo Y, Ogawa I, Zhao M, Takata T. Cytokine expression in rat molar gingival periodontal tissues after topical application of lipopolysaccharide. Histochem Cell Biol. 2001;116:57–62. doi: 10.1007/s004180100298. [DOI] [PubMed] [Google Scholar]
- 7.Tonetti MS, Imboden MA, Gerber L, Lang NP, Laissue J, Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun. 1994;62:4005–14. doi: 10.1128/iai.62.9.4005-4014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonetti MS, Imboden MA, Lang NP. Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J Periodontol. 1998;69:1139–47. doi: 10.1902/jop.1998.69.10.1139. [DOI] [PubMed] [Google Scholar]
- 9.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–96. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 10.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–9. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–94. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 12.Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–6. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–13. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 14.Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–22. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallin RP, Lundqvist A, More SH, von Bonin A, Kiessling R, Ljunggren HG. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130–5. doi: 10.1016/s1471-4906(01)02168-8. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212–9. [PubMed] [Google Scholar]
- 17.Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–7. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- 18.Kol A, Sukhova GK, Lichtman AH, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation. 1998;98:300–7. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- 19.Skeen MJ, Miller MA, Shinnick TM, Ziegler HK. Regulation of murine macrophage IL-12 production. Activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J Immunol. 1996;156:1196–206. [PubMed] [Google Scholar]
- 20.Kol A, Bourcier T, Lichtman AH, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest. 1999;103:571–7. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabeta K, Yamazaki K, Hotokezaka H, Yoshie H, Hara K. Elevated humoral immune response to heat shock protein 60 (hsp60) family in periodontitis patients. Clin Exp Immunol. 2000;120:285–93. doi: 10.1046/j.1365-2249.2000.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazaki K, Ohsawa Y, Tabeta K, Ito H, Ueki K, Oda T, Yoshie H, Seymour GJ. Accumulation of human heat shock protein 60-reactive T cells in the gingival tissues of periodontitis patients. Infect Immun. 2002;70:2492–501. doi: 10.1128/IAI.70.5.2492-2501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueki K, Tabeta K, Yoshie H, Yamazaki K. Self-heat shock protein 60 induces tumour necrosis factor-alpha in monocyte-derived macrophage: possible role in chronic inflammatory periodontal disease. Clin Exp Immunol. 2002;127:72–7. doi: 10.1046/j.1365-2249.2002.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundqvist C, Baranov V, Teglund S, Hammarstrom S, Hammarstrom ML. Cytokine profile and ultrastructure of intraepithelial gamma delta T cells in chronically inflamed human gingiva suggest a cytotoxic effector function. J Immunol. 1994;153:2302–12. [PubMed] [Google Scholar]
- 25.Zhang L, Koivisto L, Heino J, Uitto VJ. Bacterial heat shock protein 60 may increase epithelial cell migration through activation of MAP kinases and inhibition of alpha6beta4 integrin expression. Biochem Biophys Res Commun. 2004;319:1088–95. doi: 10.1016/j.bbrc.2004.04.202. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Pelech S, Uitto VJ. Bacterial GroEL-like heat shock protein 60 protects epithelial cells from stress-induced death through activation of ERK and inhibition of caspase 3. Exp Cell Res. 2004;292:231–40. doi: 10.1016/j.yexcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Pelech SL, Mayrand D, Grenier D, Heino J, Uitto VJ. Bacterial heat shock protein-60 increases epithelial cell proliferation through the ERK1/2 MAP kinases. Exp Cell Res. 2001;266:11–20. doi: 10.1006/excr.2001.5199. [DOI] [PubMed] [Google Scholar]
- 28.Scherle PA, Jones EA, Favata MF, Daulerio AJ, Covington MB, Nurnberg SA, Magolda RL, Trzaskos JM. Inhibition of MAP kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J Immunol. 1998;161:5681–6. [PubMed] [Google Scholar]
- 29.Curry JL, Qin JZ, Bonish B, Carrick R, Bacon P, Panella J, Robinson J, Nickoloff BJ. Innate immune-related receptors in normal and psoriatic skin. Arch Pathol Laboratory Med. 2003;127:178–86. doi: 10.5858/2003-127-178-IIRRIN. [DOI] [PubMed] [Google Scholar]
- 30.Gaston JS. Heat shock proteins and innate immunity. Clin Exp Immunol. 2002;127:1–3. doi: 10.1046/j.1365-2249.2002.01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirono S, Dibrov E, Hurtado C, Kostenuk A, Ducas R, Pierce GN. Chlamydia pneumoniae stimulates proliferation of vascular smooth muscle cells through induction of endogenous heat shock protein 60. Circ Res. 2003;93:710–6. doi: 10.1161/01.RES.0000095720.46043.F2. [DOI] [PubMed] [Google Scholar]
- 32.Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–76. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- 33.Paramonov N, Bailey D, Rangarajan M, Hashim A, Kelly G, Curtis MA, Hounsell EF. Structural analysis of the polysaccharide from the lipopolysaccharide of Porphyromonas gingivalis strain W50. Eur J Biochem. 2001;268:4698–707. doi: 10.1046/j.1432-1327.2001.02397.x. [DOI] [PubMed] [Google Scholar]
- 34.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–76. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimura A, Hara Y, Kaneko T, Kato I. Secretion of IL-1 beta, TNF-alpha, IL-8 and IL-1ra by human polymorphonuclear leukocytes in response to lipopolysaccharides from periodontopathic bacteria. J Periodontal Res. 1997;32:279–86. doi: 10.1111/j.1600-0765.1997.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 36.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 37.Hightower LE, Guidon PT., Jr Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–66. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- 38.van Eden W, Koets A, van Kooten P, Prakken B, van der Zee R. Immunopotentiating heat shock proteins. negotiators between innate danger and control of autoimmunity. Vaccine. 2003;21:897–901. doi: 10.1016/s0264-410x(02)00538-8. [DOI] [PubMed] [Google Scholar]
- 39.Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P, Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol. 2001;167:4844–52. doi: 10.4049/jimmunol.167.9.4844. [DOI] [PubMed] [Google Scholar]
- 40.Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, Stoppacciaro A, Vile RG. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol. 1999;163:1398–408. [PubMed] [Google Scholar]
- 41.Cutler CW, Jotwani R, Pulendran B. Dendritic cells: immune saviors or Achilles heel? Infect Immun. 2001;69:4703–8. doi: 10.1128/IAI.69.8.4703-4708.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]