Lung injury resulting from the systemic inflammatory response syndrome (SIRS), be it infectious (sepsis) or not, remains a significant contributor to patient morbidity and mortality worldwide [1]. While the mortality from acute respiratory distress syndrome (ARDS) has decreased to between 30 and 40%, approximately 20% will die of refractory hypoxaemia and the rest from the multiple organ dysfunction syndrome (MODS) [2]. Thus, with the prevalence of lung injury remaining substantial and the acquisition of lung injury not being inconsequential, investigators continue to search for mechanisms, management strategies and therapeutic treatments that will benefit those patients in need.
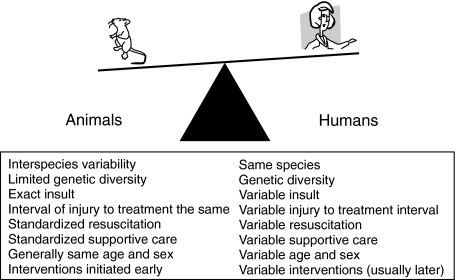
Models of lung injury used by investigators internationally are numerous in their insults of initiation, maintenance and host organisms utilized. Despite their diversity, it is extremely difficult to mimic lung injury encountered at the bedside. Examples of inherent differences just among species are numerous and do not allow replication of human disease, but rather provide to some degree in vivo biological proof of principle (Fig. 1) [3,4].
Fig. 1.
Imbalance between animal models and humans [3,4]. Inherent differences between animals and humans makes modelling lung injury challenging. Thus, the efforts to mimic the bedside situation by utilizing animal models must be conducted carefully and the conclusions interpreted with caution.
Patients afflicted with lung injury more commonly than not encounter more than ‘one-hit’ modulating the immunological response to injury by increasing duration and amplitude of the inflammatory response. In fact, many second ‘hits’ occur after proinflammatory responses (SIRS) have waned and patients manifest a compensatory anti-inflammatory responses (CARS) with suppressed immunity and diminished resistance to infection. This scenario seemingly places the patient at risk for manifesting clinically significant lung injury and MODS [5].
While human studies lack clarity in this area, recently published animal studies have been designed with ‘two-hits’ to better mimic and allow for insight into the ‘yin and yang’ of immuno-inflammatory responses due to injury. Recently, Murphy et al. tested whether changes in innate immune cell reactivity following two-hit injury contributes to the development of heightened inflammation and organ dysfunction [6]. Using a rodent model of thermal injury followed by intraperitoneal administration of endotoxin at either 1 or 7 days, the investigators demonstrated significantly elevated Toll-like receptor 4 (TLR4), a family of pattern recognition receptors that respond to endotoxin, mediated increases in interleukin (IL)-1β, tumour necrosis factor (TNF)-α and interleukin (IL)-6 in spleen cells stimulated ex vivo by endotoxin. While increases in cytokines concentrations were significant at day 1, the response was twofold greater in magnitude at day 7. In support of enhanced TLR-4 responses was the observation that 75% of the animals died within 48 h when endotoxin was administered to animals on day 7, but not on day 1. To determine cause of death, vital organs were assessed for severity of injury after 7 days. Lung and liver from the endotoxin–thermally injured animals demonstrated the most significant injury. In an attempt to relate mortality and pathology, selected serum and organ-derived cytokines and chemokines were measured serially in endotoxin-treated animals. Interestingly, no significant increases were observed in the injured animals or sham animals at day 1. However, in the day 7 group, cytokine (TNF-α, IL-6, IL-10) levels were significantly higher and remained there for much longer intervals as compared to sham animals. At day 7, both chemokines [macrophage inflammatory protein (MIP)-1α), keratinocyte-derived chemokine, monocyte chemoatractant protein-1] and cytokines (TNF-α, IL-1β, IL-6, IL-10) measured in lung tissue extracts of endotoxin-challenged animals were significantly elevated as compared to their sham cohorts. Blocking TNF-α with soluble TNF-R55-Ig infusion 2 h prior to endotoxin challenge at day 7 post-injury resulted in a dramatic reduction in mortality. In conclusion, the authors provided evidence that a second-hit could enhance injury, and that the elapsed time from which the second-hit occurred was pivotal in evoking innate immunological responses necessary for instigating injury. To strengthen the two-hit model argument, Rizoli et al. sought to assess the influence of shock followed by endotoxin instillation on C-X-C chemokine responses [7]. These families of chemokines are potent neutrophil chemoattractants implicated in neutrophil influx to acutely inflamed lungs. Rodents were subjected to haemorrhagic shock by blood withdrawal subsequently reducing mean arterial pressure (MAP) to 50 mmHg within 15 min. The MAP was maintained between 35 and 40 mmHg for 60 min, after which time the animals were resuscitated over a 2 h interval. At 1 h post-resuscitation the animals underwent tracheostomy for mechanical ventilation, and either endotoxin or normal saline was administered intratracheally. Animals exposed to both hits demonstrated significantly greater lung injury reflected by increased transpulmonary albumin flux, increased neutrophil counts and cytokine-induced neutrophil chemoattractant (CINC) concentrations compared to sham animals and those animals given only normal saline. Additionally, anti-CINC antibodies administered 10 min prior to endotoxin instillation, significantly reduced lung neutrophil sequestration. The authors conclude that lung injury was more significant with a second hit, and that augmented release of CINC was responsible for the observed injury.
In this issue of Clinical and Experimental Immunology, Vuichard et al. utilize another variation of the two-hit model in an attempt to better characterize endotoxin-mediated lung injury, in hopes of better reflecting the ‘clinical situation’. Endotoxin was instilled intratracheally into rats, followed by placing the animals in a chamber with either an oxygen concentration of 21% (normoxia) or 10% (hypoxia) within 60 min of endotoxin instillation. While an additional animals receiving hypoxia without endotoxin were tested, these data were de-emphasized due to insignificant findings when compared to the endotoxin–normoxia and endotoxin–hypoxia animals. Pulmonary inflammatory responses were measured at 2, 4, 6 and 8 h. In animals exposed to endotoxin–hypoxia, lung myeloperoxidase (MPO), neutrophil counts, TNF-α, MIP-1β and surfactant protein-B were measured in bronchoalveolar lavage fluid (BALF) and all were significantly increased as compared to animals exposed to endotoxin–noromoxia. It was interesting that when macrophages were depleted using clodronate liposomes, lung MPO, neutrophil counts, TNF-α and MIP-1β measured at 6 h were significantly reduced, thus implicating the alveolar macrophage as the major effector cell. In addition to providing mechanistic insights into the complex disease that is lung injury, this experimental design better mimics the clinical condition. Insults occurring via an inflammatory nidus followed by a secondary insult such as hypoxia or severe and protracted hypotension is commonly encountered in the critically ill patient. Also, the observation of compartmentalization (alveolar versus interstitial) was fascinating. Measurements derived from BALF were significantly altered (except for MPO) by this two-hit model compared to measurements derived from lung homogenates. This may have arisen from the mode of inducing injury, time interval between the first and second hit (short at 60 min) and the total experimental time (8 h). Nevertheless, the study by Vuichard et al., as well as the previously cited studies, epitomizes legitimate efforts to more effectively model experimentation to enhance patient care. With that said, is the two-hit model really necessary?
There is a diverse body of literature aimed at determining the ideal ‘clinically relevant’ animal models for the study of ALI (acute lung injury) and ARDS. The difficulty in determination of the single best choice for these studies lies in a lack of uniformity in regards to methodology and clinical endpoints. Direct comparison of one- versus two-hit models of lung injury is compounded further by the lack of definition for lung injury that appropriately mimics ARDS in human patients; i.e. is it enough to study specific inflammatory and histological changes that correspond to ARDS or must the development, progression and resolution of the syndrome also mirror the human clinical scenario? Vuichard and colleagues accurately point out that, based on the complexity of the illness, two-hit models of ALI should more accurately reflect comorbidities and risk factors found in patients. These authors therefore utilized intratracheal endotoxin and hypoxia to enhance acute lung injury. While the use of hypoxia in combination with endotoxin represents a relatively new (if not unique) two-hit model for ARDS [8,9], the use of endotoxin alone or in combination with a second hit has been well utilized as a model for lung injury (Table 1).
Table 1. Examples of endotoxin models of ARDS and ALI.
| Animal model | Endotoxin typea | Route | Dose (mg/kg) | Second-hit | Ref. |
|---|---|---|---|---|---|
| Rat | NR | i.t. | 0·75 | Hypoxiab | Vuichard 2005 |
| Rat | 0111:B4 | i.t. | 0·03 | Haemorrhageb | [7] |
| Rat | ST | i.p. | 15·0 | ∔ | [20] |
| Rat | 0111:B4 | i.v. | 0·20/hd | ∔ | [21] |
| Rat | 026:B6 | i.t. | 0·005–0·5e | ∔ | [10] |
| Rat | 0111:B4 | i.t. | 5·0 | ∔ | [13] |
| Mouse | FP | i.p. | ∔ | Acid aspirationc | [14] |
| Mouse | 0111:B4 | i.p. | 15·0 | ∔ | [11] |
| Mouse | 0128:B12 | Aerosol | 0·1–1·0e | ∔ | [12] |
| Mouse | 0111:B6 | i.p. | 1·0 | ∔ | [22] |
| Pig | FP | i.p. | ∔ | Ischaemia/reperfusionb | [18] |
| Pig | FP | i.p. | ∔ | Haemorrhagec | [15] |
| Pig | 0111:B4 | i.v. | 2500/hd | ∔ | [23] |
| Pig | 0111:B4 | i.v. | 0·20 | ∔ | [24] |
Escherichia coli.
Introduction of a second-hit increased inflammation of model
Introduction of second-hit had no effect on inflammation of model.
Constant infusion;
dose–response; NR = not reported; FP = fecal peritonitis; i.t. = intratracheal; i.p. = intraperitoneal; i.v. = intravenous; ST = Salmonella typhimurium.; ARDS = acute respiratory distress syndrome; ALI = acute lung injury.
Indeed, within the parameters of inflammation as characterized by Vuichard et al. there are a number of single-hit endotoxin models that closely parallel their findings. Increasing doses of endotoxin (5–5000 µg/ml/kg) instilled intratracheally in the rat has been shown to increase airway hyperinflation and oedema, induce the production of TNF-α, MCP-1, IL-6 and IL-1β, and trigger neutrophilia [10]. Intraperitoneal injection of endotoxin into mice has also been reported to display the key features of ARDS including increased pulmonary oedema, neutrophil infiltration, inflammatory cytokine and chemokine expression and mortality [11]. Interestingly the use of aerosolized endotoxin in mice has demonstrated that low dose exposure (100 µg/ml) causes a rapid and transient activation of macrophages with a concomitant spike in neutrophilic inflammation within the airspace [12], and in a rat model a single high dose (5 mg/kg) intratracheal injection of endotoxin identified the macrophage as the ‘pivotal’ cell type responsible for both inflammation and repair during lung injury [13]. These latter data correlate well with the clodronate-mediated macrophage depletion data presented by Vuichard et al. The arguments for a single-hit endotoxin model of lung injury are clear: reproducibility, rapid onset of clinical signs and lack of expense make these uncomplicated models extremely attractive. Similarly, several articles have reported that the introduction of a second-hit had no impact on inflammation or increased lung injury [14,15].
However, despite the appeal of the single-hit model, the reasoning for the use of a two-hit model is still extremely logical. The development of ALI and ARDS in human patients is complex and rarely occurs as the result of a single instigating factor. Therefore the concept of priming the immune response to react exuberantly to a second stimulus makes eminent sense for a model system [16]. A number of two-hit models have been proposed with apparent success. The use of haemorrhagic shock prior to the introduction of low dose (0·03 mg/kg) intratracheal endotoxin in rats produced significant increases in neutrophil infiltration as well as protein leakage across the lung epithelial barrier as compared to endotoxin alone [7]. Similarly, this same model was utilized to determine that an antioxidative or anti-inflammatory agent applied after haemorrhagic shock might ameliorate effectively the development of serious lung injury [17]. Development of two-hit models has become extremely sophisticated. Recently Steinberg and colleagues [18], created a two-hit model utilizing ischaemia reperfusion injury and fecal peritonitis to initiate ARDS in pigs. These authors have argued against the use of endotoxin in favour of bacterial sepsis, on the grounds that endotoxin speeds up the development of lung disease in such a way as to make it irrelevant to the human condition. They point out that a number of human clinical trials using anti-inflammatory treatment strategies proven efficacious in acute animal models of ARDS have failed to improve lung function and mortality [18]. The failure of these interventions to provide any significant improvement to patients with ALI and ARDS underscores the importance for the development of a relevant animal model (either single-hit or two-hit) that mirror the clinical and physiological development of ARDS in humans.
At the end of the day, stating definitively that two-hit models reign superior to one-hit models may be premature. Vuichard and colleagues should be complimented for their work, but significant controversy still remains and will require further investigation. The one-hit model's simplicity, combined with reduced resources expended in a ‘do more with less’ environment, will continue to be preferred by many laboratories. While bodies such as the National Heart, Lung and Blood Institute are recommending two-hit models, prospective investigations designed to directly compare one- versus two-hit models head-to-head must be highly encouraged [19].
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999;281:354–60. doi: 10.1001/jama.281.4.354. [DOI] [PubMed] [Google Scholar]
- 3.Marshall JC. Modeling MODS: what can be learned from animal models of the multiple–organ dysfunction syndrome? Intens Care Med. 2005;31:605–8. doi: 10.1007/s00134-005-2595-3. [DOI] [PubMed] [Google Scholar]
- 4.Esmon CT. Why do animal models (sometimes) fail to mimic human sepsis? Crit Care Med. 2004;32:S219–S222. doi: 10.1097/01.ccm.0000127036.27343.48. [DOI] [PubMed] [Google Scholar]
- 5.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–44. doi: 10.1016/s1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 6.Murphy TJ, Paterson HM, Kriynovich S, et al. Linking the ‘two-hit’ response following injury to enhanced TLR4 reactivity. J Leukoc Biol. 2005;77:16–23. doi: 10.1189/jlb.0704382. [DOI] [PubMed] [Google Scholar]
- 7.Fan J, Marshall JC, Jimenez M, Shek PN, Zagorski J, Rotstein OD. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J Immunol. 1998;161:440–7. [PubMed] [Google Scholar]
- 8.Agorreta J, Garayoa M, Montuenga LM, Zulueta JJ. Effects of acute hypoxia and lipopolysaccharide on nitric oxide synthase-2 expression in acute lung injury. Am J Respir Crit Care Med. 2003;168:287–96. doi: 10.1164/rccm.200209-1027OC. [DOI] [PubMed] [Google Scholar]
- 9.Agorreta J, Zulueta JJ, Montuenga LM, Garayoa M. Adrenomedullin expression in a rat model of acute lung injury induced by hypoxia and LPS. Am J Physiol Lung Cell Mol Physiol. 2005;288:L536–L545. doi: 10.1152/ajplung.00314.2004. [DOI] [PubMed] [Google Scholar]
- 10.Jansson AH, Eriksson C, Wang X. Lung inflammatory responses and hyperinflation induced by an intratracheal exposure to lipopolysaccharide in rats. Lung. 2004;182:163–71. doi: 10.1007/s00408-004-1803-1. [DOI] [PubMed] [Google Scholar]
- 11.Kabir K, Gelinas JP, Chen M, et al. Characterization of a murine model of endotoxin-induced acute lung injury. Shock. 2002;17:300–3. doi: 10.1097/00024382-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Larsson R, Rocksen D, Lilliehook B, Jonsson A, Bucht A. Dose-dependent activation of lymphocytes in endotoxin-induced airway inflammation. Infect Immun. 2000;68:6962–9. doi: 10.1128/iai.68.12.6962-6969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domenici L, Pieri L, Galle MB, Romagnoli P, Adembri C. Evolution of endotoxin-induced lung injury in the rat beyond the acute phase. Pathobiology. 2004;71:59–69. doi: 10.1159/000074418. [DOI] [PubMed] [Google Scholar]
- 14.Nemzek JA, Call DR, Ebong SJ, Newcomb DE, Bolgos GL, Remick DG. Immunopathology of a two-hit murine model of acid aspiration lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;278:L512–L520. doi: 10.1152/ajplung.2000.278.3.L512. [DOI] [PubMed] [Google Scholar]
- 15.Lyden SP, Patton JH, Jr, Ragsdale DN, Croce MA, Fabian TC, Proctor KG. Transient inhibition of CD18-dependent leukocyte functions after hemorrhage and polymicrobial sepsis. Surgery. 1998;123:679–91. [PubMed] [Google Scholar]
- 16.Rotstein OD. Modeling the two-hit hypothesis for evaluating strategies to prevent organ injury after shock/resuscitation. J Trauma. 2003;54:S203–S206. doi: 10.1097/01.TA.0000064512.62949.92. [DOI] [PubMed] [Google Scholar]
- 17.Zhou R, Hu DY, Liu LM, Zhou XW. Protective effects of apocynin on ‘two-hit’ injury induced by hemorrhagic shock and lipopolysaccharide. Acta Pharmacol Sin. 2002;23:1023–8. [PubMed] [Google Scholar]
- 18.Steinberg J, Halter J, Schiller H, Gatto L, Nieman G. The development of acute respiratory distress syndrome after gut ischemia/reperfusion injury followed by fecal peritonitis in pigs: a clinically relevant model. Shock. 2005;23:129–37. doi: 10.1097/01.shk.0000148053.66645.2e. [DOI] [PubMed] [Google Scholar]
- 19.Matthay MA, Zimmerman GA, Esmon C, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute Working Group. Am J Respir Crit Care Med. 2003;167:1027–35. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 20.Fischer LG, Hollmann MW, Horstman DJ, Rich GF. Cyclooxygenase inhibitors attenuate bradykinin-induced vasoconstriction in septic isolated rat lungs. Anesth Analg. 2000;90:625–31. doi: 10.1097/00000539-200003000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Simons RK, Maier RV, Chi EY. Pulmonary effects of continuous endotoxin infusion in the rat. Circ Shock. 1991;33:233–43. [PubMed] [Google Scholar]
- 22.Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005;288:L333–L341. doi: 10.1152/ajplung.00334.2004. [DOI] [PubMed] [Google Scholar]
- 23.Odenstedt H, Aneman A, Karason S, Stenqvist O, Lundin S. Acute hemodynamic changes during lung recruitment in lavage and endotoxin-induced ALI. Intens Care Med. 2005;31:112–20. doi: 10.1007/s00134-004-2496-x. [DOI] [PubMed] [Google Scholar]
- 24.Ogura H, Offner PJ, Saitoh D, et al. The pulmonary effect of nitric oxide synthase inhibition following endotoxemia in a swine model. Arch Surg. 1994;129:1233–9. doi: 10.1001/archsurg.1994.01420360023002. [DOI] [PubMed] [Google Scholar]