Abstract
Organ-specific lymphocyte homing is dependent on the expression of tissue-specific homing receptors and selected chemokine receptors. During the effector phase of an immune response, IgA and IgG antibody-secreting cells (ASC) are differently distributed in the body. Still, B cell expression of L-selectin and the mucosal homing receptor integrin α4β7 is not related to the isotype produced, but only to the site of antigen encounter. In this study, we examined if differences in chemokine responsiveness between IgA+ and IgG+ B cells could explain their different tissue localization. Circulating CD19+ B cells were isolated and their expression of IgA, IgG, and selected chemokine receptors was determined by flow cytometry. Few Ig+ cells expressed CCR2, CCR3, or CCR9, and there was no difference in the expression of these receptors between IgA+ and IgG+ cells. In contrast, CCR4, CCR5, and CXCR3 was expressed on significantly more IgG+ than IgA+ cells. The function of chemokine receptors on memory B cells and ASC was then tested in the transwell system. IgG+ memory cells migrated to a higher extent than IgA+ cells towards the CXCR3 ligand CXCL11/I-TAC, while there was only a small migration towards the CCR4 ligand CCL17/TARC and the CCR9 ligand CCL25/TECK. ASC migrated poorly to all chemokines tested. In conclusion, this study shows that IgG+ and IgA+ memory B cells have a differential expression of the Th1 associated chemokine receptor CXCR3, as well as of CCR4 and CCR5. In contrast, none of the studied chemokine receptors was preferentially expressed by IgA+ cells.
Keywords: Chemokine receptor, B cell, lymphocyte trafficking, human, antibody-secreting cell
Introduction
Lymphocyte circulation through secondary lymphoid tissues and nonlymphoid tissues serves to ensure contact of naive and memory cells with antigen and to distribute effector lymphocytes to their target tissues. Organ-specific lymphocyte homing is dependent on the expression of tissue-specific adhesion molecules on both the circulating lymphocyte and the endothelial cells. Lymphocytes roll along the vessel wall in postcapillary venules, a process mediated by loose interactions between selectins and their carbohydrate ligands. Chemokines induce firm adhesion of rolling leucocytes by activating surface integrins and are also thought to help direct subsequent transmigration through endothelial cells into surrounding tissues [1,2]. Chemokines are a large family of small chemotactic proteins. They can be broadly divided into homeostatic and inflammatory chemokines. The former mediate recruitment of naive and memory lymphocytes to lymphoid tissues and guide these cells to the correct microenvironment within the lymph node, while the latter are up-regulated by inflammatory stimuli, and preferentially recruit neutrophils and effector lymphocytes [3,4].
Lymphocyte recruitment to the gastrointestinal tract is in part mediated by integrin α4β7, that interacts with mucosal addressin cellular adhesion molecule-1 on endothelial cells within the lamina propria of the gut [5,6]. Chemokine responsiveness has recently been identified as another important component of tissue-specific lymphocyte migration. Thus, the chemokine receptor CCR9 is specifically expressed on a subset of gut-homing T cells expressing integrin α4β7, and its ligand CCL25/TECK is constitutively expressed by the small intestinal epithelium [7,8]. Furthermore, murine IgA-secreting cells from gastrointestinal organs specifically migrate towards CCL25 [9] and CCR9 interactions appear to contribute to localization of IgA+ plasma cells to the small intestine [10]. Another recently described chemokine, CCL28/MEC, is expressed in most mucosal tissues and its receptor CCR10 is expressed on IgA+ plasmablasts from all mucosal sites examined [11–13]. Other combinations of adhesion molecules, chemokines and chemokine receptors make up ‘recognition codes’ for other parts of the body. Thus, cutaneous lymphocyte antigen (CLA) mediates binding of skin-seeking lymphocytes to endothelial E-selectin [14]. CLA+ cells also express CCR8, the receptor for CCL1/I-309, which is produced in the skin [15].
We and others have previously shown that circulating B cells activated by mucosal immunization or infection carry the mucosal homing receptor α4β7 and home back to mucosal tissues [16–19]. Both IgA+ and IgG+ cells induced by various mucosal immunizations express α4β7, however, the final distribution of these cells in the body following immunization is fundamentally different. While IgA-secreting vaccine-specific cells can be found in the intestinal mucosa and mucosa-associated exocrine glands, IgG+ cells preferentially migrate to lymph nodes, spleen, and the bone marrow, even after mucosal immunization [20–24]. Thus, expression of α4β7 alone cannot explain the differential localization of IgA+ and IgG+ B cells. In the current study, we compared the expression and function of chemokine receptors on IgA+ and IgG+ memory B cells and antibody-secreting cells (ASC).
Materials and methods
Isolation of B cells
CD19+ B cells were isolated from heparinized blood collected from healthy Swedish volunteers using anti-CD19 antibody coated Dynabeads (Dynal AS, Oslo, Norway). Peripheral blood mononuclear cells were isolated by gradient centrifugation on Ficoll-Paque (Amersham Biosciences AB, Uppsala, Sweden) and incubated with CD19 Dynabeads at a 1 : 1 ratio for 30 min with end-over-end rotation at 4 °C. Cells binding to the beads were isolated and washed on a magnet. Positively selected B cells were released from beads by incubation with CD19 detachabeads for 1 h at room temperature. This procedure yielded a cell suspension containing 97 ± 4% CD19+ cells, most of which were small, resting lymphocytes.
Lymphocytes were isolated from the gastric mucosa of three patients undergoing gastroectomy due to gastric adenocarcinoma. Gastric tissue comprising both the antrum and corpus region was collected from a site at least 5 cm from the tumour, and lamina propria lymphocytes isolated by collagenase treatment of tissues after removal of the epithelial layer by treatment with EDTA [25]. Isolated lymphocytes were kept at 37 °C over night before chemotaxis assays. All patients gave informed consent to participate in the study, which was approved by the Human Research Ethical Committee at Sahlgrenska University Hospital.
Flow cytometry analyses
The surface expression of IgA and IgG on the isolated B cells was first examined using FITC-labelled rabbit anti-human IgA and IgG antibodies (Dako Cytomation, Solna, Sweden) gating on the small, resting B cell population or on the large, recently activated population. The IgA-specific antibody preparation had to be absorbed with IgA-deficient human serum (Sigma-Aldrich Sweden AB, Stockholm, Sweden) before use, to remove unspecific binding, presumably to surface IgM. In subsequent samples, IgA or IgG-specific antibodies were combined with phycoerythrin (PE)-labelled appropriate isotype controls for the different chemokine receptor antibodies. Quadrants were placed to allow approximately 0·5% of all B cells to fall into the upper two quadrants. PE-labelled mouse antibodies to CCR2, CCR3, CCR4, CCR5, CCR6, CXCR2, CXCR4 (all from R & D Systems, Inc., Abingdon, UK) and CD27 (BD Biosciences, San Jose, CA, USA) were used to determine the expression of the respective cell surface molecules. Antibodies to CXCR3 (R & D) and CCR9 (clone GPR-9·6, kindly provided by Dr M. Briskin, Millenium Pharmaceuticals, Inc.) were biotinylated using the method of Heitzmann and Richards [26]. PE-labelled extravidin (Serotec, Oxford, UK) was used to detect biotinylated antibodies.
In a second set of experiments, simultaneous detection of integrin α4β7 was performed using biotinylated mAb ACT-1 (kindly provided by Dr D. Picarella, Millenium), followed by PE-Cy5-labelled extravidin (BD Pharmingen). In these experiments, PE-labelled antibodies to CXCR3 and CCR9 (R & D) were employed.
Chemotaxis assays
Analysis of B cell migration to selected chemokines was performed in 24-well transwells with 5 µm polycarbonate membranes (Costar Corning Inc., Corning, NY, USA). B cells were resuspended in RPMI 1640 medium supplemented with 0·5% fetal calf serum, and 5 × 105 B cells were added to the upper chamber and allowed to migrate for 2·5 h at 37°C. The cells migrating to the lower chamber, containing serial dilutions of CXCL11, CCL17, CCL25, or CCL28 (all from Peprotech EC Ltd, London, UK), were resuspended and washed off with ice cold buffer, pooled, and then divided for flow cytometry and ELISPOT assays. CXCL13/BCA-1 (Peprotech) was used a positive control at 3 µg/ml [27,28], and consistently induced a large B cell migration (on average 130-fold higher than spontaneous migration) comprising equal proportions of IgA+ and IgG+ cells. Incubation at 37°C for 2·5 h under conditions mimicking those of the migration assay did not change the frequencies of IgA+ or IgG+ cells.
Half of the migrating B cells from each assay condition were used for enumeration and analysis of surface Ig expression by flow cytometry. Migrating cells were identified by scatter gating on lymphocytes combined with CD19 expression, and enumerated using Truecount beads (BD Biosciences). Their expression of IgA and IgG was determined as above.
ELISPOT detection of ASC
Antibody-secretion by migrating B cells was detected in two-colour ELISPOT assays as previously described [29]. Briefly, the total frequencies of IgA- and IgG-secreting cells, irrespective of specificity, were determined in wells coated with goat antibodies to the F(ab)2 fragment of human IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). Half of the migrating B cells from each assay condition were used for ELISPOT assays, and they were equally divided into 2 wells. Cells were then incubated over night at 37 °C in a humidified atmosphere containing 7% CO2, and the assay was developed by the addition of HRP-conjugated goat antibodies to human IgA and AP-conjugated goat antibodies to human IgG (both from Southern Biotech, Birmingham, AL, USA) for 4 h, followed by chromogen substrates [29]. The number of spots, each representing the former position of an ASC, was determined under low magnification. Incubation at 37°C for 2·5 h under conditions mimicking those of the migration assay did not change the frequencies of IgA- or IgG-secreting cells.
Statistical analyses
Differences in chemokine receptor expression between IgA+ and IgG+ cells were analysed using the Wilcoxon signed rank test.
Results
Chemokine receptor expression on circulating IgA+ and IgG+ memory B cells
Circulating CD19+ B cells were isolated using magnetic beads and their expression of IgA and IgG, as well as selected chemokine receptors, was determined by flow cytometry. Two gates were used for the analyses, one defining small, resting naïve and memory B cells and one defining large, recently activated and effector B cells.
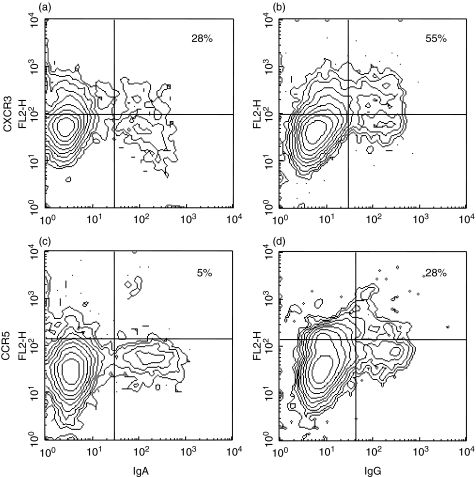
Within the population of small lymphocytes, IgG+ cells were more abundant than IgA+ cells, 8·2 ± 3·7%versus 6·0 ± 4·5%, respectively. These cells were defined as memory cells, since they had undergone isotype switching to IgA or IgG. In addition, most IgA+ and IgG+ cells coexpressed CD27, confirming their memory cell status [30,31]. Very few of the memory B cells expressed CCR2, CCR3, and CCR9, and there was no difference in the expression between IgA+ and IgG+ cells (Table 1). In contrast, the large majority of circulating memory B cells expressed CCR6 and to a smaller extent CXCR4, but again, there was no difference in chemokine receptor expression between IgA+ and IgG+ B cells. On the other hand, expression of CXCR3 differed significantly between IgA+ and IgG+ cells (P < 0·01) (Table 1, Fig. 1). Even though the absolute numbers of Ig+ cells expressing CXCR3 varied among individuals, the fraction of IgG+ cells expressing CXCR3 was larger than that of IgA+ cells in all the volunteers. In this limited material, the mean fluorescence intensity (MFI) was somewhat higher on IgG+ than IgA+ cells (Table 2). A similar trend was seen for expression of CCR5 (P < 0·01) and CCR4 (P < 0·05). These receptors were also found on more IgG+ than IgA+ cells, even though the fraction of Ig+ cells expressing them was lower (Table 1, Fig. 1). However, in the case of CCR4 and CCR5 positive cells, there was a tendency of IgA+ cells to have a higher MFI (Table 2).
Table 1.
Expression of chemokine receptors on circulating IgA+ and IgG+ small and large B cells.
| Small B cells | Large B cells | |||||
|---|---|---|---|---|---|---|
| n | IgA+ (%) | IgG+ (%) | n | IgA+ (%) | IgG+ (%) | |
| CCR2 | 8 | 5·2 ± 2·0† | 7·0 ± 2·1 | 7 | 5·1 ± 1·1 | 16·9 ± 2·6 |
| CCR3 | 8 | 2·9 ± 0·6 | 5·0 ± 1·3 | 7 | 4·5 ± 1·2 | 6·2 ± 1·5 |
| CCR4 | 8 | 5·0 ± 1·7 | 13·8 ± 2·4* | 4 | 11·6 ± 5·6 | 25·9 ± 10·4 |
| CCR5 | 11 | 9·3 ± 1·5 | 27·6 ± 6·0** | 10 | 14·5 ± 4·0 | 35·0 ± 6·0** |
| CCR6 | 8 | 90·5 ± 2·1 | 80·2 ± 7·2 | 7 | 75·3 ± 4·9 | 85·7 ± 4·4* |
| CCR9 | 7 | 7·9 ± 1·4 | 6·3 ± 1·5 | 6 | 14·0 ± 4·7 | 17·2 ± 5·9 |
| CXCR3 | 11 | 36·3 ± 4·5 | 66·8 ± 3·9** | 8 | 60·8 ± 7·0 | 79·4 ± 4·1* |
| CXCR4 | 7 | 60·4 ± 10·0 | 62·0 ± 13·7 | 7 | 56·4 ± 6·1 | 62·1 ± 10·9 |
Percent of all IgA+ or IgG+ cells expressing the respective chemokine receptors, data are expressed as mean ± SEM.
P < 0·05 when comparing IgA+ and IgG+ cells;
P < 0·01 when comparing IgA+ and IgG+ cells
Fig. 1.
Chemokine receptor expression by memory IgA+ and IgG+ B cells. Circulating CD19+ B cells were isolated from healthy volunteers and the expression of CXCR3 (a,b) and CCR5 (c,d) on IgA+ (a,c) and IgG+ (b,d) small memory cells was determined by flow cytometry. The figure shows one representative experiment from the data presented in Table 1. Numbers indicate the percentage of IgA+ or IgG+ expressing the indicated chemokine receptor.
Table 2.
Mean fluorescence intensity of chemokine receptor staining on IgA+ and IgG+ small and large B cells.
| Small B cells | Large B cells | |||
|---|---|---|---|---|
| IgA+ | IgG+ | IgA+ | IgG+ | |
| CCR4 | 132 ± 30† | 93 ± 12 | 149 ± 5 | 78 ± 13 |
| CCR5 | 151 ± 58 | 99 ± 14 | 183 ± 64 | 128 ± 8 |
| CCR6 | 65 ± 5 | 85 ± 11 | 350 ± 134 | 211 ± 34 |
| CXCR3 | 92 ± 40 | 170 ± 32 | 121 ± 29 | 124 ± 25 |
Mean fluorescence intensity of staining for the respective chemokine receptors on expressing IgA+ and IgG+ cells, data are expressed as mean ± SEM.
When the large B cells were analysed, they were found to contain a larger fraction of IgG+ (21·4 ± 11·2%) and IgA+ (15·8 ± 10·9%) cells compared to the small resting B cells. There was a somewhat higher expression of most chemokine receptors on the large lymphocytes, but generally the distribution pattern of the chemokine receptors examined was similar in the large and small B cell subsets (Table 1). As in the small lymphocyte population, there were significantly more IgG+ than IgA+ cells expressing CXCR3 (P < 0·05) and CCR5 (P < 0·01), while there was no significant difference in the CCR4 expression between IgA+ and IgG+ cells. In addition, more large IgG+ cells expressed CCR6 than their IgA+ counterparts (P < 0·05; Table 1).
Expression of integrin α4β7 on IgA+ and IgG+ B cells expressing different chemokine receptors
In order to determine if certain chemokine receptors are preferentially expressed in the context of the mucosal homing receptor integrin α4β7, we analysed the expression of α4β7 on IgA+ and IgG+ B cells expressing different chemokine receptors. As would be expected, more IgA+ than IgG+ cells expressed α4β7 (72 ± 15% of all IgA+ cells versus 57 ± 14% IgG+ cells). Next, the expression of α4β7 on cells that were either IgA+ or IgG+ and also expressed CXCR3, CCR4, CCR9, or CXCR4 was analysed. Expression of CXCR3 and CXCR4 did not seem to be influenced by α4β7 expression, since the expression of α4β7 on IgA+ and IgG+ memory B cells expressing CXCR3 or CXCR4 was the same as on IgA+ and IgG+ cells in general (Table 3). In contrast, a high proportion of both IgA+ and IgG+ cells expressing CCR4 also expressed α4β7. Finally, the expression of α4β7 on IgG+ CCR9+ cells was lower than on the whole population of IgG+ cells (Table 3).
Table 3.
Expression of integrin α4β7 on IgA+ and IgG+ cells also expressing different chemokine receptors.
| IgA+ cells | IgG+ cells | |
|---|---|---|
| CCR4 | 87 ± 10a | 87 ± 4 |
| CCR9 | 74 ± 4 | 36 ± 3 |
| CXCR3 | 73 ± 5 | 57 ± 8 |
| CXCR4 | 71 ± 5 | 53 ± 7 |
Percent of α4β7+ cells among IgA+ or IgG+ cells also expressing the respective chemokine receptors, data are expressed as mean ± SEM calculated from 4 individuals.
Migration of IgA+ and IgG+ memory B cells
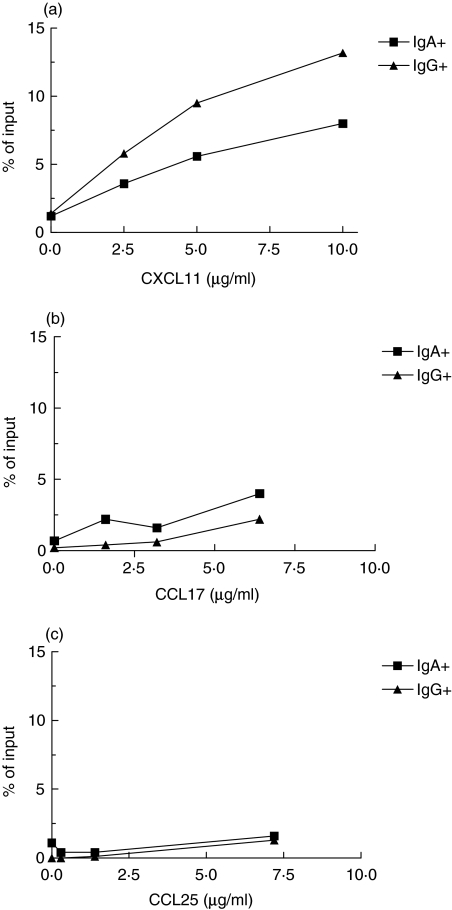
To test the functional significance of the differential expression of CXCR3 and CCR4 expression on IgA+ and IgG+ B cells, their ability to respond to the CXCR3 ligand CXCL11 and CCR4 ligand CCL17 was assessed in chemotaxis assays using the transwell system. Most migrating B cells belonged to the small lymphocyte population, and the large B cells that migrated were too few to be enumerated properly. CXCL11 induced a dose-dependent migration of small B cells, that was on average 35-fold larger than the spontaneous migration at the optimal concentration (10 µg/ml) when all cells, regardless of isotype commitment, were included. Both IgA+ and IgG+ cells migrated in response to CXCL11, but with higher chemokine concentrations, the migration of IgG+ cells was more pronounced than that of IgA+ cells (Fig. 2). CCL17 induced a much smaller increase in B cell migration (on average 6-fold, based on all CD19+ cells), and there were no consistent differences in migration between IgG+ and IgA+ B cells (Fig. 2).
Fig. 2.
Migration of IgA+ and IgG+ memory B cells. Circulating CD19+ B cells were isolated from healthy volunteers and the migration of IgA+ (▪) and IgG+ (▴) cells towards increasing concentrations of (a) CXCL11 (b) CCL17, and (c) CCL25 was analysed in the transwell system, using flow cytometry to enumerate and characterize migrating cells. Assays were carried out in duplicates and the figure shows one representative experiment out of four.
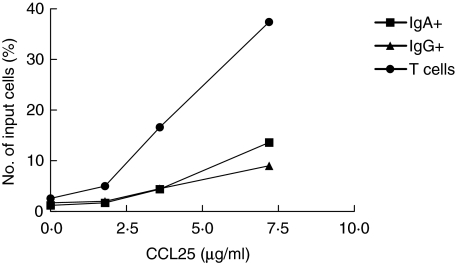
The migration of human B cells in response to CCL25 was also examined, even though CCR9 expression was low on circulating memory B cells. A small but significant proportion of circulating B cells responded to CCL25 (on average 8-fold increased migration of CD19+ cells compared to the spontaneous migration). IgG+ and IgA+ cells responded similarly to CCL25 (Fig. 2), as would be predicted from their CCR9 expression. To ascertain that the conditions employed would allow for efficient migration to CCL25, we examined the migration of lymphocytes isolated from stomach lamina propria to CCL25. These assays showed a substantial migration of T cells, and to a lesser extent also of B cells, to CCL25 (Fig. 3). In parallel, migration towards the other mucosal chemokine, CCL28, was also evaluated. CCL28 induced only a very small increase in the migration of circulating CD19+ cells (on average 2·5-fold), and the migration of IgG+ and IgA+ cells was similar and did not exceed that level at any of the concentrations used (data not shown).
Fig. 3.
Migration of lamina propria lymphocytes. Lamina propria lymphocytes were isolated from normal gastric mucosa and the migration of IgA+ B cells (▪), IgG+ B cells (▴) and T cells (•) in response to increasing concentrations of CCL25 monitored in the transwell system, using flow cytometry to enumerate and characterize migrating cells. One representative experiment out of three is shown.
Migration of ASC
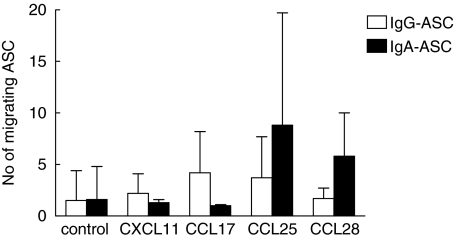
In order to assess the migration of circulating plasmablasts, which might be fundamentally different from that of memory cells, ELISPOT assays were performed to detect ASC among the migrating cells from the transwell assays. The CD19+ cells from blood usually contained more IgA-ASC (mean 501 ASC/105 B cells, range 90–2012) than IgG-ASC (mean 249 ASC/105 B cells, range 28–600). Very few ASC migrated spontaneously to the lower chamber (mean 0·5% of IgA-ASC and 0·7% of IgG-ASC). All of the chemokines tested induced only a modest migration of ASC above the spontaneous migration with a relatively large individual variation (Fig. 4). None of the chemokines examined induced a consistent recruitment of ASC secreting IgA or IgG, but there was a tendency, although not statistically significant, towards a larger recruitment of IgA-ASC when using CCL25 or CCL28.
Fig. 4.
Chemokine responsiveness of circulating IgA- and IgG-secreting cells. Circulating CD19+ B cells were isolated from healthy volunteers and the migration of ASC towards selected chemokines analysed using ELISPOT. The number of IgG-secreting (□) and IgA-secreting (▪) cells is expressed as mean ± SD of the number of ASC migrating towards optimal concentrations of CXCL11 (5 µg/ml), CCL17 (3 µg/ml), CCL25 (7 µg/ml) and CCL28 (2·5 µg/ml) and are pooled from 3 to 5 experiments.
Discussion
In this article, we show that circulating IgG+ human memory B cells express CXCR3, CCR5 and CCR4 to a larger extent than their IgA+ counterparts. Furthermore, IgG+ cells migrate more efficiently towards the CXCR3 ligand CXCL11 than IgA+ cells. Together, these results indicate a potential role for chemokine receptors in mediating the selective localization of IgG+ and IgA+ B cells to distinct anatomical compartments and in particular for CXCR3, CCR5 and CCR4 in regulating IgG+ B cell trafficking.
The migration of circulating memory and effector lymphocytes to their final effector organs is mediated by both homing receptor recognition of endothelial ligands and binding of chemokines to their respective receptors on the lymphocytes [1,2]. Apart from the constitutive organ-specific production of chemokines mediating selective lymphocyte homing, chemokines mediate recruitment of leucocytes into inflamed tissues [32]. In many inflammatory conditions, recruitment of T cells is accomplished by the IFN-γ-inducible chemokines CXCL11, CXCL10/IP-10 and CXCL9/Mig, all signalling through CXCR3, which is often coexpressed with CCR5, recognizing CCL5/RANTES and CCL8/MCP-2. CXCR3+ T cells are found in many human Th1 driven inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease and multiple sclerosis [33,34]. Indeed, IgG+ cells are often ubiquitous in such sites [35–37], suggesting that CXCR3 is utilized also in vivo, and that the local cytokine production during Th1 dominated inflammation results in recruitment of selected B cell populations. On the other hand, CXCR3 and CCR5 are found on most CD4+ T cells infiltrating nonlymphoid tissues [38,39], and have therefore been suggested to retain cells after entry into the tissue, rather than determining tissue-specificity of lymphocyte homing [39]. However, our finding that CXCL11 induces a robust migration of circulating B cells suggest that it could also act during recruitment. CXCR3 mediated B cell chemotaxis was actually among the strongest in this study, exceeded only by the positive control CXCL13.
The memory B cell responsiveness to CXCL11 was not shared to any larger extent by the effector B cells actively secreting IgG. In addition, IgG-ASC responded poorly to all chemokines tested and had no selectivity towards any particular chemokine. Indeed, the low migration of ASC towards CXCL11 is puzzling, since this chemokine has been shown to efficiently attract spleenic IgG-ASC induced by i.p. immunization in an animal model [40]. Apart from species differences, there are several possible explanations to these discrepancies. First, the circulating ASC are not plasma cells, but rather terminally differentiating plasmablasts [41], which may respond differently to chemokines than the fully matured plasma cells present in tissues. Secondly, it may well be that IgG-ASC induced by antigens encountered at different sites respond differently to chemokines. In our experiments, all ASC, regardless of antigen specificity and activation site, were assayed, and the specific migration by a small subset of cells might therefore be diluted by a larger number of unresponsive cells. Thirdly, i.p. induced ASC were only responsive to CXCL11 during a narrow window in time while leaving the spleen for the bone marrow [40]. If all these parameters influence ASC migration, the cells in the circulation of an unimmunized human corresponding to spleenic ASC induced by i.p. immunization a few days ago is probably a minute fraction of the circulating pool.
Recently, it was demonstrated in humans that CCL25 preferentially attracts small intestinal T cells [42]. In the mouse, CCL25 also attracts IgA-ASC from intestinal associated lymphoid tissues, while CCL28 recruits IgA+ effector cells from most lymphoid organs [9,10,13]. Few corresponding studies have been performed in humans, but Kunkel et al. [43] showed that CCR10, a receptor for CCL28, is selectively expressed by IgA+ plasmablast. It was also shown that tonsillar IgA-ASC migrate in response to CCL28, but circulating ASC were not examined. Our results clearly show that although CCL25 induced some migration of circulating B cells, there was no selective recruitment of IgA+ memory cells and only a slight increase in effector cell migration. On the other hand, mucosal T cells and to a lesser extent IgA+ and IgG+ B cells, were readily attracted by CCL25 under the same circumstances. These findings are probably explained by the small number of circulating human IgA+ cells expressing CCR9. The observation that CCL25 only recruits resident mucosal cells and not circulating B cells may indicate that this chemokine functions as a retention signal in the tissue rather than as an attractor of circulating B cells. Alternatively, only a minor, selected population of circulating IgA+ cells might be attracted by CCL25, along the same line as discussed for CXCL11. The B cell responses to CCL28 were similar to the CCL25 responses, only a small migration of circulating memory and effector cells, but no selective recruitment of IgA+ cells. CCL28 can bind and signal through CCR3 and CCR10, but lymphocyte recruitment to mucosal sites is believed to be mediated by CCR10 [12]. We could not analyse the expression of CCR10, since proper reagents were not available, but a previous study showed that it is virtually absent on circulating memory B cells [43], as is the expression of CCR3 in this study. In view of these observations, the poor responses to CCL28 by memory B cells are not surprising. On the other hand, more than half of the IgA+ plasmablasts in the human circulation express CCR10 [43]. Nonetheless, very few circulating IgA-ASC responded to CCL28, and possibly additional signals are needed to induce migration of these cells.
Chemokine responsiveness alone cannot determine tissue specificity, but act within the context of leucocyte and endothelial adhesion molecules [1,2], and one may speculate that specific chemokine receptors are associated with specific adhesion molecules. We therefore examined the expression of the mucosal homing receptor integrin α4β7 on B cells expressing different chemokine receptors. Expression of CXCR3 and CXCR4 did not seem to correlate with α4β7 expression, but somewhat surprisingly, there was a strong association between CCR4 and α4β7 expression, both on IgA+ and IgG+ cells. CCR4 is usually associated with cutaneous, but not intestinal, T cells [39,44]. Therefore, the migratory pathways of memory B cells expressing α4β7 and CCR4 remain elusive at this point, but one may speculate that they circulate through the spleen. Spleenic marginal zone endothelial cells express MAdCAM-1, and the CCR4 ligand CCL22/MDC is readily induced in spleenocytes [45–47]. Our analyses also showed that only a small fraction of CCR9+ IgG+ cells expressed α4β7. These cells can probably not migrate to the small intestinal lamina propria, even though they express CCR9, but may instead be destined for organized lymphoid follicles in the mucosa, utilizing L-selectin for interactions with high endothelial venules [48,49].
In conclusion, this study shows that IgG+ and IgA+ memory B cells have a differential expression of chemokine receptors CXCR3, CCR5, and CCR4. These receptors were all preferentially expressed on IgG+ cells, while no positive association was found between IgA expression and any of the studied chemokine receptors.
Acknowledgments
We are grateful to all volunteers who participated in this study, and to Dr M. Briskin and Dr D. Picarella (Millenium Pharmaceuticals, Inc.) for the generous gift of the CCR9- and α4β7-specific mAb. Magdalena Granung and the staff at the surgery department at Sahlgrenska University Hospital are gratefully acknowledged for their help with collection of clinical material. This study was supported by the Swedish Research Council (grant 06X-13428), the Swedish Cancer Foundation, Clas Groschinsky's Foundation, Magn. Bergwall's Foundation, Professor Nanna Swartz’ Foundation, the Swedish Medical Association, and Längmanska kulturfonden.
References
- 1.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 2.Buther EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 3.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 4.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regulatory Integrative Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 5.Hu MC-T, Crowe DT, Weissman IL, Holzmann B. Cloning and expression of mouse integrin βp (β7): a functional role in Peyer's patch-specific lymphocyte homing. Proc Natl Acad Sci USA. 1992;89:8254–8. doi: 10.1073/pnas.89.17.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berlin C, Berg EL, Briskin MJ, et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 7.Kunkel EJ, Campbell JJ, Haraldsen G, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–7. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zabel BA, Agace WW, Campbell JJ, et al. Human G protein-coupled receptor GPR-9–6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241–55. doi: 10.1084/jem.190.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowman EP, Kuklin NA, Youngman KR, Lazarus NH, Kunkel EJ, Pan J, Greenberg HB, Butcher EC. The intestinal chemokine thymus-expressed chemokine (CCL25) attracts IgA antibody-secreting cells. J Exp Med. 2002;195:269–75. doi: 10.1084/jem.20010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pabst O, Ohl L, Wendland M, Wurbel M-A, Kremmer E, Malissen B, Förster R. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J Exp Med. 2004;199:411–6. doi: 10.1084/jem.20030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Soto H, Oldham ER, et al. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2) J Biol Chem. 2000;275:22313–23. doi: 10.1074/jbc.M001461200. [DOI] [PubMed] [Google Scholar]
- 12.Pan J, Kunkel EJ, Gosslar U, et al. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J Immunol. 2000;165:2943–9. doi: 10.4049/jimmunol.165.6.2943. [DOI] [PubMed] [Google Scholar]
- 13.Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol. 2003;170:3799–805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- 14.Berg EL, Yoshino T, Rott LS, Robinsin MK, Warnock RA, Kishimoto TK, Picker LJ, Butcher EC. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–6. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaerli P, Ebert L, Willimann K, Blaser A, Roos RS, Loetscher P, Moser B. A skin-selective homing mechanism for human immune surveillance T cells. J Exp Med. 2004;199:1265–75. doi: 10.1084/jem.20032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantele JM, Arvilommi H, Kontiainen S, Salmi S, Jalkanen S, Savilahti E, Westerholm M, Kantele A. Mucosally activated circulating human B cells in diarrhea express homing receptors directing them back to the gut. Gastroenterol. 1996;110:1061–7. doi: 10.1053/gast.1996.v110.pm8612994. [DOI] [PubMed] [Google Scholar]
- 17.Quiding-Järbrink M, Nordström I, Granströn G, et al. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunization. A molecular basis for the compartmentalization of effector B cell responses. J Clin Invest. 1997;99:1281–6. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantele A, Zivny J, Häkkinen M, Elson CO, Mestecky J. Differential homing commitments of antigen-specific T cells after oral or parenteral immunization in humans. J Immunol. 1999;162:5173–7. [PubMed] [Google Scholar]
- 19.Quiding-Järbrink M, Ahlstedt I, Lindholm C, Johansson E-L, Lönroth H. Homing commitment of lymphocytes activated in the human gastric and intestinal mucosa. Gut. 2001;49:519–25. doi: 10.1136/gut.49.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quiding M, Nordström I, Kilander A, Andersson G, Hansson L-Å, Holmgren J, Czerkinsky C. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses, gamma-interferon production, and evokes local immunological memory. J Clin Invest. 1991;88:143–8. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czerkinsky C, Svennerholm A-M, Quiding M, Johnsson R, Holmgren J. Antibody-producing cells in peripheral blood and salivary glands after oral cholera vaccination in humans. Infect Immun. 1991;59:996–1001. doi: 10.1128/iai.59.3.996-1001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eriksson K, Quiding-Järbrink M, Osek J, Nordström I, Hjulström M, Holmgren J, Czerkinsky C. Anatomical segmentation of the intestinal immune response in non-human primates. Oral and rectal immunization induce differential homing of B cells to sites defined by their source of vascularisation. Infect Immun. 1999;67:6210–2. doi: 10.1128/iai.67.11.6210-6212.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benner R, Hijmans W, Haaijman JJ. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin Exp Immunol. 1981;46:1–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Dilosa RM, Maeda K, Masuda A, Szakal AK, Tew JG. Germinal center B cells and antibody production in the bone marrow. J Immunol. 1991;146:4071–7. [PubMed] [Google Scholar]
- 25.Bergin PJ, Edebo A, Wen S, et al. Increased production of matrix metalloproteinases in Helicobacter pylori associated human gastritis. Helicobacter. 2004;9:201–10. doi: 10.1111/j.1083-4389.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 26.Heitzmann H, Richards FM. Use of the avidin-biotin complex for specific staining of biological membranes in electron microscopy. Proc Natl Acad Sci USA. 1974;71:3537–41. doi: 10.1073/pnas.71.9.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JC, Williams LT. A B-cell homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 28.Legler DF, Loetscher M, Roos RS, Clarc-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187:655–60. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czerkinsky C, Moldoveanu Z, Mestecky J, Nilsson L-Å, Ouchterlony Ö. A novel two colour ELISPOT assay. I. Simultaneous detection of distinct types of antibody-secreting cells. J Immunol Meth. 1988;115:31–7. doi: 10.1016/0022-1759(88)90306-7. [DOI] [PubMed] [Google Scholar]
- 30.Agematsu K, Hokibara S, Nagumo H, Komiyama A. CD27: a memory B-cell marker. Immunol Today. 2000;21:204–9. doi: 10.1016/s0167-5699(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 31.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig) M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–9. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin S, Rottman RB, Myers P, et al. The chemokine receptors CXR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balashov K, Rottman J, Weiner H, Hancock WW. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1, and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96:6873–8. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandtzaeg P. Inflammatory Bowel disease: clinics and pathology. Do inflammatory bowel disease and periodontal disease have similar immunopathogeneses? Acta Odent Scand. 2001;59:235–43. doi: 10.1080/00016350152509265. [DOI] [PubMed] [Google Scholar]
- 36.Ebersole JL. Humoral immune responses in gingival crevice fluid: local and systemic implications. Periodontol. 2000 2003;31:135–66. doi: 10.1034/j.1600-0757.2003.03109.x. [DOI] [PubMed] [Google Scholar]
- 37.Berek C, Kim H-J. B-cell activation and development within chronically inflamed synovium in rheumatoid and reactive arthritis. Semin Immunol. 1997;9:261–8. doi: 10.1006/smim.1997.0076. [DOI] [PubMed] [Google Scholar]
- 38.Agace WW, Roberts AI, Wu L, Greineder C, Ebert EC, Parker CM. Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur J Immunol. 2000;30:819–26. doi: 10.1002/1521-4141(200003)30:3<819::AID-IMMU819>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 39.Kunkel EJ, Boisvert J, Murphy K, et al. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol. 2002;160:347–55. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hauser AJ, Debes GF, Arce S, Cassese G, Hamann A, Radbruch A, Manz RA. Chemotactic responsiveness towards ligands for CXCR3 and CXCR4 is regulated on plasmablasts during the time course of a memory immune response. J Immunol. 2002;169:1277–82. doi: 10.4049/jimmunol.169.3.1277. [DOI] [PubMed] [Google Scholar]
- 41.Quiding-Järbrink M, Lakew M, Nordström I, Banchereau J, Butcher EC, Holmgren J, Czerkinsky C. Human circulating specific antibody-forming cells after systemic and mucosal immunizations. differential homing commitments and cell surface differentiation markers. Eur J Immunol. 1995;25:322–7. doi: 10.1002/eji.1830250203. [DOI] [PubMed] [Google Scholar]
- 42.Papadakis KA, Prehn J, Nelson V, Cheng L, Binder SW, Ponath PD, Andrew DP, Targan SR. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. 2000;165:5069–76. doi: 10.4049/jimmunol.165.9.5069. [DOI] [PubMed] [Google Scholar]
- 43.Kunkel EJ, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, Butcher EC. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J Clin Invest. 2003;111:1001–10. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell JJ, Haraldsen G, Pan J, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory cells. Nature. 1999;400:776–80. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 45.Shyjan AM, Bertagnolli M, Kenney CJ, Briskin MJ. Human mucosal addressin cell adhesion molecule-1 (MAdCAM-1) demonstrates structural and functional similarities to the alpha 4 beta 7-integrin binding domains of murine MAdCAM-1, but extreme divergence of mucin-like sequences. J Immunol. 1996;156:2851–7. [PubMed] [Google Scholar]
- 46.Kuroda E, Sugiura T, Okada K, Zeki K, Yamasita U. Prostaglandin E2 up-regulates macrophage-derived chemokine production but suppresses IFN-inducible protein-10 production by APC. J Immunol. 2001;166:1650–8. doi: 10.4049/jimmunol.166.3.1650. [DOI] [PubMed] [Google Scholar]
- 47.Jiang X, Jing H, Ganea D. VIP and PACAB down-regulate CXCL10 (IP-10) and up-regulate CCL22 (MDC) in spleen cells. J Neuroimmunol. 2002;133:81–94. doi: 10.1016/s0165-5728(02)00365-x. [DOI] [PubMed] [Google Scholar]
- 48.Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori. Proc Natl Acad Sci U S A. 2004;101:17807–12. doi: 10.1073/pnas.0407503101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. 1-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993;366:695–8. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]