Abstract
Herbal medicine has become an increasing popular therapeutic alternative among patients suffering from various inflammatory disorders. The Salvia miltiorrhizae water-soluble extract (SME) have been shown to possess antioxidant and anti-inflammatory properties in vitro. However, the mechanism of action and impact of SME on LPS-induced gene expression is still unknown. We report that SME significantly abrogated LPS-induced IκB phosphorylation/degradation, NF-κB transcriptional activity and ICAM-1 gene expression in rat IEC-18 cells. Chromatin immunoprecipitation assay demonstrated that LPS-induced RelA recruitment to the ICAM-1 gene promoter was inhibited by SME. Moreover, in vitro kinase assay showed that SME directly inhibits LPS induced IκB kinase (IKK) activity in IEC-18 cells. To investigate the physiological relevance of SME inhibitory activity on NF-κB signalling, we used small intestinal explants and primary intestinal epithelial cells derived from a transgenic mouse expressing the enhanced green fluorescent protein (EGFP) under the transcriptional control of NF-κB cis-elements (cis-NF-κBEGFP). SME significantly blocked LPS-induced EGFP expression and IκBα phosphorylation in intestinal explants and primary IECs, respectively. However, salvianolic acid B, an activate component of SME did not inhibit NF-κB transcriptional activity and IκB phosphorylation/degradation in IEC-18 cells. These results indicate that SME blocks LPS-induced NF-κB signalling pathway by targeting the IKK complex in intestinal epithelial cells. Modulation of bacterial product-mediated NF-κB signalling by natural plant extracts may represent an attractive strategy towards the prevention and treatment of intestinal inflammation.
Keywords: Salvia miltiorrhizae, NF-κB IκB kinase intestinal epithelial cell transgenic mouse
Introduction
Intestinal epithelial cells (IECs) are the first line of defense against noxious intraluminal agents including microorganisms and toxic antigens [1,2]. Among the potential stimuli of IECs, bacteria and bacterial products (lipopolysaccharide [LPS], peptidoglycans) are the most important due to their high content in the intestinal lumen [1,3]. Although IECs are less responsive to LPS than monocytes/macrophages, numerous groups have shown that endotoxin triggers a proinflammatory gene transcriptional program in some various IEC [4–9], including rat small intestinal cell line IEC-6 [1,10,11]. Moreover, immunohistochemistry analysis clearly showed enhanced RelA phosphorylation in the intestinal epithelium of bacteria-monoassociated gnotobiotic rats [10]. Thus, luminal endotoxin may participate in various intestinal inflammatory disorders by virtue of its ability to trigger a complex gene program in the intestinal epithelium. Modulation of bacteria and bacterial product-induced gene expression in the intestine may have significant impact in intestinal inflammatory disorders. The signalling events leading to LPS-induced NF-κB activity and pro-inflammatory gene expression require the participation of the toll-like receptor (TLR)-4 and the coreceptors CD-14 and MD-2 [4,12,13]. Down-stream of TLR4 lies numerous kinases and adapter proteins that control LPS signalling to various effector targets, including NF-κB. LPS-induced NF-κB activation requires the recruitment of MyD88 (myeloid differentiation primary response gene 88) and IRAK-1 (IL-1 receptor associated kinase-1). Subsequently, IRAK-1 phosphorylates TRAF 6 (TNF receptor-associated factor 6), which then activates TAK1 (TGF-β activated kinase 1)-TAB (TGF-β activated kinase 1-binding protein) complex [13]. The signal then reaches the IκB kinase (IKK) complex, which phosphorylates the cytoplasmic NF-κB inhibitor IκBα at serine residues 32 and 36, causing its ubiquitination and degradation. Elimination of IκB releases NF-κB and permits the nuclear translocation of the transcription factor, binding to NF-κB-promoter elements and induction of proinflammatory gene transcription [12]. A challenging task for the intestinal immune system is to swiftly response to pathogenic microorganism while remaining tolerant to the high bacteria load found in the intestine. Not surprisingly, dysregulated innate/adaptive response to the host endogenous microflora is associated with intestinal inflammatory disorders such as inflammatory bowel diseases (IBD). A common feature of patients with IBD and of various experimental models of IBD is the dysregulated production of numerous inflammatory mediators, including (IL)-1, IL-6, IL8, IL-12, and tumour necrosis factor (TNF)-α[14,15]. Since NF-κB is involved in the transcriptional regulation of these various proinflammatory molecules, there is a growing interest in targeting this signalling pathway for therapeutic purpose [16,17]. NF-κB regulates the expression of ICAM-1, a cell surface glycoprotein that plays a pivotal role in the recruitment of leucocytes at the sites of intestinal inflammation. This adhesion molecule is up-regulated in the inflamed intestinal mucosa [18–20]. Recent studies showed that a topically administered enema formulation of ISIS 2302, an antisense inhibitor of ICAM-1, improved the clinical symptoms in patients with mild to moderately active distal ulcerative colitis and chronic unremitting pouchitis, respectively [21,22]. An interesting approach for the modulation of NF-κB activity in vivo is the use of natural plant extracts. A growing segment of patients with IBD already relied on alternative medicine, including the use of natural plant extract and/or dietary supplements.
Salvia miltiorrhizae Bunge, known as Danshen, has long been used as a traditional oriental medicine for cardiovascular disease [23]. Salvia miltiorrhizae extracts contain lipid-soluble diterpene quinones (tanshinones) and water-soluble phenolic acid derivatives such as salvianolic acid A, B and lithospermic acid B [24,25]. Recent articles have shown that water-soluble extract of Salvia miltiorrhizae suppressed neutrophil-endothelial adhesion [26], and salvianolic acid B inhibited ICAM-1 expression in TNF-α-treated human endothelial cells [27]. However, the specific mechanism through which Salvia miltiorrhizae water-soluble extract mediates its anti-inflammatory effects is not clear. We investigated the effect of Salvia miltiorrhizae water-soluble extract (SME) on LPS-induced NF-κB signalling and proinflammatory gene expression in IECs. We report that SME prevents the activation of NF-κB signalling by targeting IKK complex in IECs in vitro, and in small intestine explants and primary IECs derived from a transgenic mice expressing the enhanced green fluorescent protein (EGFP) under the transcriptional control of NF-κB cis-elements (cis-NF-κBEGFP). These findings suggest that SME represent a new plant extract capable of inhibiting IKK activity in IECs.
Materials and methods
Salvia miltiorrhizae water-soluble extract (SME) and salvianolic acid B (Sal B)
Dry roots of Salvia miltiorrhizae were extracted with 70% ethanol and the extract obtained was filtered and concentrated to obtain 8 : 1 powdered extract. This extract (Lot # SLV 02210, Narula Research, Chapel Hill, NC, USA) which contains water-soluble polyphenolic acids including salvianolic acids was dissolved in PBS to a final concentration of 50 mg/ml. Purified salvianolic acid B was purchased from Ivy Fine Chemicals (Cherry Hill, NJ, USA) and was dissolved in PBS to a final concentration of 20 mM.
Cell culture and treatment of IEC
The rat nontransformed small intestinal cell line IEC-18 (ATCC CRL 1589, Manassas, VA,USA) was used between passages 5 and 15. Cells were grown as described previously [11]. Cells were pretreated for 1 h with various concentration of SME (0–1 mg/ml), Sal B (50–100 µM) or with PBS vehicle (1%), after which they were stimulated with LPS (10 µg/ml; from E. coli serotype O111:B4, Sigma) for various time.
Animals
Cis-NF-κBEGFP mice (BALB/c background) were used between 8 and 10 weeks of age. Cis-NF-κBEGFP mice expressed the EGFP gene under the transcriptional control of NF-κB cis-elements, thus responding to various NF-κB inducers, including LPS [28]. Animal experiments were performed in accordance with the guidelines of the institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Real-time RT-PCR analysis
RNA was isolated using Trizol (Invitrogen, Carlsbad, CA, USA), and 1 µg of total RNA was reverse-transcribed as described previously [29]. Real-time RT-PCR was performed using an ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA, USA) with specific primers for rat ICAM-1. As an endogenous control, 18S ribosomal RNA primers were used. All primers were designed by Primer Express v2·0 (Applied Biosystems). The sequence of primers used were as follows: rat ICAM-1 (forward primer) 5-CGGG ATGGTGAAGTCTGTCAA-3, ICAM-1 (reverse primer) 5-TGCACGTCCCTGGTGATACTC-3; 18S-RRNA (forward primer) 5-CGCCGCTAGAGGTGAAATTCT-3, 18S-RRNA (reverse primer) 5-CATTCTTGGCAAATGCTTTCG-3. PCR was conducted using SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer's instructions. Thermal cycler conditions were as follows: one cycle of 10 min, 95 °C, and 40 cycles of denaturation (15 s, 95 °C) and combined annealing/extension (1 min, 60 °C). The specificity of the amplicon was tested via melting curve analysis. A serial dilution of an external standard (positive samples; LPS treated) was used to generate a calibration curve to determine signal intensity of each sample according to their threshold cycle. The amplifications were performed in triplicates and the data was normalized to the 18S ribosomal subunit.
Adenoviral infection and NF-κB-luciferase reporter assay
IEC-18 cells were infected for 16 h with an adenoviral vector encoding an NF-κB-luciferase reporter gene (Ad5κB-LUC) as described previously [11]. Where indicated cells were infected with Ad5IκBαAA and Ad5dnIKKβ to selectively block NF-κB activation, or Ad5wtNIK to induce NF-κB activation at a multiplicity of infection (m.o.i) of 50. The Ad5dnIKKβ and Ad5wtNIK constructs were described previously [30]. The Ad5GFP containing GFP was used as a viral negative control. The adenoviruses were washed off, and fresh medium containing serum was added. Cells were pretreated with various concentration of SME or Sal B and then stimulated with LPS (10 µg/ml) for 12 h. Cell extracts were prepared using luciferase cell lysis buffer (PharMingen, San Diego, CA, USA). Luciferase assays were performed using an Lmax luminometer microplate reader (Molecular Devices, Sunnyvale, CA, USA), and results were normalized for extract protein concentrations measured with the Bio-Rad protein assay kit (Bio-Rad).
Western blot analysis
IEC-18 cells were stimulated with LPS (10 µg/ml) for various times (0–1 h). The cells were lysed in 1X Laemmli buffer, and 20 µg of protein was subjected to electrophoresis on 10% SDS-polyacrylamide gels as described previously [11]. Where indicated IEC-18 cells were pretreated for 1 h with SME (500 µg/ml) or Sal B (100 µM). Anti-phosphoserine IκBα (Cell Signalling, Beverly, MA, USA), anti-IκBα (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and antiβ-actin (ICN, Costa Mesa, CA, USA) were used to detect immunoreactive phospho-IκBα, IκBα, and β-actin, respectively, using an enhanced chemiluminescence detection kit (Amersham Biosciences, Arlington Heights, IL, USA).
RelA localization by immunofluorescence
SME (500 µg/ml)-pretreated IEC-18 cells were stimulated with LPS (10 µg/ml) for 30 min, after which they were fixed with 100% ice-cold methanol. RelA immunofluorescence was performed as described previously [29]. Briefly, cells were blocked with 10% nonimmune goat serum (NGS) for 30 min, then probed with rabbit anti-RelA Ab (Rockland, Gilberville, PA, USA; diluted 1 : 200) in 10% NGS for 45 min, followed by rhodamine isothiocyanate-conjugated goat antirabbit IgG Ab (Jackson ImmunoResearch, West Grove, PA, USA; diluted 1 : 100) in 10% NGS for 30 min. RelA was visualized with a fluorescent light microscope.
Chromatin immunoprecipitation (ChIP) assay
IEC-18 cells were pretreated with SME (500 µg/ml) and then stimulated with LPS (10 µg/ml) for 30 min, washed in cold PBS and fixed by adding formaldehyde to a final concentration of 1%. Nuclear extraction and chromatin immunoprecipitation were performed as described previously [10]. Briefly, cells were lysed after formaldehyde fixation in L1 lysis buffer (50 mM Tris [pH 8·0], 2 mM EDTA, 0·1% Nonidet P-40, and 10% glycerol) supplemented with protease inhibitors. Nuclei were pelleted and resuspended in 300 µl of L2 lysis buffer (50 mM Tris [pH 8·0], 0·1% SDS, and 5 mM EDTA). Chromatin was sheared by sonication (3 times for 10 s at one-fifth of maximum power), centrifuged, and diluted in dilution buffer (50 mM Tris [pH 8·0], 5 mM EDTA, 0·2 M NaCl, and 0·5% Nonidet P-40). Extracts were precleared for 3 h with salmon sperm-saturated protein A/G-agarose (ssProtein A/G). Immunoprecipitation was carried out overnight at 4 °C using 5 µl of anti-phosphoserine RelA (Cell Signalling). Immune complexes were collected with ssProtein A/G for 30 min and washed three times in washing buffer (20 mM Tris [pH 8·0], 0·1% SDS, 0·5 M NaCl, 2 mM EDTA, and 1% Nonidet P-40) and once in 0·5 M LiCl, followed by three washes with TE buffer. Immune complexes were extracted 3 times with 100 µl of extraction buffer (TE buffer containing 2% SDS). DNA cross-links were reverted by heating for 8 h at 65°C. After proteinase K (100 µg for 2 h) digestion, DNA was extracted with phenol/chloroform and precipitated in ethanol. DNA isolated from an aliquot of the total nuclear extract was used as a loading control for the PCR (input control). PCR was performed with total DNA (1 µl, input control) and immunoprecipitated DNA (2 µl) using the following rat ICAM-1 promoter-specific primers: ICAM-1 promoter (forward primer) 5-CTTCTCTCCCGGACTCTCCT-3 AND ICAM-1 (reverse primer) 5-ATGAGGGCTTCGGTATTTCC-3.
In vitro IκB kinase assay
IEC-18 cells were pretreated for 1 h with SME (500 µg/ml) and then stimulated with LPS (10 µg/ml) for 20 min or infected with Ad5wtNIK (m.o.i 50) for 16 h. IKK activity on serine IκBα phosphorylation was determined by immunocomplex kinase assay as described previously [29,30]. Briefly, IEC-18 cells were lysed in Triton lysis buffer containing protease and phosphatase inhibitors and then cleared by cenrifugation at 18 000 g for 10 min. Three hundred micrograms of whole cell extract were immunoprecipitated with anti-IKKγ (Santa Cruz Biotechnology)/protein-A beads and the kinase reaction was performed by incubating 25 µl of kinase buffer containing 20 mM Hepes (pH 7·7), 10 mM MgCl2, 5 mM dithiothreitol, 50 µM ATP, and 5 µCi of [γ-32P] ATP (ICN) with GST-IκBα substrate (amino acid 1–54) for 30 min at 30 °C. Substrate proteins were resolved by gel electrophoresis, and phosphate incorporation was assessed by autoradiography and PhosphorImager analysis (Amersham Biosciences).
Alternatively, the effect of SME on IKK activity was directly measured. Immunoprecipitated IKK complexes from LPS (10 µg/ml)-stimulated IEC-18 cells were incubated with various concentrations of SME or control PBS vehicle and the kinase reactions were performed as described above.
Cytotoxicity assay
IEC-18 cells were pretreated with various concentration of SME and then stimulated with LPS. Cytotoxicity was measured using the LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes, Eugene, OR, USA) according to the manufacturer's specifications. Positive control dead cells were generated by treating IEC-18 cells with methanol for 30 min. Fluorescence in cell samples was measured with appropriate excitation and emission filters using a SpectraMax Gemini XS spectrofluorometer (Molecular Devices) according to the manufacturer's instructions.
Murine small intestinal explants and primary IEC study
Cis-NF-κBEGFP mice were euthanized, dissected and the small intestines were flushed and sectioned into 2 cm parts. The small intestinal explants were incubated in RPMI-1640 with 10% FBS and antibiotics. The explants were pretreated for 1 h with SME (500 µg/ml) and then stimulated with LPS (10 µg/ml) for 12 h. The sections were imaged using either a CCD camera in a light-tight imaging box with a dual filtered light source and emission filters specific for EGFP (LT-99D2 Illumatools, Lightools Research, Encinitas, CA, USA), or alternatively, scanned using a PhosphorImager with fluorescence settings (Typhoon 8600, Amersham Biosciences). Fluorescent intensity was quantified by the densitometric analysis using ImageQuant software ver 2·2.
To study SEM effects on primary intestinal epithelial cells, the small intestine from Cis-NF-κBEGFP mice was sliced open and washed 3 times in PBS and twice in 3 mM EDTA and 0·5 mM DTT (solution A). The small intestine was incubated for 90 min at room temperature in solution A with shaking, and then the resulting supernatant wase passed over nylon filter. The cellular suspension was centrifuged, washed, and resuspended in RPMI-1640 with 10% FBS and antibiotics. Cells were pretreated for 1 h with SME (500 µg/ml) and then stimulated with LPS (10 µg/ml) for various times.
Statistical analysis
All data were expressed as the means for a series of experiments ± SEM. Data were analysed by nonparametric t-tests or Wilcoxon rank sum tests. A 2-tailed P-value of <0·05 was considered statistically significant.
Results
SME abrogates LPS-induced ICAM-1 mRNA expression and NF-κB transcriptional activity in IECs
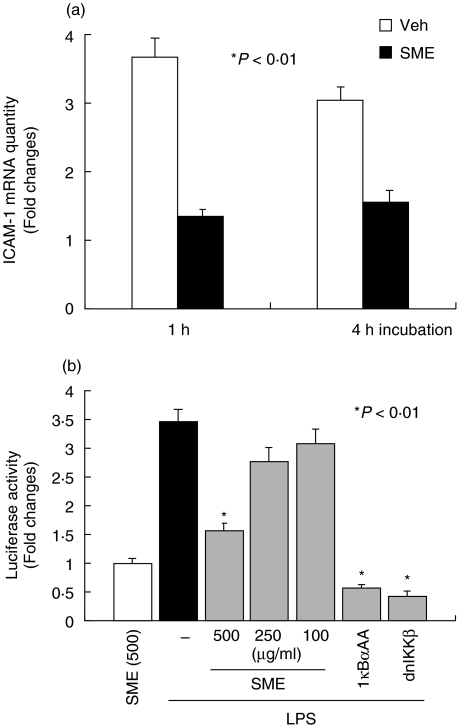
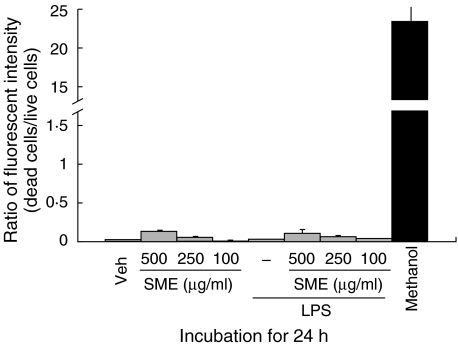
We first evaluated the effects of SME on LPS-induced ICAM-1 gene expression in the rat nontransformed intestinal cell line IEC-18. Cells were pretreated with SME (500 µg/ml) for 1 h, stimulated with LPS (10 µg/ml) for up to 4 h and ICAM-1 gene expression was measured by real-time RT-PCR using an ABI Prism 7700 sequence detection system. As seen in Fig. 1a, LPS-induced ICAM-1 mRNA expression is inhibited by SME treatment (64% and 49% inhibition at 1 h and 4 h poststimulation, respectively). A critical downstream effector pathway induced by LPS is the NF-κB transcriptional system. Thus we next assessed whether SME abrogates LPS-induced NF-κB transcriptional activity using a luciferase reporter gene assay. As shown in Fig. 1b, SME dose-dependently blocked LPS-induced NF-κB transcriptional activity in IEC-18 cells. As expected, the molecular inhibitory approach using Ad5IκBαAA and Ad5dnIKKβ blocked LPS-induced NF-κB activity in IEC-18 cells. To rule out the possibility that SME-mediated inhibition of LPS-induced gene expression is due to a cytotoxic effect, we measured SME-induced cytotoxicity in LPS-stimulated IEC-18 cells. SME-treated cells exhibited minimal cytotoxicity as compared to methanol-treated control, suggesting that the SME is not significantly altering cell viability (Fig. 2).
Fig. 1.
Salvia miltiorrhizae extract (SME) abrogates LPS-induced ICAM-1 mRNA expression and NF-κB transcriptional activity. (a) IEC-18 cells were pretreated with SME (500 µg/ml) for 1 h, and then stimulated with LPS (10 µg/ml) for up to 4 h. Total RNA (1 µg) was extracted, reverse-transcribed, and amplified with an ABI Prism 7700 sequence detection system using specific rat ICAM-1 primers. Relative quantification was performed by comparison of threshold cycle values of samples with 18S ribosomal RNA. (b) IEC-18 cells were infected for 16 h with Ad5κB-LUC, and cells were coinfected for an additional 12 h with Ad5IκBαAA, Ad5dnIKKβ, and control Ad5GFP (m.o.i. of 50). Cells were stimulated with LPS (10 µg/ml) for 12 h in the presence or absence of various concentrations of Salvia miltiorrhizae extract (SME). Cell extracts were prepared, luciferase assays were performed, and results were normalized to extract protein concentrations. Data are expressed as fold changes over control determined as the mean of three independent experiments measured in triplicate (values are mean ± SEM). Veh, PBS vehicle.
Fig. 2.
Salvia miltiorrhizae extract (SME) does not induce cytotoxicity in IECs. IEC-18 cells were pretreated with SME (500 µg/ml) for 1 h, and then stimulated with LPS (10 µg/ml) for 24 h. Simultaneous two-colour fluorescence determination of live and dead cells was carried out with two probes that measured two recognized parameters of cell viability: calcein AM for intracellular esterase activity and ethidium homodimers for plasma membrane integrity. Cells were incubated with methanol (100%) for 30 min to generate a positive control for cell death. Fluorescence in cell samples was measured with appropriate excitation and emission filters using a spectrofluorometer. Results are representative of four independent experiments (values are mean ± SEM).
SME blocks LPS-induced IκBα phosphorylation/degradation and RelA nuclear translocation
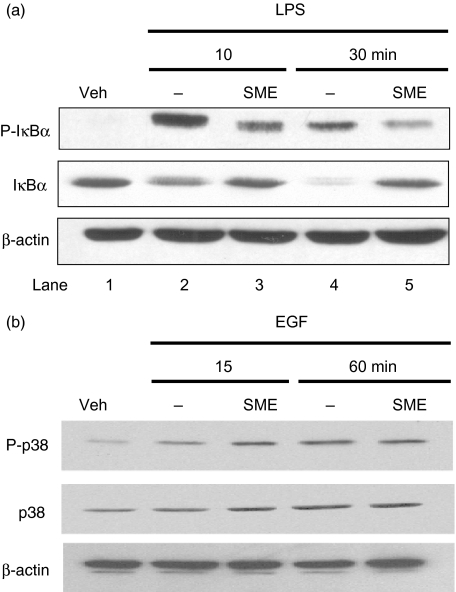
To dissect the effect of SME on LPS-induced NF-κB signal transduction, we determined the phosphorylation levels of various down-stream signalling proteins involved in NF-κB pathway using Western blot analysis. Figure 3a shows that LPS induced IκBα phosphorylation (first panel) and triggered IκBα degradation (second panel) in IEC-18 cells, which are blocked in SME-pretreated cells (compared lanes 3 and 5 with lanes 2 and 4). As opposed, EGF-induced p38 phosphorylation is not inhibited in SME-treated cells (Fig. 3b), suggesting that this botanical extract is not a pan inhibitor of kinase activity.
Fig. 3.
Salvia miltiorrhizae extract (SME) blocks LPS-induced IκBα phosphorylation/degradation. (a) IEC-18 cells were stimulated with LPS (10 µg/ml) for various times (0–30 min). Where indicated, IEC-18 cells were pretreated for 1 h with SME (500 µg/ml). Total protein was extracted, and 20 µg of protein was subjected to SDS-PAGE followed by phospho-IκBα, IκBα, and β-actin immunoblotting using the ECL technique. Results are representative of three independent experiments. Veh, PBS vehicle. (b) IEC-18 cells were stimulated with EGF (10 ng/ml) for various times (0–60 min). Where indicated, IEC-18 cells were pretreated for 1 h with SME (500 µg/ml). Total protein was extracted, and 20 µg of protein was subjected to SDS-PAGE followed by phospho-p38, p38, and β-actin immunoblotting using the ECL technique. Results are representative of three independent experiments. Veh, PBS vehicle.
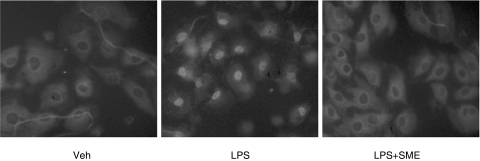
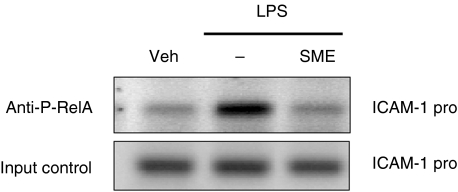
Accordingly, SME significantly suppressed LPS-induced RelA nuclear translocation in IEC-18 cells as determined by immunofluorescence staining (Fig. 4). To evaluate the functional impact of decreased nuclear RelA in SME-treated cells, we carried out chromatin immunoprecipitation (ChIP) analysis on the ICAM-1 gene promoter. IEC-18 cells were stimulated with LPS (10 µg/ml) in the presence or absence of SME (500 µg/ml), and RelA recruitment to the ICAM-1 promoter region determined by PCR. As seen in Fig. 5, LPS-induced RelA recruitment to the ICAM-1 gene promoter was inhibited in SME treated cells, whereas similar levels were amplified from the total pool of genomic DNA (input control). Taken together, SME blocks NF-κB signalling through inhibition of IκBα phosphorylation/degradation, RelA nuclear translocation and binding to the ICAM-1 gene promoter.
Fig. 4.
Salvia miltiorrhizae extract (SME) inhibits LPS-induced RelA nuclear translocation. IEC-18 cells were pretreated with SME (500 µg/ml) for 1 h, and then stimulated with LPS (10 µg/ml) for 30 min. RelA localization was visualized using an anti-RelA primary Ab followed by a rhodamine-conjugated detection Ab. This immunofluorescence is representative of two independent experiments. Veh, PBS vehicle.
Fig. 5.
Salvia miltiorrhizae extract (SME) blocks LPS-induced NF-κB recruitment to the ICAM-1 promoter in IECs. IEC-18 cells were stimulated with LPS for 30 min in the presence or absence of SME (500 µg/ml), and ChIP assay was performed using an antiphospho-RelA Ab as described under ‘Materials and methods’. PCR was performed using specific primers for the rat ICAM-1 promoter. Results are representative of two independent experiments. Veh, PBS vehicle.
SME directly inhibits LPS-induced IκB kinase activity in IECs
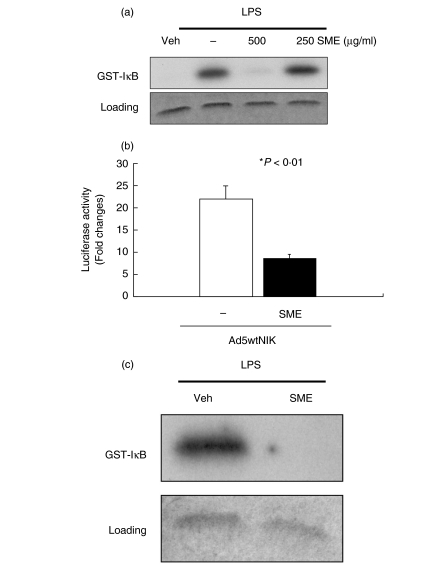
LPS-induced serine 32 and 36 IκBα phosphorylation is triggered by activation of the IKK complex [23]. To determine the impact of SME on IKK activity, we performed an in vitro kinase assay. IEC-18 cells were pretreated with various doses of SME (250 and 500 µg/ml), stimulated with LPS (10 µg/ml) for 20 min and IKKγ was immunoprecipitated, and then kinase activity was measured using a GST-IκB (1–54) substrate. As shown in Fig. 6a, SME (500 µg/ml) significantly inhibited LPS-induced IKK activity in IEC-18 cells. Since the MAP3K NF-κB-inducing kinase (NIK) participates in LPS-induced IKK activity [31], we investigated the effect of SME on NIK-induced NF-κB activation. In accordance with previous reports [30,32], adenoviral gene delivery of NIK (Ad5wtNIK) strongly induced (>20-fold) NF-κB transcriptional activity (Fig. 6b), which was significantly inhibited by SME treatment. Since NIK directly activates the IKK complex, this finding suggests that SME interferes with signalling events proximal to NIK-IKK. We next tested whether SME directly impact on IKK activity in IEC-18 cells by using a cell free kinase assay system. SME (500 µg/ml) was added to LPS-stimulated (20min) IKKγ immunoprecipitated beads and kinase activity was determined using a GST-IκB substrate. As shown in Fig. 6c, LPS-induced IKK activity is blocked by SME, suggesting that this extract directly blocks IKK activity in IEC-18 cells.
Fig. 6.
Salvia miltiorrhizae extract (SME) directly inhibits LPS-induced IκB kinase activity in IECs. (a) IEC-18 cells were pretreated with SME (500 µg/ml) for 1 h, and then stimulated with LPS (10 µg/ml) for 20 min. Whole cell extract was immunoprecipitated with anti-IKKγ/protein-A beads and the kinase reaction was performed using GST-IκB (1–54) as a substrate as described under ‘Materials and methods’. Substrate protein was resolved by gel electrophoresis, and phosphate incorporation was assessed by autoradiography and PhosphorImager analysis. Coomassie Blue staining shows equal loading (lower blot). (b) IEC-18 cells were coinfected with Ad5κB-LUC and Ad5wtNIK in the presence or absence of SME (500 µg/ml). Luciferase assay was measured, and results were normalized for extract protein concentrations. Values are mean ± SEM. (c) To evaluate whether SME inhibits IKK directly, immunoprecipitated IKK from LPS-stimulated (20min) IEC-18 cells was aliquoted into kinase reaction buffer in presence of various concentrations of SME or PBS vehicle. IKK kinase assay was performed as described above. These results are representative of at least two independent experiments. Veh, PBS vehicle.
SME inhibits LPS-induced NF-κB signalling in murine small intestinal explants and in primary IECs
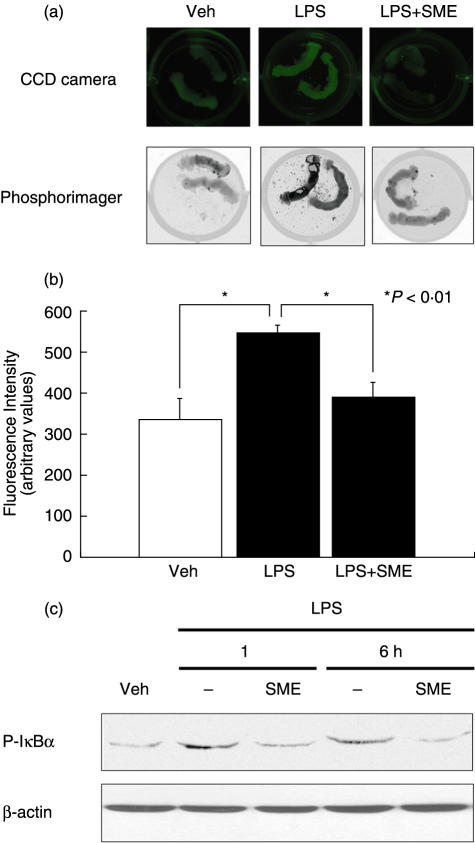
To verify the physiological relevance of SME-mediated inhibition of LPS-induced NF-κB signalling, we used small intestinal explants derived from a transgenic mouse expressing the EGFP under the transcriptional control of NF-κB cis-elements (cis-NF-κBEGFP). We have shown that this mouse displayed enhanced EGFP expression in numerous tissues following induction of NF-κB signalling [28]. The small intestine from cis-NF-κBEGFP was sectioned and pretreated with SME for 1 h, and then stimulated with LPS for 12 h. EGFP expression levels were determined using either a CCD specific EGFP imaging camera or a PhosphorImager. LPS induced EGFP expression level (64%) compared to unstimulated intestinal explant, and was inhibited by 74% in SME-treated explant (Fig. 7a,b).
Fig. 7.
Salvia miltiorrhizae extract (SME) inhibits LPS-induced NF-κB signalling in murine small intestinal explants and in primary intestinal epithelial cells. (a) Small intestinal segments derived from cis-NF-κBEGFP mice were pretreated for 1 h with SME (500 µg/ml) and then stimulated with LPS (10 µg/ml) for 12 h. The segments were imaged using either a CCD camera with a filtered light source and emission filters specific for EGFP or alternatively, scanned using a PhosphorImager with fluorescence settings. (b) Fluorescence intensity was quantified by densitometric analysis using ImageQuant software ver 2·2. (c) Small intestinal epithelial cells isolated from cis-NF-κBEGFP mice were pretreated for 1 h with SME (500 µg/ml) and then stimulated with LPS (10 µg/ml) for various times. Cell extracts were obtained and IκB phosphorylation was analysed by Western blotting. Results are representative of at least two independent experiments (values are mean ± SEM).
To determine whether SME blocks LPS-induced NF-κB signalling in primary IECs, we isolated IECs from the small intestine of cis-NF-κBEGFP mice. SME blocked LPS-induced IκBα phosphorylation in primary IECs in a manner similar to that observed in IEC-18 cells (Fig. 7c). Altogether, these finding indicate that SME inhibits LPS-induced NF-κB signalling pathway through blockade of IKK activity.
Salvianolic acid B does not inhibit LPS-induced NF-κB transcriptional activity and IκBα phosphorylation/degradation in IECs
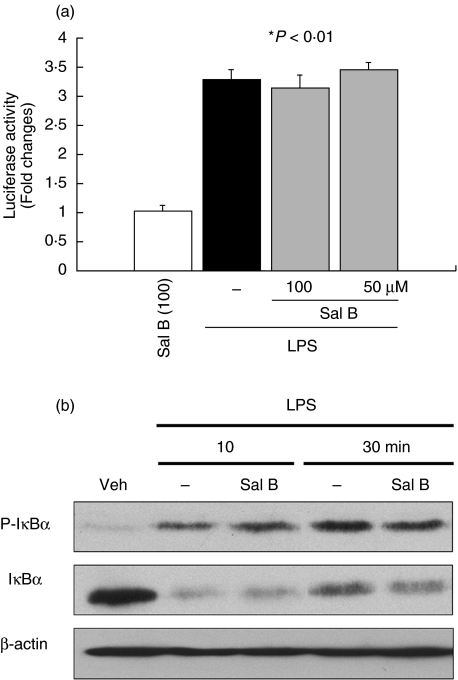
Salvianolic acid B, a phenolic constituent of SME, has been showed to inhibit TNF-α induced ICAM-1 gene expression in endothelial cells [4]. Thus, we next investigated whether this compound could mediate SME inhibitory effect. As seen in Fig. 8a, LPS-induced NF-κB transcriptional activity is not blocked in salvianolic acid B-treated IEC-18 cells. Moreover, LPS-induced IκBα phosphorylation (first panel) and IκBα degradation (second panel) are not inhibited in salvianolic acid B-treated IEC-18 cells (Fig. 8b) This indicates that SME-mediated blockade of LPS-induced NF-kB activity is independent of salvianolic acid B.
Fig. 8.
Salvianolic acid B (Sal B) does not inhibit LPS-induced NF-κB transcriptional activity and IκBα phosphorylation/degradation in IEC-18 cells. (a) IEC-18 cells were infected for 16 h with Ad5κB-LUC, and cells were stimulated with LPS (10 µg/ml) for 12 h in the presence or absence of various concentrations of Sal B. Cell extracts were prepared, luciferase assays were performed, and results were normalized to extract protein concentrations. (b) IEC-18 cells were stimulated with LPS (10 µg/ml) for various times (0–30 min) pretreated with Sal B (100 µM). Total protein was extracted, and 20 µg of protein was subjected to SDS-PAGE followed by phospho-IκBα, IκBα, and β-actin immunoblotting using the ECL technique. Results are representative of two independent experiments. Veh, PBS vehicle.
Discussion
In this study, we investigated the effect of the SME on LPS-induced NF-κB signalling in IECs. We showed that SME strongly inhibited LPS-induced NF-κB transcriptional activity and ICAM-1 gene expression. This effect was mediated through direct inhibition of IKK activity, causing a strong reduction of LPS-induced IκBα phosphorylation/degradation and RelA nuclear translocation. This was accompanied by reduced RelA recruitment to the ICAM-1 gene promoter in LPS-stimulated cells. To investigate the physiological relevance of SME-mediated inhibition of NF-κB activity, we used the cis-NF-κBEGFP transgenic mice recently engineered in our laboratory [28]. This transgenic mouse allows the dynamic assessment of NF-κB activity through measurement of EGFP expression levels using specialized fluorescence-detecting camera. Using this approach, we demonstrate for the first time that SME blocks LPS-induced EGFP expression levels, thus NF-κB activation in small intestine explants. Therefore, SME blocks LPS-induced NF-κB activity both in cell lines and in primary intestinal epithelial cells.
Enhanced EFGP expression in LPS-stimulated intestinal explants is not a direct proof that intestinal epithelial cells are responsible for this effect. Interestingly, we have shown that intraperitoneal injection of LPS in cis-NF-κBEGFP transgenic mice lead to increase EGFP accumulation mainly in the duodenum [28]. Using immunohistochemistry and confocal analysis, we have identified the EGFP positive cells as being lamina propria mononuclear cells (monocytes and lymphocytes) and IEC, although the latter represent a weak fraction of the total EGFP positive cells. Similarly, we showed here that primary cells isolated from cis-NF-κBEGFP transgenic mice displayed enhanced IκB phosphorylation in response to LPS. SME inhibited LPS-induced IκB phosphorylation suggesting that decreased EGFP expression levels in small intestinal explants are partially due to decreased NF-κB signalling in IEC. Further work will be necessary to identify the cells targeted by SME in vivo.
For centuries, natural plant extracts have been used as remedies to treat numerous medical conditions in Eastern countries. Although incorporation of natural plant extracts as alternative medicine has been slow in the United States, the past decade has seen a rapid user increase among the population [33]. Indeed, a survey indicated that herbal medicine users have increased by more than 400% in the United States between 1990 and 1997 [34], and 16% of prescription drug users have taken natural products/supplements [35]. Despite this tremendous grow of herbal medicine users, lack of empirical data showing efficacy, safety and mechanism of action preclude their incorporation in mainstream medicine. In this study, we demonstrate the efficacy and nontoxic inhibitory effects of the water-soluble Salvia miltiorrhizae extract on LPS-induced NF-κB signalling pathway in both IEC and in an intestinal explant culture system. The water-soluble extract of Salvia miltiorrhizae has been used clinically in China to alleviate certain diseases including myocarditis and myocardial infarction, cerebral vascular diseases, and chronic hepatitis [23,36–38]. Since our data demonstrate that water-soluble extract of Salvia miltiorrhizae blocks innate signalling to NF-κB, this suggests that this extract may be beneficial in treating inflammatory condition involving dysregulated innate/adaptive immune response. Moreover, considering that dysregulated NF-κB activation associates with the development of IBD, SME may represent an attractive approach for the treatment/prevention of intestinal inflammation. Further investigations are needed to document the safety and efficacy of SME on treating colitis in experimental models of intestinal inflammation.
In this study, we showed that the water-soluble fraction of Salvia miltiorrhizae blocks LPS-induced IKK activity in IEC. The hydrophilic fraction of Salvia miltiorrhizae contains several natural phenolic compounds including salvianolic acid A, salvianolic acid B, lithospermate B, rosmarinic acid, protocatechualdehyde, protocatechuic acid, caffeic acid and danshensu [25]. TNF-α-induced VCAM and ICAM-1 expression is inhibited in salvianolic acid B-treated human aortic endothelial cells [27], which correlated with decrease expression of the p65 NF-κB subunit. Interestingly, we found that salvianolic acid B did not inhibit LPS-induced NF-κB transcriptional activity and IκBα phosphorylation/degradation in IEC-18 cells. Thus, the active component responsible for SME-mediated inhibition in IEC remains to be identified. Further investigation will be necessary to identify a specific compound mediating IKK inhibition in water-soluble fraction of Salvia miltiorrhizae. However, it should be stressed that the biological activity of a natural plant extract may be strikingly different from its isolated purified constituent. Indeed, the complex mixture of compounds present in numerous natural plant extracts are likely responsible for their various biological activities, including additive and synergistic effects [39].
Cytokine and bacterial product signalling converge on the IKK complex to trigger IκB phosphorylation and ultimately NF-κB activity in numerous cell systems. Using adenoviral gene delivery, we found that SME blocked NIK-induced NF-κB transcriptional activity in IEC-18 cells, suggesting that this extract mediates its suppressive effects downstream of NIK. Since SME strongly blocked LPS-induced IKK activity in IEC-18, this suggests that this extract mainly mediates its effects through this kinase complex. This is in line with the inhibitory effect of SME on IL-1β and TNF-α-induced ICAM-1 gene expression (Kim and Jobin, unpublished data), which all utilized IKK as a common signal transducer to induce NF-κB activity. Thus, the anti-inflammatory activities of some natural plant extracts and pure constituents may be mediated through IKK inhibition [40,41].
In conclusion, we show that SME abrogates LPS-induced NF-κB signalling and proinflammatory gene expression in intestinal epithelial cells through the direct inhibition of IKK activity. Modulation of bacterial product-mediated NF-κB signalling by natural plant extracts may represent an attractive strategy towards the prevention and treatment of intestinal inflammation.
Acknowledgments
The authors wish to thank Brigitte Allard for technical expertise. This work was supported by NIH ROI grants DK 47700 and the Broad Medical Foundation to C. Jobin and by NIH P30 DK34987 for the CGIBD.
References
- 1.Haller D, Jobin C. Interaction between resident luminal bacteria and the host: Can a healthy relationship turn sour? J Pediatr Gastroenterol Nutr. 2004;38:123–36. doi: 10.1097/00005176-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Rolfe RD. The role of probiotic cultures in the control of gastrointestinal health. J Nutr. 2000;130(Suppl.):396S–402S. doi: 10.1093/jn/130.2.396S. [DOI] [PubMed] [Google Scholar]
- 3.Kraehenbuhl JP, Corbett M. Immunology. Keeping the gut microflora at bay. Science. 2004;303:1624–5. doi: 10.1126/science.1096222. [DOI] [PubMed] [Google Scholar]
- 4.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–72. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 5.Cetin S, Dunklebarger J, Li J, et al. Endotoxin differentially modulates the basolateral and apical sodium/proton exchangers (NHE) in enterocytes. Surgery. 2004;136:375–83. doi: 10.1016/j.surg.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Haller D, Holt L, Parlesak A, Zanga J, Bauerlein A, Sartor RB, Jobin C. Differential effect of immune cells on non-pathogenic Gram-negative bacteria-induced nuclear factor-κB activation and pro-inflammatory gene expression in intestinal epithelial cells. Immunology. 2004;112:310–20. doi: 10.1111/j.1365-2567.2004.01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YS, Kim JS, Jung HC, Song IS. The effects of thalidomide on the stimulation of NF-κB activity and TNF-α production by lipopolysaccharide in a human colonic epithelial cell line. Mol Cells. 2004;17:210–6. [PubMed] [Google Scholar]
- 8.Kojima K, Musch MW, Ropeleski MJ, Boone DL, Ma A, Chang EB. Escherichia coli LPS induces heat shock protein 25 in intestinal epithelial cells through MAP kinase activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G645–52. doi: 10.1152/ajpgi.00080.2003. [DOI] [PubMed] [Google Scholar]
- 9.Vora P, Youdim A, Thomas LS, et al. β-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol. 2004;173:5398–405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- 10.Haller D, Holt L, Kim SC, Schwabe RF, Sartor RB, Jobin C. Transforming growth factor-β 1 inhibits non-pathogenic Gram negative bacteria-induced NF-κB recruitment to the interleukin-6 gene promoter in intestinal epithelial cells through modulation of histone acetylation. J Biol Chem. 2003;278:23851–60. doi: 10.1074/jbc.M300075200. [DOI] [PubMed] [Google Scholar]
- 11.Haller D, Russo MP, Sartor RB, Jobin C. IKKβ and phosphatidylinositol 3-Kinase/Akt participate in non-pathogenic Gram-negative enteric bacteria-induced RelA phosphorylation and NF-κB activation in both primary and intestinal epithelial cell lines. J Biol Chem. 2002;277:38168–78. doi: 10.1074/jbc.M205737200. [DOI] [PubMed] [Google Scholar]
- 12.Karin M. The beginning of the end. IκB kinase (IKK) and NF-κB activation. J Biol Chem. 1999;274:27339–42. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 14.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–33. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 15.Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–98. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 16.Jobin C, Sartor RB. The IκB/NF-κB system. a key determinant of mucosal inflammation and protection. Am J Physiol Cell Physiol. 2000;278:C451–62. doi: 10.1152/ajpcell.2000.278.3.C451. [DOI] [PubMed] [Google Scholar]
- 17.Jobin C, Sartor RB. NF-κB signaling proteins as therapeutic targets for inflammatory bowel diseases. Inflamm Bowel Dis. 2000;6:206–13. doi: 10.1097/00054725-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Hou J, Baichwal V, Cao Z. Regulatory elements and transcription factors controlling basal and cytokine-induced expression of the gene encoding intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1994;91:11641–5. doi: 10.1073/pnas.91.24.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura S, Ohtani H, Watanabe Y, Fukushima K, Matsumoto T, Kitano A, Kobayashi K, Nagura H. In situ expression of the cell adhesion molecules in inflammatory bowel disease. Evidence of immunologic activation of vascular endothelial cells. Laboratory Invest. 1993;69:77–85. [PubMed] [Google Scholar]
- 20.Fiocchi C. Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am J Physiol Gastrointest Liver Physiol. 1997;273:G769–75. doi: 10.1152/ajpgi.1997.273.4.G769. [DOI] [PubMed] [Google Scholar]
- 21.van Deventer SJ, Tami JA, Wedel MK. A randomised, controlled, double blind, escalating dose study of alicaforsen enema in active ulcerative colitis. Gut. 2004;53:1646–51. doi: 10.1136/gut.2003.036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miner P, Wedel M, Bane B, Bradley J. An enema formulation of alicaforsen, an antisense inhibitor of intercellular adhesion molecule-1, in the treatment of chronic, unremitting pouchitis. Aliment Pharmacol Ther. 2004;19:281–6. doi: 10.1111/j.1365-2036.2004.01863.x. [DOI] [PubMed] [Google Scholar]
- 23.Yagi A, Fujimoto K, Tanonaka K, Hirai K, Takeo S. Possible active components of tan-shen (Salvia miltiorrhiza) for protection of the myocardium against ischemia-induced derangements. Planta Med. 1989;55:51–4. doi: 10.1055/s-2006-961824. [DOI] [PubMed] [Google Scholar]
- 24.Fung KP, Zeng LH, Wu J, Wong HN, Lee CM, Hon PM, Chang HM, Wu TW. Demonstration of the myocardial salvage effect of lithospermic acid B isolated from the aqueous extract of Salvia miltiorrhiza. Life Sci. 1993;52:239–44. doi: 10.1016/0024-3205(93)90471-e. [DOI] [PubMed] [Google Scholar]
- 25.Liu GT, Zhang TM, Wang BE, Wang YW. Protective action of seven natural phenolic compounds against peroxidative damage to biomembranes. Biochem Pharmacol. 1992;43:147–52. doi: 10.1016/0006-2952(92)90271-j. [DOI] [PubMed] [Google Scholar]
- 26.Ren de CGH, Zhang JT. Inhibitory effect of the water-soluble extract of Salvia miltiorrhiza on neutrophil-endothelial adhesion. Jpn J Pharmacol. 2002;90:276–80. doi: 10.1254/jjp.90.276. [DOI] [PubMed] [Google Scholar]
- 27.Chen YH, Lin SJ, Ku HH, Shiao MS, Lin FY, Chen JW, Chen YL. Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-α-treated human aortic endothelial cells. J Cell Biochem. 2001;82:512–21. doi: 10.1002/jcb.1176. [DOI] [PubMed] [Google Scholar]
- 28.Magness ST, Jijon H, Van Houten N, Sharpless NE, Brenner DA, Jobin C. In vivo pattern of lipopolysaccharide and anti-CD-3-induced NF-κB activation using a novel gene targeted enhanced GFP reporter gene mouse. J Immunol. 2004;173:1561–70. doi: 10.4049/jimmunol.173.3.1561. [DOI] [PubMed] [Google Scholar]
- 29.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor IκB kinase activity. J Immunol. 1999;163:3474–83. [PubMed] [Google Scholar]
- 30.Russo MP, Schwabe RF, Sartor RB, Jobin C. NF-κB-inducing kinase restores defective IκB kinase activity and NF-κB signaling in intestinal epithelial cells. Cell Signal. 2004;16:741–50. doi: 10.1016/j.cellsig.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Hatada EN, Krappmann D, Scheidereit C. NF-κB and the innate immune response. Curr Opin Immunol. 2000;12:52–8. doi: 10.1016/s0952-7915(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 32.Jijon H, Allard B, Jobin C. NF-κB inducing kinase activates NF-κB transcriptional activity independently of IκB kinase γ through a p38 MAPK-dependent RelA phosphorylation pathway. Cell Signal. 2004;16:1023–32. doi: 10.1016/j.cellsig.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Bent S, Ko R. Commonly used herbal medicines in the United States: a review. Am J Med. 2004;116:478–85. doi: 10.1016/j.amjmed.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 34.De Smet PA. Herbal remedies. N Engl J Med. 2002;347:2046–56. doi: 10.1056/NEJMra020398. [DOI] [PubMed] [Google Scholar]
- 35.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. J Am Med Assoc. 2002;287:337–44. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 36.Tang MK, Ren DC, Du Zhang JTG. H. Effect of salvianolic acids from Radix Salviae miltiorrhizae on regional cerebral blood flow and platelet aggregation in rats. Phytomedicine. 2002;9:405–9. doi: 10.1078/09447110260571634. [DOI] [PubMed] [Google Scholar]
- 37.Zhao BL, Jiang W, Zhao Y, Hou JW, Xin WJ. Scavenging effects of salvia miltiorrhiza on free radicals and its protection for myocardial mitochondrial membranes from ischemia-reperfusion injury. Biochem Mol Biol Int. 1996;38:1171–82. [PubMed] [Google Scholar]
- 38.Wasser S, Ho JM, Ang HK, Tan CE. Salvia miltiorrhiza reduces experimentally-induced hepatic fibrosis in rats. J Hepatol. 1998;29:760–71. doi: 10.1016/s0168-8278(98)80257-2. [DOI] [PubMed] [Google Scholar]
- 39.Liu RH. Protective role of phytochemicals in whole foods: implications for chronic disease prevention. Appl Biotechnol Food Sci Policy. 2003;1:39–46. [Google Scholar]
- 40.Bremner P, Heinrich M. Natural products as targeted modulators of the nuclear factor-κB pathway. J Pharm Pharmacol. 2002;54:453–72. doi: 10.1211/0022357021778637. [DOI] [PubMed] [Google Scholar]
- 41.Karin M, Yamamoto Y, Wang QM. The IKK NF-κB system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]