Abstract
Eotaxin-2/CCL24 and eotaxin-3/CCL26 are CC chemokines and their receptor, CC chemokine receptor 3 is preferentially expressed on eosinophils. It was reported that vascular endothelial cells and dermal fibroblasts produced CCL26. However, the regulation of CCL24 and CCL26 production in keratinocytes has not been well documented. We investigated the expression and production of CCL24 and CCL26 in the human keratinocyte cell line, HaCaT cells. Reverse transcription and polymerase chain reaction was performed using these cells and Enzyme-linked immunosorbent assay was carried out using supernatant of these cells. The production of CCL24 in HaCaT cells was slightly enhanced by IL-4 and that of CCL26 was strongly enhanced by IL-4 and IL-13. Furthermore, TNF-α generated a synergistic effect on IL-4 enhanced CCL26 production. Dexamethasone, IFN-γ and the p38 mitogen-activated protein kinase inhibitor SB202190 inhibited IL-4 enhanced CCL26 production. IL-4 enhanced production of CCL26 was inhibited by leflunomide and JAK inhibitor 1, but not by JAK3 inhibitor, which indicates that it is mediated by JAK1-STAT6-dependent pathway. This result also strongly suggests the involvement of the type 2 IL-4 receptor in IL-4 enhanced production of CCL26. These results suggest that keratinocytes are involved in the migration of CC chemokine receptor 3 positive cells such as eosinophils in a Th2-dominant situation like atopic dermatitis.
Keywords: interleukin-4 receptor, Janus-activated kinase 1, p38 mitogen-activated protein kinase, signal transducer and activator of transcription 6
Introduction
Chemokines are small secreted proteins that mainly regulate leucocyte trafficking [1]. Based on the position of the first two of cysteine residues, chemokines are classified into four subfamilies: CXC, CC, C, and CX3C. Keratinocytes form the outer component of skin and are known to play an important role to skin inflammation [2]. They synthesize many chemokines including members of the CC and CXC subfamilies, such as regulated on activation, normal T cell expressed and secreted (RANTES)/CCL5 [3], eotaxin/CCL11 [4], thymus and activation-regulated chemokine (TARC)/CCL17 [5], macrophage-derived chemokine (MDC)/CCL22 [6], gamma-interferon-inducible protein-10 (IP-10)/CXCL10 and monokine induced by gamma-interferon (MIG)/CXCL9 [7].
Both eotaxin-2/CCL24 and eotaxin-3/CCL26 are CC chemokines and functional ligands for CC chemokine receptor 3 (CCR3), which is preferentially expressed on eosinophils. After identification of CCL11 as a relatively selective chemokine for eosinophils [8], CCL24 was cloned and showed chemotactic activity for eosinophils in a similar concentration range as CCL11 [9]. More recently a new member of the eotaxins, CCL26 has been cloned [10]. Only 34% of the amino acids in CCL24 and CCL26 are identical, but these two chemokines have a common characteristic feature in terms of chemotaxis for eosinophils via the CCR3 [9,10]. We were interested in how these eotaxins are regulated by Th1 and Th2 cytokines.
High levels of CCL11 and CCL24 mRNA were found in bronchial samples obtained from patients with bronchial asthma and CCL26 mRNA expression was up-regulated in these patients 24 h after allergen challenge [11]. Moreover, endothelial cells and dermal fibroblasts produced CCL26 after stimulation with interleukin (IL)-4 and IL-13 [10,12]. We reported that serum levels of CCL26, but not CCL24, were significantly elevated in patients with atopic dermatitis [13]. These results suggest the important role of eotaxins for eosinophil infiltration in a Th2-polarized condition. Although keratinocytes have a pivotal role for immune responses in inflammatory skin diseases, little is known about the production of CCL24 and CCL26 in human keratinocytes. To clarify this, we investigated the regulation of CCL24 and CCL26 production in a human keratinocyte cell line, HaCaT cells after stimulation with various cytokines.
Materials and methods
Samples and reagents
CCL24 and CCL26 immunoassay kits, recombinant IL-4, recombinant IL-13 and antihuman IL-4 antibody (AF-204-NA) were purchased from R & D Systems (Minneapolis, MN, USA). Recombinant interferon (IFN)-γ and recombinant tumour necrosis factor (TNF)-α were purchased from PeproTech (RockyHill, NJ, USA). Dexamethasone was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Janus-activated kinase (JAK) inhibitor 1 which inhibits JAK1, JAK2, JAK3 and Tyk2, JAK3 inhibitor 1, SB202190 (p38 mitogen-activated protein (MAP) kinase inhibitor), the c-Jun N-terminal kinase (JNK) inhibitor 2, PD153035 (epidermal growth factor receptor (EGFR) inhibitor), parthenolide (nuclear factor (NF)-κB inhibitor) and LY294002 (phosphatidyl inositol 3 (PI3)-kinase inhibitor) were purchased from Calbiochem (Darmstadt, Germany). PD98059 (extracellular signal-regulated kinase (ERK) inhibitor) was purchased from Alexis Biochemicals (Lausen, Switzerland). Leflunomide, an inhibitor of JAK3 and signal transducer and activation of transcription (STAT) 6, was obtained from Sigma-Aldrich (St. Louis, MO, USA). HaCaT cells were kindly provided by Dr Toshio Kuroki (Institute of Molecular Oncology, Showa University, Japan) with permission from Dr N. Fusenig (Institute Fur Zell- und Tumourbiologie. Deutshes Kresforschungeszentrum, Heidelberg, Germany).
Cell culture
HaCaT cells were cultured in 150 cm2 cell culture flasks (Corning, Corning, NY, USA) at 37°C, 5% CO2 in minimum essential medium eagle (Sigma, St. Louis, MO, USA) containing 10% fetal bovine serum, penicillin G sodium, streptomycin sulphate and amphotericin B. The cells received fresh medium every 3 days and were subcultured every 10 days. Fortieth- to seventieth-passage cells were used in all experiments. When confluence was achieved, the cells were trypsinized, washed and resuspended in minimum essential medium eagle with 10% fetal bovine serum at 1 × 106 cells/ml, and 1 ml was added to each well of the six-well plates (Becton Dickinson, Franklin Lakes, NJ, USA). When the cells reached confluence, the medium was replaced with 1 ml minimum essential medium eagle without fetal bovine serum. Stimuli were added simultaneously. When we used antihuman IL-4 antibody, we added IL-4 after 3 h. Then, the cells were incubated at 37°C and 5% CO2. After 24 h, supernatants were collected, centrifuged to remove cell debris and stored at −20°C until enzyme-linked immunosorbent assay was performed. Cells were trypsinized, pelleted, and stored at −80°C until reverse transcription and polymerase chain reaction was carried out.
Reverse transcription and polymerase chain reaction
Total RNA was isolated using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. DNAse I was used to avoid genomic DNA contamination. Reverse transcription and polymerase chain reaction (RT-PCR) was performed using Superscript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA, USA). Primer pairs specific for the different components were: 5′-AAC TCC GAA ACA ATT GTG ACT CAG CTG (sense) and 5′-GTA ACT CTG GGA GGA AAC ACC CTC TCC (antisense) for CCL26, 5′-GCG TCT CCG ACT ACA TGA GC (sense) and 5′-GGT TGC TCC AGG TCA GCA GC (antisense) for the 140 kDa IL-4 binding protein (IL-4Rα), 5′-AGG ATG ACA AAC TCT GGA G (sense) and 5′-CTC AAG GTC ACA GTG AAG G (antisense) for IL-13 receptor α1(IL-13Rα1), 5′-CAG CCT ACC AAC CTC ACT CT (sense) and 5′-GTC CTG GAG CTG AAC AAC AA (antisense) for IL-2 receptor γ chain (IL-2Rγ), 5′-TGA AGG TCG GAG TCA ACG GAT TTG GT (sense) and 5′-CAT GTG GGC CAT GAG GTC CAC CAC (antisense) for human G3PDH. The mixture was predenatured for 5 min at 94°C and then subjected to 35 cycles of 94°C 1 min, 62°C (CCL26) or 58°C (the other components) 1 min, 72°C 1 min, then 72°C 7 min. Amplified DNA fragments were judged from 2% agarose gel electrophoresis. The expected fragment length was 152 bp for CCL26, 336 bp for IL-4Rα, 358 bp for IL-13Rα1, 156 bp for IL-2Rγ and 283 bp for human G3PDH, respectively. One representative experiment of three is shown.
Enzyme-linked immunosorbent assay
The supernatant levels of CCL24 and CCL26 were measured according to the manufacturer's instructions. Each culture condition was tested in triplicate and representative data from three experiments are shown. Optical densities were measured at 450 nm with a Bio-Rad Model 550 microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA). The concentrations were calculated from the standard curve generated by a curve-fitting program. The minimum detectable dose of CCL24 and CCL26 was 1·83 pg/ml and 2·33 pg/ml, respectively.
Statistical analyses
Data were analysed using Mann—Whitney's U-test. A P-value less than 0·05 was considered to be statistically significant.
Results
CCL26 production in HaCaT cells was not enhanced by TNF-α or IFN-γ, but strongly enhanced by IL-4 and IL-13
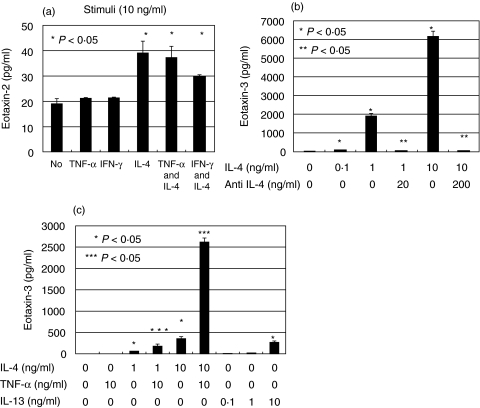
We stimulated HaCaT cells with proinflammatory cytokines such as TNF-α, IFN-γ and IL-4. Enzyme-linked immunosorbent assay (ELISA) showed that when HaCaT cells were cultured without cytokine, the supernatant CCL24 level was 19·1 ± 2·0 pg/ml (mean ± standard error). When cultured with IL-4 (10 ng/ml), it was slightly increased to 39·2 ± 4·6 pg/ml (P < 0·05). Culture with TNF-α (10 ng/ml) or IFN-γ (10 ng/ml), however, did not result in a change in CCL24 level (21·2 ± 0·3 pg/ml and 21·5 ± 0·3 pg/ml, respectively). Neither TNF-α nor IFN-γ had a synergistic effect on IL-4 enhanced CCL24 production (Fig. 1a).
Fig. 1.
CCL24 and CCL26 production in HaCaT cells were determined by ELISA. (a) CCL24 production was determined when they were stimulated by various stimuli. (b) CCL26 production was determined when they were stimulated by IL-4 and antihuman IL-4 antibody. (c) CCL26 production was determined when they were stimulated by TNF-α, IL-4 and IL-13. The error bars indicate standard error. ‘No’ means no stimulation. *P < 0·05 for the stimulus effect; **P < 0·05 for the inhibitory effect. ***P < 0·05 for the synergistic effect of TNF-α and IL-4.
ELISA for CCL26 concentration showed that when HaCaT cells were cultured without cytokine, the supernatant CCL26 level was 37·0 ± 8·8 pg/ml. When cultured with IL-4 (0·1, 1 and 10 ng/ml), it was increased in a dose-dependent manner (78·6 ± 0·5, 1919·2 ± 99·3, 6165·7 ± 288·0 pg/ml, respectively, P < 0·05). When cultured with IL-4 (1, 10 ng/ml) and antihuman IL-4 antibody (20, 200 ng/ml), it was decreased to 36·1 ± 2·4, 51·4 ± 6·6, respectively (Fig. 1b). Anti-human IL-4 antibody significantly neutralized the bioactivity of IL-4 (P < 0·05). IL-13 also enhanced CCL26 production dose dependently (P < 0·05), although it was less potent than IL-4 (Fig. 1c). When stimulated by IFN-γ (10 ng/ml), it was decreased to 20·8 ± 1·8 pg/ml (P < 0·05) (data not shown). Culture with TNF-α (10 ng/ml) did not result in a change in CCL26 level (40·4 ± 3·2 pg/ml). However, TNF-α and IL-4 synergistically up-regulated CCL26 production (Fig. 1c). Primary human keratinocytes were also examined, but they did not produce CCL24 or CCL26 even if stimulated by IL-4 and TNF-α (data not shown).
CCL26 mRNA in HaCaT cells was not up-regulated by TNF-α or IFN-γ, but strongly up-regulated by IL-4 and IL-13
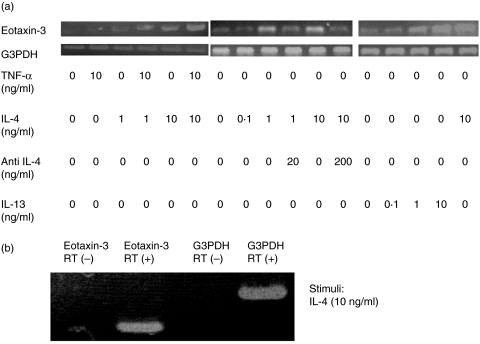
RT-PCR revealed that CCL26 mRNA was weakly expressed in HaCaT cells cultured without cytokines. Culture with TNF-α (10 ng/ml) did not result in a change in CCL26 mRNA level. However, when cells were cultured with IL-4, the level was strongly up-regulated. TNF-α and IL-4 synergistically up-regulated CCL26 expression (Fig. 2a). Culture with various concentrations of IL-4 (0·1 ng/ml, 1 ng/ml, 10 ng/ml) up-regulated CCL26 expression in a dose-dependent manner and antihuman IL-4 antibody weakened this up-regulation (Fig. 2a). IL-13 also enhanced expression of CCL26 mRNA dose dependently (Fig. 2a). When we used reaction products from an RT reaction in which the reverse transcriptase enzyme was omitted, neither CCL26 mRNA nor G3PDH mRNA was detected (Fig. 2b). Therefore the results in Fig. 2a were not due to genomic DNA contamination. These results were parallel to those of ELISA, which indicate that CCL26 production by these cytokines is regulated at the RNA level.
Fig. 2.
(a) CCL26 mRNA expression in HaCaT cells was determined by RT-PCR when they were stimulated with TNF-α, IL-4, IL-13 and antihuman IL-4 antibody. These results were parallel to those of ELISA. (b) CCL26 mRNA and G3PDH mRNA expression in IL-4-stimulated HaCaT cells were determined when the reverse transcriptase enzyme was omitted. RT means reverse transcriptase.
Dexamethasone, IFN-γ and SB202190 inhibited IL-4 enhanced CCL26 production in HaCaT cells
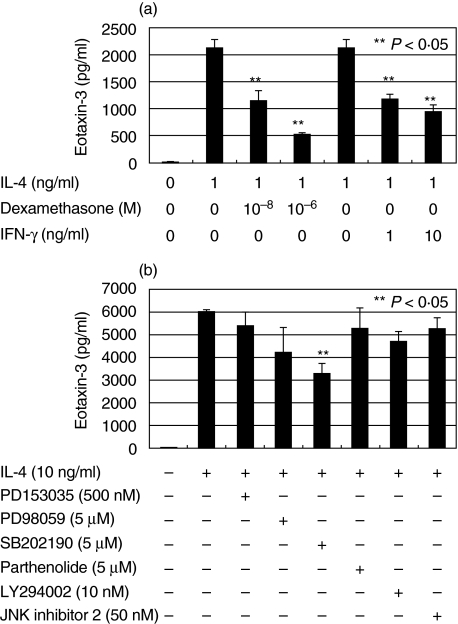
We examined the effects of dexamethasone (1 × 10−8 and 1 × 10−6 M) or IFN-γ (1 and 10 ng/ml) on the IL-4-enhanced production of CCL26. Dexamethasone and IFN-γ significantly and dose-dependently inhibited IL-4 enhanced CCL26 production (P < 0·05) (Fig. 3a).
Fig. 3.
CCL26 production in HaCaT cells was determined by ELISA. (a) Dexamethasone and IFN-γ significantly inhibited IL-4-enhanced CCL26 production. (b) SB202190, but not other reagents, significantly inhibited the IL-4 enhanced CCL26 production. These inhibitors, at the indicated concentrations, were effective in suppressing other cytokines in HaCaT cells. The error bars indicate standard error. **P < 0·05 for the inhibitory effect.
We also tested inhibitors of signalling molecules; PD153035 500 nM for EGFR, PD98059 5 µM for ERK, SB202190 5 µM for p38 MAP kinase, JNK inhibitor 2 50 nM, Parthenolide 5 µM for NF-κB, LY294002 10 nM for PI3-kinase. These inhibitors, at the indicated concentrations, are effective in suppressing other cytokines in HaCaT cells (data not shown). Only the p38 MAP kinase inhibitor, SB202190, inhibited IL-4-enhanced production of CCL26 by HaCaT cells by almost 50% (P < 0·05) (Fig. 3b), which indicates that IL-4-enhanced CCL26 production in these cells is partially dependent on p38 MAP kinase but not on ERK, JNK, NF-κB, EGFR or PI3-kinase.
IL-4-enhanced CCL26 production in HaCaT cells was inhibited by leflunomide and JAK inhibitor 1 dose-dependently, but not by JAK3 inhibitor 1
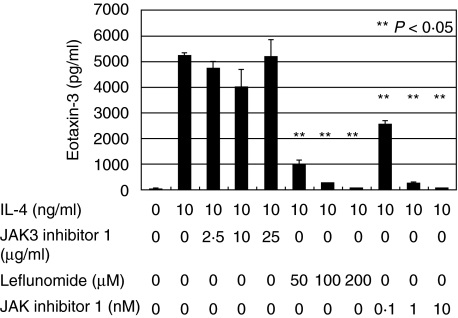
IL-4 binds to the type 1 and type 2 IL-4 receptors, and IL-13 binds to the type 2 IL-4 receptor. The type 1 IL-4 receptor contains IL-4Rα and IL-2Rγ, whereas the type 2 IL-4 receptor contains IL-4Rα and IL-13Rα1. JAK1, JAK2 and signal transducer and activator of transcription (STAT) 6 bind to IL-4Rα. JAK3 binds to IL-2Rγ, and Tyk2 binds to IL-13Rα1[14]. In order to examine which of these receptor components are involved in CCL26 mRNA expression in HaCaT cells, we tried JAK3 inhibitor 1, JAK inhibitor 1 (an inhibitor of JAK1, JAK2, JAK3 and Tyk2) and leflunomide (an inhibitor of JAK3 and STAT6) [15] on IL-4-enhanced CCL26 production. ELISA disclosed that when the cells were cultured with IL-4 (10 ng/ml) and JAK3 inhibitor 1 (2·5 µg/ml, 10 µg/ml, 25 µg/ml), JAK3 inhibitor 1 did not down-regulate the levels of supernatant CCL26. When cultured with IL-4 (10 ng/ml) and various concentrations of leflunomide (50 µM, 100 µM, 200 µM) or JAK inhibitor 1 (0·1 nM, 1 nM, 10 nM), it was decreased in a dose dependent manner (985·1 ± 175·0, 238·2 ± 15·7, 79·1 ± 7·4, 2562·4 ± 116·2, 250·1 ± 24·9, 63·5 ± 0·4 pg/ml, respectively, P < 0·05) (Fig. 4). These results indicate that IL-4-enhanced production of CCL26 in HaCaT cells was mediated by JAK1-STAT6 dependent pathway, and suggest the predominant utilization of type 2 IL-4 receptor over type 1 IL-4 receptor.
Fig. 4.
CCL26 production in HaCaT cells was determined by ELISA when they were stimulated with IL-4, leflunomide, JAK inhibitor 1 or JAK3 inhibitor 1. Leflunomide and JAK inhibitor 1, but not JAK3 inhibitor 1, significantly inhibited the IL-4-enhanced CCL26 production from HaCaT cells. The error bars indicate standard error. **P < 0·05 for the inhibitory effect.
Leflunomide and JAK inhibitor 1, but not JAK3 inhibitor 1, inhibited IL-4 enhanced CCL26 mRNA expression in HaCaT cells
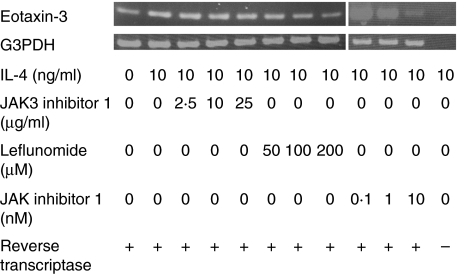
RT-PCR demonstrated that when the cells were cultured with IL-4 (10 ng/ml) and JAK3 inhibitor 1 (2·5 µg/ml, 10 µg/ml, 25 µg/ml), IL-4 enhanced CCL26 mRNA expression was not inhibited. When cultured with IL-4 (10 ng/ml) and various concentrations of JAK inhibitor 1 (0·1 nM, 1 nM, 10 nM) or leflunomide (50 µM, 100 µM, 200 µM), CCL26 mRNA expression was down-regulated in a dose-dependent manner (Fig. 5). When we used reaction products from an RT reaction in which the reverse transcriptase enzyme was omitted, neither CCL26 mRNA nor G3PDH mRNA was detected (Fig. 5). Therefore the results in Fig. 5 were not due to genomic DNA contamination. These results are parallel to those of ELISA, which indicate that regulation of CCL26 production occurs at the RNA level.
Fig. 5.
CCL26 mRNA expression in HaCaT cells was determined by RT-PCR when they were stimulated with IL-4, leflunomide, JAK inhibitor 1 and JAK3 inhibitor 1. Leflunomide and JAK inhibitor 1, but not JAK3 inhibitor 1, inhibited IL-4-enhanced CCL26 mRNA expression dose-dependently. When the reverse transcriptase enzyme was omitted, neither CCL26 mRNA nor G3PDH mRNA was detected.
HaCaT expressed components of IL-4 receptors
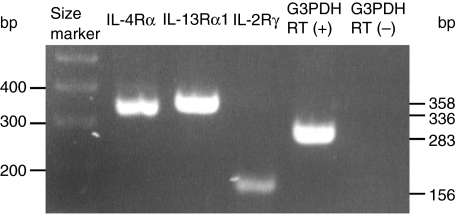
In order to examine the existence of each chain of IL-4 receptor in HaCaT cells, we performed RT-PCR utilizing primers specific for each chain of type 1 and 2 IL-4 receptor. RT-PCR revealed that HaCaT cells weakly expressed mRNA of IL-2Rγ and strongly expressed IL-4Rα and IL-13Rα1(Fig. 6). When we used reaction products from an RT reaction in which the reverse transcriptase enzyme was omitted, G3PDH mRNA was not detected (Fig. 6). Therefore the results in Fig. 6 were not due to genomic DNA contamination. This result suggests the predominance of type 2 IL-4 receptor over type 1 IL-4 receptor in HaCaT cells.
Fig. 6.
Expression of components of IL-4 receptors in HaCaT cells was determined by RT-PCR. HaCaT cells weakly expressed mRNA of IL-2Rγ and strongly expressed IL-4Rα and IL-13Rα1. When the reverse transcriptase enzyme was omitted, G3PDH mRNA was not detected. RT means reverse transcriptase.
Discussion
Eosinophils are involved in allergic diseases, such as asthma and atopic dermatitis (AD) [16]. Elucidation of the mechanisms underlying the accumulation of eosinophils in inflamed tissues is of critical importance for understanding the onset and progress of these eosinophilic diseases. The serum CCL11 levels were significantly correlated with the disease activity of AD [17]. Immunoreactivity and transcripts of CCL11 and CCR3 were significantly increased in lesional skin from AD [18]. Increased expression of CCL11, CCL24 and CCL26 was detected in bronchial mucosa of asthmatic patients [19]. These results suggest that all the three eotaxins are involved in the Th2-polarized situations such as bronchial asthma and AD. In human keratinocytes, CCL11 expression was synergistically up-regulated by TNF-α and IL-4 [20], and the blister fluid of bullous pemphigoid contained high levels of CCL11 associated with skin infiltration of eosinophils [4]. In this study we showed that CCL26 production was also synergistically up-regulated by TNF-α and IL-4.
In the previous study, we showed that serum levels of CCL26, but not of CCL24, were significantly elevated in AD patients [13]. Although we did not show whether keratinocytes in AD patients produced CCL26 in vivo, our previous and present study may indicate that CCL26, rather than CCL24, produced by keratinocytes is involved in the development of a Th2-dominant inflammatory skin disease like AD. This is the first report describing the regulation of CCL24 and CCL26 production in a human keratinocyte cell line, HaCaT cells by various cytokines. We also examined primary human keratinocytes, however, they did not produce these chemokines. The discrepancy of these results is due to the difference in the nature of primary human keratinocytes and HaCaT cells. It is true that there is limitation for presuming the in vivo function of CCL26 from only HaCaT cell data, however, we used these cells instead of primary human keratinocytes in order to analyse the signal pathways of IL-4 enhanced CCL26 production by keratinocytes.
IL-4- or IL-13-dependent CCL11 release from airway smooth muscle cells was abolished when inhibitors of both p42/p44 ERK and p38 MAP kinase pathways were combined in STAT6 antisense oligodeoxynucleotide-transfected cells [21]. Regulation of all three members of eotaxins by IL-4 has been reported to depend on STAT6 activation in various tissues such as murine airway, murine lung and human dermal fibroblasts [22–24]. In our study, IL-4-enhanced CCL26 production in HaCaT cells also depends on STAT6 pathway. However, CCL24 production was only weakly up-regulated even after IL-4 stimulation, suggesting that only STAT6 activation in HaCaT cells is not sufficient for strong enhancement of CCL24. This finding may reflect the fact that only the level of CCL26, but not CCL24, was elevated in sera from AD patients, the Th2 type inflammatory skin disease [13]. Moreover, this may suggest that production of eotaxins is differently regulated in various cell types.
We have shown that IL-4 enhanced production of CCL26 in HaCaT cells is mediated by JAK1-STAT6-dependent pathway mainly through the type 2 IL-4 receptor and that IFN-γ suppressed the IL-4 enhanced CCL26 production. In this regards, IFN-γ is known to induce a suppressor of cytokine signalling (SOCS)-1 and SOCS-3, known inhibitors of IL-4 signalling. They suppress IL-4 signalling by inhibiting JAK1 activity or type 1 and type 2 IL-4 receptors [25–27], which suggests similar mechanism of suppression of IL-4 signalling by IFN-γ in HaCaT cells. This awaits further clarification.
In this study, we showed that dexamethasone inhibited CCL26 production in HaCaT cells. Banwell et al. [12] also reported that dexamethasone was a potent inhibitor of CCL26 mRNA expression in lung epithelial cells. One possible explanation would be dexamethasone suppresses IL-4-enhanced transactivation of a STAT6-responsive promoter without affecting IL-4 stimulated STAT6 DNA-binding, as reported by Biola et al.[28] in lymphocytes.
IL-4 modulates NF-κB activity [29], and activates JNK [30], p38 MAP kinase [31], ERK [32], and PI3-kinase [33] in certain cell types. Blockade of the epidermal growth factor receptor (EGFR) changes chemokine expression in keratinocytes [34]. Therefore, we also examined inhibitors of EGFR (PD153035), ERK (PD98059), p38 (SB202190), JNK (JNK inhibitor 2), NF-κB (Parthenolide), or PI3-kinase (LY294002) on IL-4-enhanced CCL26 production. Jean-Baptiste et al. [20] showed that expression of CCL11 parallels epidermal eosinophil accumulation in the vesiculobullous stage of incontinentia pigmenti which was attributed to the mutation of NF-κB essential modulator, suggesting the involvement of in NF-κB in expression of CCL11 in keratinocytes. An inhibitor of NF-κB inhibited IL-13- and IL-4-enhanced CCL11 gene expressions, whereas enhanced CCL26 gene expression in bronchial epithelial cells [35]. Our results demonstrated that IL-4-enhanced production of CCL26 in HaCaT cells involved the p38 MAP kinase-dependent pathway, in addition to the JAK1/STAT6-dependent pathway, but not the EGFR, ERK, JNK or NF-κB dependent pathway. These findings add new data which strengthen the hypothesis induced from many studies that IL-4-dependent regulation of all the three eotaxins occurs through STAT6 in common in a variety of cell types, and is also dependent on other signalling molecules p38, NF-κB, ERK, or PI3-kinase, which varies in different cell types and eotaxins. All the three eotaxins may be differentially regulated in a variety of tissues, which makes CCR3-mediated inflammation complex, however, inhibition of STAT6 seems to be an effective way to attenuate IL-4-mediated production of all of them in a wide range of tissues.
In the previous study, we disclosed that CCL17 and CCL22 production by HaCaT cells was enhanced by TNF-α and IFN-γ, but restricted by IL-4 [5,6]. Thus in keratinocytes, the production of Th2 chemokines CCL17 and CCL22 was accelerated by Th1 cytokine IFN-γ and reduced by Th2 cytokine IL-4. In contrast, our present study showed that CCL26 production in keratinocytes was increased by IL-4 and decreased by IFN-γ. These results indicate that production of CC chemokines which seems to play important roles in the pathogenesis of AD is differently regulated by cytokines in keratinocytes. AD is considered to be a Th2-dominant disease especially in the acute phase, but it is reported that in the chronic phase, Th1 cells also infiltrate the lesional skin and play a role in the pathogenesis of the disease [36]. Th2- and Th1-type immune responses are not mutually exclusive, but a human inflammatory disease like AD is based on interactions between different Th-cell subsets. The difference in regulation of CC chemokine production by keratinocytes may contribute to maintaining the Th1/Th2 balance in an inflammatory skin disease like AD.
In conclusion, keratinocytes could be involved in the migration of CCR3 positive cells such as eosinophils, through the production of CCL26, in a Th2-dominant inflammatory skin disease like AD.
Acknowledgments
This work was supported by Health Science Research Grants from the Ministry of Health, Labor and Welfare of Japan and by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Yoshie O, Imai T, Nomiyama H. Chemokines in immunity. Adv Immunol. 2001;78:57–109. doi: 10.1016/s0065-2776(01)78002-9. [DOI] [PubMed] [Google Scholar]
- 2.Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nicholoff BJ. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–4. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- 3.Wakugawa M, Nakamura K, Akatsuka M, Nakagawa H, Tamaki K. Interferon-gamma-induced RANTES producing by human keratinocytes is enhanced by IL-1 β, TNF-α, IL-4 and IL-13 and is inhibited by dexamethasone and tacrolimus. Dermatology. 2001;202:239–45. doi: 10.1159/000051644. [DOI] [PubMed] [Google Scholar]
- 4.Wakugawa M, Nakamura K, Hino H, et al. Elevated levels of eotaxin and interleukin-5 in blister fluid of bullous pemphigoid: correlation with tissue eosinophilia. Br J Dermatol. 2000;143:112–6. doi: 10.1046/j.1365-2133.2000.03599.x. [DOI] [PubMed] [Google Scholar]
- 5.Kakinuma T, Nakamura K, Wakugawa M, et al. IL-4, but not IL-13, modulates TARC (thymus and activation-regulated chemokine)/ CCL17 and IP-10 (interferon-induced protein of 10 kDa)/CXCL10 release by TNF-α and IFN-γ in HaCaT cell line. Cytokine. 2002;20:1–6. doi: 10.1006/cyto.2002.1965. [DOI] [PubMed] [Google Scholar]
- 6.Xiao T, Kagami S, Saeki H, et al. Both IL-4 and IL-13 inhibit the TNF-α and IFN-γ enhanced MDC production in a human keratinocyte cell line, HaCaT cells. J Dermatol Sci. 2003;31:111–7. doi: 10.1016/s0923-1811(02)00149-4. [DOI] [PubMed] [Google Scholar]
- 7.Albanesi C, Scarponi C, Sebastiani S, Cavani A, Federici M, De Pita O, Puddu P, Girolomoni G. IL-4 enhances keratinocyte expression of CXCR3 agonistic chemokines. J Immunol. 2000;165:1395–402. doi: 10.4049/jimmunol.165.3.1395. [DOI] [PubMed] [Google Scholar]
- 8.Jose PJ, Griffiths-Johnson DA, Collins PD, et al. Eotaxin. A potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med. 1994;179:881–7. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forssmann U, Uguccioni M, Loetscher P, et al. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J Exp Med. 1997;185:2172–6. doi: 10.1084/jem.185.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinkai A, Yoshisue H, Koike M, et al. A novel human CC chemokine, eotaxin-3, which is expressed in IL-4-stimulated vascular endothelial cells, exhibits potent activity toward eosinophils. J Immunol. 1999;163:1602–10. [PubMed] [Google Scholar]
- 11.Berkman N, Ohnona S, Chung FK, Breuer R. Eotaxin-3 but not eotaxin gene expression is upregulated in asthmatics 24 hours after allergen challenge. Am J Respir Cell Mol Biol. 2001;24:682–7. doi: 10.1165/ajrcmb.24.6.4301. [DOI] [PubMed] [Google Scholar]
- 12.Banwell ME, Tolley NS, Williams TJ, Mitchell TJ. Regulation of human eotaxin-3/CCL26 expression: modulation by cytokines and glucocorticoids. Cytokine. 2002;17:317–23. doi: 10.1006/cyto.2002.1021. [DOI] [PubMed] [Google Scholar]
- 13.Kagami S, Kakinuma T, Saeki H, et al. Significant elevation of serum levels of eotaxin-3/CCL26, but not of eotaxin-2/CCL24, in patients with atopic dermatitis: Serum eotaxin-3/CCL26 levels reflect the disease activity of atopic dermatitis. Clin Exp Immunol. 2003;134:309–13. doi: 10.1046/j.1365-2249.2003.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murata T, Noguchi PD, Puri RK. IL-13 induces phosphorylation and activation of JAK2 janus kinase in human colon carcinoma cell lines: Similarities between IL-4 and IL-13 signaling. J Immunol. 1996;156:2972–8. [PubMed] [Google Scholar]
- 15.Siemasko K, Chong AS, Jack HM, Gong H, Williams JW, Finnegan A. Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production. J Immunol. 1998;160:1581–8. [PubMed] [Google Scholar]
- 16.Venge P. Monitoring the allergic inflammation. Allergy. 2004;59:26–32. doi: 10.1046/j.1398-9995.2003.00386.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaburagi Y, Shimada Y, Nagaoka T, Hasegawa M, Takehara K, Sato S. Enhanced production of CC-chemokines (RANTES, MCP-1, MIP-1α, MIP-1β, and eotaxin) in patients with atopic dermatitis. Arch Dermatol Res. 2001;293:350–5. doi: 10.1007/s004030100230. [DOI] [PubMed] [Google Scholar]
- 18.Yawalkar N, Uguccioni M, Scharer J, et al. Enhanced expression of eotaxin and CCR3 in atopic dermatitis. J Invest Dermatol. 1999;113:43–8. doi: 10.1046/j.1523-1747.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- 19.Komiya A, Nagase H, Yamada H, et al. Concerted expression of eotaxin-1, eotaxin-2, and eotaxin-3 in human bronchial epithelial cells. Cell Immunol. 2003;225:91–100. doi: 10.1016/j.cellimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Jean-Baptiste S, O'Toole EA, Chen M, Guitart J, Paller A, Chan LS. Expression of eotaxin, an eosinophil-selective chemokine, parallels eosinophil accumulation in the vesiculobullous stage of incontinentia pigmenti. Clin Exp Immunol. 2002;127:470–8. doi: 10.1046/j.1365-2249.2002.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng Q, Matsuda T, Hirst SJ. Signaling pathways regulating interleukin-13-stimulated chemokine release from airway smooth muscle. Am J Respir Crit Care Med. 2004;169:596–603. doi: 10.1164/rccm.200307-888OC. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann N, Hogan SP, Mishra A, et al. Murine eotaxin-2: a constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J Immunol. 2000;165:5839–46. doi: 10.4049/jimmunol.165.10.5839. [DOI] [PubMed] [Google Scholar]
- 23.Hoeck J, Woisetschlager M. Activation of eotaxin-3/CCL26 gene expression in human dermal fibroblasts is mediated by STAT6. J Immunol. 2001;167:3216–22. doi: 10.4049/jimmunol.167.6.3216. [DOI] [PubMed] [Google Scholar]
- 24.Yang M, Hogan SP, Mahalingam S, et al. Eotaxin-2 and IL-5 cooperate in the lung to regulate IL-13 production and airway eosinophilia and hyperreactivity. J Allergy Clin Immunol. 2003;112:935–43. doi: 10.1016/j.jaci.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Haque SJ, Harbor PC, Williams BR. Identification of critical residues required for suppressor of cytokine signaling-specific regulation of interleukin-4 signaling. J Biol Chem. 2000;275:26500–6. doi: 10.1074/jbc.275.34.26500. [DOI] [PubMed] [Google Scholar]
- 26.Heller NM, Matsukura S, Georas SN, et al. Interferon-γ inhibits STAT6 signal transduction and gene expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2004;31:573–82. doi: 10.1165/rcmb.2004-0195OC. [DOI] [PubMed] [Google Scholar]
- 27.Sato T, Saito R, Jinushi T, et al. IFN-γ-induced SOCS-1 regulates STAT6-dependent eotaxin production triggered by IL-4 and TNF-α. Biochem Bioph Res Comm. 2004;314:468–75. doi: 10.1016/j.bbrc.2003.12.124. [DOI] [PubMed] [Google Scholar]
- 28.Biola A, Andreau K, David M, Sturm M, Haake M, Bertoglio J, et al. The glucocorticoid receptor and STAT6 physically and functionally interact in T-lymphocytes. FEBS Lett. 2000;487:229–33. doi: 10.1016/s0014-5793(00)02297-3. [DOI] [PubMed] [Google Scholar]
- 29.Molina-Holgado E, Arevalo-Martin A, Castrillo A, Bosca L, Vela JM, Guaza C. Interleukin-4 and interleukin-10 modulate nuclear factor kappaB activity and nitric oxide synthase-2 expression in Theiler's virus-infected brain astrocytes. J Neurochem. 2002;81:1242–52. doi: 10.1046/j.1471-4159.2002.00925.x. [DOI] [PubMed] [Google Scholar]
- 30.Levings MK, Bessette DC, Schrader JW. Interleukin-4 synergizes with Raf-1 to promote long-term proliferation and activation of c-jun N-terminal kinase. Blood. 1999;93:3694–702. [PubMed] [Google Scholar]
- 31.Wery-Zennaro S, Zugaza JL, Letourneur M, Bertoglio J, Pierre J. IL-4 regulation of IL-6 production involves Rac/Cdc42- and p38 MAPK-dependent pathways in keratinocytes. Oncogene. 2000;19:1596–604. doi: 10.1038/sj.onc.1203458. [DOI] [PubMed] [Google Scholar]
- 32.David M, Ford D, Bertoglio J, Maizel AL, Pierre J. Induction of the IL-13 receptor alpha2-chain by IL-4 and IL-13 in human keratinocytes: involvement of STAT6, ERK and p38 MAPK pathways. Oncogene. 2001;20:6660–8. doi: 10.1038/sj.onc.1204629. [DOI] [PubMed] [Google Scholar]
- 33.Ueno H, Sasaki K, Honda H, et al. c-Cbl is tyrosine-phosphorylated by interleukin-4 and enhances mitogenic and survival signals of interleukin-4 receptor by linking with the phosphatidylinositol 3′-kinase pathway. Blood. 1998;91:46–53. [PubMed] [Google Scholar]
- 34.Mascia F, Mariani V, Girolomoni G, Pastore S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303–12. doi: 10.1016/S0002-9440(10)63654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi I, Yamamoto S, Nishi N, et al. Regulatory mechanisms of Th2 cytokine-induced eotaxin-3 production in bronchial epithelial cells: possible role of interleukin 4 receptor and nuclear factor-kappaB. Ann Allergy Asthma Immunol. 2004;93:390–7. doi: 10.1016/S1081-1206(10)61399-3. [DOI] [PubMed] [Google Scholar]
- 36.Grewe M, Bruijnzeel-Koomen CA, Schopf E, et al. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998;19:359–61. doi: 10.1016/s0167-5699(98)01285-7. [DOI] [PubMed] [Google Scholar]