Abstract
The widely expressed tumour antigens hTERT and CYP1B1 are commonly expressed in multiple myeloma (MM) cells. Several trials targeting these antigens by immunotherapy have been initiated. The aim of this study was to explore whether patients with MM have an endogenous pre-existing immune response against recently identified epitopes from hTERT and CYP1B1. Peripheral blood T cells from 27 HLA-A*0201+ multiple myeloma patients at different stages of disease and 20 healthy HLA-A*0201+ donors were enriched and studied for the presence of hTERT- and CYP1B1-specific cytotoxic T cells using MHC tetramer detection and short-term ex vivo expansion. No significant expansion of tetramer-positive cells was detected in the peripheral blood of either MM patients or healthy controls when cells were stained with tetramers containing the dominant hTERT-derived epitope or two peptides derived from CYP1B1. A single ex vivo peptide stimulation led to the detection of a small population (0·3–0·5%) of hTERT-specific cells in two of 27 patients with MM. None of the patients or controls showed significant expansion of CYP1B1-specific cells after a single peptide stimulation. Thus, endogenous in vivo priming of T cells against hTERT and CYP1B1 is a rare event in MM patients. These results suggest that strategies targeting hTERT and CYP1B1 may have to utilize techniques to induce T cell responses from a naive precursor frequency.
Keywords: CYP1B1, hTERT, T cell immunity, tumour antigen, immuno-therapy
Introduction
Active cancer immunotherapy depends on the identification of tumour antigens that can induce a clinically efficient anti-tumour immune response [1]. Most tumour antigens have been discovered by analysing cancer patients’ anti-tumour immune responses [2,3]. Recently, more and more tumour antigens with broad tumour-associated expression and potentially wide clinical applicability have been identified by comparative expression studies and epitope deduction [4]. This approach led to the discovery of tumour antigens such as hTERT [5], survivin [6], CYP1B1 [7] or cyclin B1 [8]. While survivin-specific T cells have been detected in peripheral blood of patients with melanoma, breast cancer or chronic lymphocytic leukaemia [9], a small study in a heterogeneous group of patients with prostate, lung or bladder cancer suggested the absence of T cells reactive against the most prominent epitope (I540) derived from hTERT [10]. In contrast, vaccination against hTERT-derived epitopes has clearly demonstrated the induction of antigen-specific T cells suggesting that such T cells are not deleted from the T cell repertoire [11,12].
We recently identified the carcinogen activating cytochrome P450 1B1 (CYP1B1) as a tumour antigen recognized by cytotoxic T cells [7,13]. CYP1B1 is expressed in the vast majority of human tumours with only limited expression in normal tissue [7,14]. In an initial screen of five cancer patients of different histology and five healthy volunteers no significant spontaneous interferon (IFN)-γ response by T cells was detected against two HLA-A*0201-restricted epitopes, although fully functional CYP1B1-specific T cells could be expanded from healthy individuals and cancer patients by prolonged ex vivo stimulation [7].
To better understand whether the deduced epitopes from CYP1B1 and hTERT are targets of natural occurring tumour immunity in multiple myeloma (MM) we studied the frequencies of T cells against the three HLA-A*0201 restricted epitopes derived from CYP1B1, respectively, hTERT in 27 MM patients. MM was chosen as a model cancer because of (1) significant and homogeneous overexpression of hTERT and CYP1B1 in MM cells, (2) the lack of T cell response to a series of known tumour antigens despite demonstration of strong anti-tumour CTL response in MM patients [15,16] and (3) an intense tumour—T cell interaction at the site of disease in the bone marrow [16].
Materials and methods
Donor and patient samples
Peripheral blood from healthy volunteers and multiple myeloma patients was obtained by phlebotomy following informed consent and approval by our institute's Review Board. Peripheral blood mononuclear cells (PBMC) were purified by Ficoll-density centrifugation. CD8+ T cells were isolated using RosetteSep® (StemCell Technologies, Vancouver, Canada) according to the manufacturer's recommendations. Tetramer analysis and short-term cultures were performed from fresh material. PBMC cryopreserved in liquid nitrogen were used for enzyme-linked immunospot (ELISPOT) analysis.
In vitro antigenic stimulation
In vitro stimulation of CD8+ T cells with peptides was performed as described [17]. In brief, purified CD8+ T cells were incubated with autologous irradiated (32Gy) PBMC in the presence of peptide (1 µg/ml; New England Peptide, Fitchburg, MA, USA) and β2-microglobulin (2 µg/ml; Sigma, St Louis, MO, USA) in RPMI-1640 media supplemented with 10% human AB serum, 2 m M glutamine, 15 µg/ml gentamicin and 20 m M HEPES. Peptides used were from human telomerase reverse transcriptase (hTERT I540, ILAKFLHWL [5]), human cytochrome P450 1B1 (CYP1B1 CYP239, SLVDVMPWL [7]; and CYP246, WLQYFPNPV [13]), and Epstein—Barr virus (EBV BMLF-1 280–288, GLCTLVAML [18]). Interleukin (IL)-7 (10 ng/ml; Endogen, Woburn, MA, USA) was added on day +1 after initiation of the culture, and IL-2 (20 IU/ml; Chiron Corp, Emeryville, CA, USA) was supplemented on days +1, +4, and +7. Tetramer analysis of the cultured cells was performed on day +10.
Tetramer analysis
Tetrameric A2/peptide complexes with peptides from HTLV TAX (negative control), hTERT I540, CYP239, CYP246 and EBV were synthesized essentially as described [19], and multimerized with streptavidin—phycoerythin (SA-PE; Molecular Probes, Eugene, OR, USA). Staining of cells with tetramers and flow cytometry was performed as previously described [17]. At least 5000 CD8+ T cells were acquired and analysed.
IFN-γ ELISPOT analysis
Cryopreserved PBMC at 1 × 105 cells/well were used for IFN-γ ELISPOT analysis. The experimental procedure and data analysis was carried out as described previously [10].
Results
Rare expansion of hTERT-specific T cells in MM patients after a single antigenic stimulation
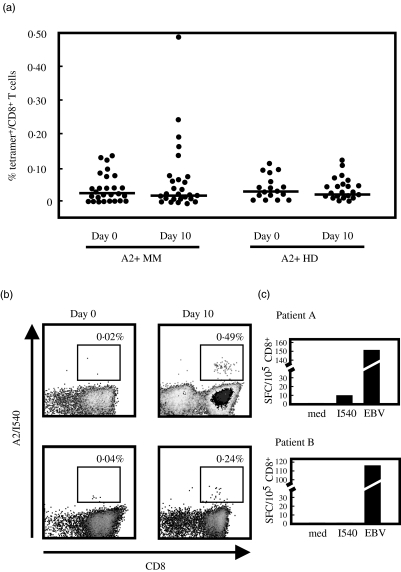
To assess immunity against the dominant I540 epitope of hTERT we studied T cell frequencies against this epitope in a cohort of 27 HLA-A*0201+ patients with MM at different stages of disease and treatment history (Table 1). 20 HLA-A*0201+ healthy volunteers served as controls. To establish sensitivity and specificity of our assay we assessed 22 HLA-A*0201− MM patients. A positive test was defined as the average percentage of tetramer+ T cells of the 22 HLA-A*0201- MM patients plus three standard deviations (SD) [20]. Using these criteria, we detected no I540-tetramer+ CD8+ T cells directly ex vivo in peripheral blood in any of the 27 HLA-A*0201+ MM patients and 20 HLA-A*0201+ healthy volunteers (Fig. 1a). Following a previously established technique to increase sensitivity of the tetramer-based assay [21] we subjected purified CD8+ T cells to a single stimulus of I540 peptide in the presence of autologous irradiated PBMC and cytokines for 10 days. A population of I540-tetramer+ cells was apparent on day 10 in only two untreated MM patients with stage I (patient A) resp. III (patient B) disease (Fig. 1b) and none of the healthy volunteers. In contrast, no increase in frequency of hTERT-specific cells was observed in the majority of MM patients and in all healthy volunteers (Fig. 1a).
Table 1. Patient characteristics.
| Total number of patients | 27 |
| Age (years) | |
| Median (range) | 58 (27–83) |
| Gender | |
| Female | 9 |
| Male | 18 |
| Stage | |
| Smouldering | 2 |
| I | 9 |
| II | 2 |
| III | 14 |
| Time from diagnosis (months) | |
| Median (range) | 12 (2–24) |
| Time from last treatment | |
| < 4 weeks | 6 |
| > 4 weeks | 12 |
| Untreated | 9 |
Fig. 1.
Detection of hTERT I540-specific T cells by tetramer in multiple myeloma (MM) patients and healthy volunteers. (a) CD8+ T cells from HLA-A*0201+ MM patients and healthy volunteers were stained with I540-tetramer and CD8 and analysed by flow cytometry. Annexin V was added to exclude dead cells. Tetramer analysis was repeated after 10 days of stimulation with I540 peptide, autologous irradiated peripheral blood mononuclear cells (PBMC) and cytokines. A tetramer containing the HTLV Tax 11–19 peptide was used as a negative control (not shown). Dots represent the percentage of I540-tetramer+ cells per total CD8+ T cells of individual patients. The line depicts the median value. (b) I540-tetramer evaluation before and after culture exemplified for the two patients with a significant expansion of cells after a single peptide stimulation. Patient A had stage I disease, patient B suffered from stage III myeloma. Both patients were diagnosed with myeloma 12 months before analysis. Percentages represent I540-tetramer+ cells per total CD8+ cells. (c) Interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) analysis of PBMC directly isolated ex vivo. Numbers reflect spot forming cells (SFC) per 105 CD8+ cells. Medium only (med) was used to determine background secretion, the EBV BMLF-1280–288 peptide was used as a positive control.
We evaluated whether the hTERT-specific T cells could be detected by IFN-γ ELISPOT analysis. Freshly isolated PBMC from patient A showed a small secretion of IFN-γ towards I540 peptide, which was about 5% of that seen in response to the positive control peptide from Epstein—Barr virus (Fig. 1c). No I540-induced IFN-γ secretion could be detected in patient B. Furthermore, IFN-γ ELISPOT analysis confirmed the absence of I540-reactive T cells in the remaining MM patients and healthy donors tested (data not shown).
In summary, using this sensitive tetramer-based assay no spontaneous reactivity to hTERT-derived epitope I540 could be detected in the majority of MM patients and in healthy volunteers. There may be rare cases of low-frequency responses in some MM patients, which are sensitively picked up by tetramer evaluation after single peptide stimulation. Alternatively, we occasionally might observe true priming of naive T cells, as has also been shown during the 10-day culture period using the HTLV-derived TAX11-19 peptide in HTLV seronegative individuals (data not shown).
Lack of spontaneous reactivity against CYP1B1 in MM patients
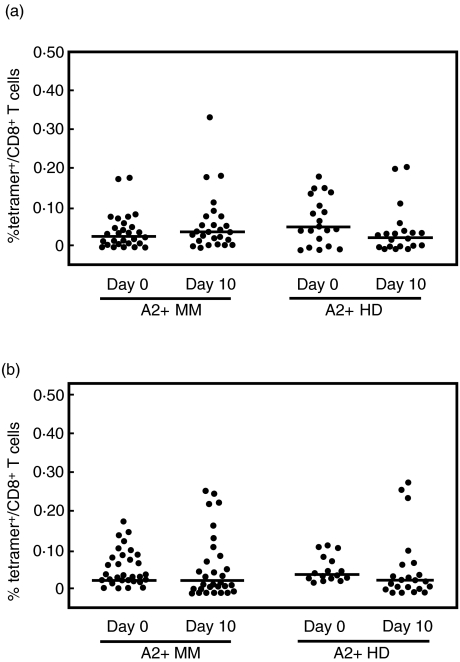
Because CYP1B1 is expressed at high levels in multiple myeloma cells and CYP1B1-specific CTL lines can be generated from cancer patients we asked whether CYP1B1-reactive T cells may be pre-existing in MM patients. Initial screening using IFN-γ ELISPOT analysis suggested no overt CYP1B1-directed reactivity [7]. To increase sensitivity we applied the tetramer approach in conjunction with a one-time antigenic stimulation to detect even low-level in vivo reactivity against CYP1B1. We assessed T cells specific for the previously described HLA-A*0201 restricted epitope CYP239 [7] and a newly identified HLA-A*0201 epitope CYP246 [13]. CYP239 tetramer+ cells were detected only in two HLA-A2+ MM patients and one healthy individual (Fig. 2a). Ex vivo expansion revealed CYP239 tetramer+ T cells in three MM patients and two healthy individuals (Fig. 2a); however, frequencies were just above the level of detection. Although CTL responses against CYP246 could be induced by 4-week long-term in vitro expansion [13], we were unable to detect CYP246 tetramer+ cells in MM patients or healthy individuals at baseline (Fig. 2b), and there was only a slight increase in four MM patients and three healthy individuals after stimulation. In conclusion, while T cells reactive to the recently identified epitopes from hTERT and CYP1B1 exist, expansion of these cells in vivo in our cohort of HLA-A*0201+ MM patients and healthy individuals is a rare event.
Fig. 2.
Evaluation of T cell responses to CYP1B1-derived epitopes in HLA-A*0201+ multiple myeloma (MM) patients and healthy volunteers. T cells specific for CYP239 (a) and CYP246 (b) were enumerated by tetramer staining before and after a 10-day stimulation with peptide, autologous irradiated peripheral blood mononuclear cells (PBMC) and cytokines. The percentage of tetramer+ cells per CD8+ cells for each patient is shown. Lines reflect the median value.
Discussion
Broadly expressed tumour antigens identified by comparative expression studies and epitope deduction may serve as target structures for widely applicable tumour immunotherapy. However, questions remain about the endogenous T cell responses against such antigens in cancer patients. Here, we show in a cohort of 27 patients with MM that T cells specific for the recently identified epitopes from the tumour antigens hTERT and CYP1B1 are of low prevalence in the majority of MM patients. There may be, however, occasional endogenous priming of T cells against the hTERT-derived epitope I540, as seen in two patients, which is reliably detected by tetramer analysis after a single ex vivo stimulation. Interestingly, both patients had not received anti-myeloma therapy prior to analysis, but differed in their stage of disease (stages I and III, respectively). In contrast, seven of nine untreated patients did not show T cells reactive with the I540-tetramer, precluding a general response against hTERT in untreated myeloma patients. Although CYP1B1 protein is expressed at high levels in MM cells, patients with MM do not mount a significant spontaneous T cell response against the HLA-A*0201 restricted epitopes CYP239 and CYP246.
We and others have previously addressed the question whether humoral or cellular immune responses against hTERT are naturally occurring in cancer patients. While spontaneous auto-antibodies against hTERT have been detected in hepatocellular carcinoma and gastric cancer patients [22], no spontaneous T cell reactivity against hTERT was observed in cancer patients either directly ex vivo[7,10] or after one restimulation with tumour lysate-pulsed dendritic cells [16]. The apparent lack of recognition by the endogenous immune response is further documented by our data showing that only in rare cases hTERT-specific T cells may have been primed in vivo. In contrast, fully functional hTERT- as well as CYP1B1-specific T cells could be expanded from patients with MM by repetitive stimulation with peptide pulsed on antigen-presenting cells over a 4-week period [7,10]. Moreover, vaccination with hTERT-loaded ex vivo generated dendritic cells induced fully functional hTERT-specific T cells in vivo in end-stage cancer patients [12]. Furthermore, dendritic cells transfected with hTERT-RNA induced functional T cell responses in patients with metastatic prostate cancer [23]. These findings might indicate that hTERT and CYP1B1 are suitable therapeutic targets particularly as in vivo loss of function of hTERT or CYP1B1-specific T cells − as observed for other tumour-antigen specific T cells [24] − may not be as critical. However, the lack of pre-existing immunity will most certainly require very efficient vaccination strategies to expand clinically relevant numbers of T cells in vivo.
Our experimental approach is based on epitope-restricted T cell responses and therefore does not necessarily reflect the entire T cell reactivity against hTERT and CYP1B1 in MM patients. We cannot rule out that immunodominant cytotoxic T cell responses directed against other HLA-A*0201-restricted epitopes or epitopes restricted to other HLA alleles may mask T cell reactivity against I540, CYP239 and CYP246. However, with respect to therapeutic targeting this issue appears less prominent.
An alternative explanation for the lack of an expanded pool of hTERT and CYP1B1-specific CD8+ T cells might be a generally reduced CD8+ T cell response in MM patients. We have shown recently that MM patients also show a decreased frequency and reactivity to influenza A and Epstein—Barr viral antigen stimulation [17]. This was not related to prior anti-myeloma treatment. Nevertheless, anti-viral memory T cell responses were present in these patients and could be expanded at a two- to threefold reduced level. Similar functional deficits may be operative for hTERT- and CYP1B1-specific T cells.
Acknowledgments
We are indebted to our patients for their commitment to this study. We thank Drs P. Richardson and R. Schlossman as well as D. Doss RN, for referral of patients and C. Hancock and R. Rich for administrative support. We thank I. Menezes, K. Beul and K. Hoar for excellent technical assistance. B.M. was supported by the Deutsche Forschungsgemeinschaft and the Multiple Myeloma Research Foundation. This work was supported by a Senior Research Award of the Multiple Myeloma Research Foundation (J.L.S.), a Special Fellowship of the Leukaemia and Lymphoma Society of America (J.L.S.), a Translational Research Award by the Leukaemia and Lymphoma Society of America (J.L.S.), a Fellowship of the Leukaemia Research Foundation of America (M.S.v. B.-B.), NIH grants K08-CA-88444–01 (K.S.A.), P01-CA-66996 and P01-CA-78378 (L.M.N.), and the Damon Runyon Cancer Research Fund (R.H.V.).
References
- 1.Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–70. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 2.Boon T, De Plaen E, Lurquin C, et al. Identification of tumour rejection antigens recognized by T lymphocytes [Review] Cancer Surv. 1992;13:23–37. [PubMed] [Google Scholar]
- 3.Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–7. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 4.Schultze JL, Vonderheide RH. From cancer genomics to cancer immunotherapy: toward second-generation tumor antigens. Trends Immunol. 2001;22:516–23. doi: 10.1016/s1471-4906(01)02015-4. [DOI] [PubMed] [Google Scholar]
- 5.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–9. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 6.Andersen MH, Pedersen LO, Becker JC, Straten PT. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Cancer Res. 2001;61:869–72. [PubMed] [Google Scholar]
- 7.Maecker B, Sherr DH, Vonderheide RH, et al. The shared tumor-associated antigen cytochrome P450 1B1 is recognized by specific cytotoxic T cells. Blood. 2003;102:3287–94. doi: 10.1182/blood-2003-05-1374. [DOI] [PubMed] [Google Scholar]
- 8.Kao H, Marto JA, Hoffmann TK, et al. Identification of Cyclin B1 as a shared human epithelial tumor-associated antigen recognized by T cells. J Exp Med. 2001;194:1313–24. doi: 10.1084/jem.194.9.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen MH, Pedersen LO, Capeller B, Brocker EB, Becker JC, thor Straten P. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 2001;61:5964–8. [PubMed] [Google Scholar]
- 10.Vonderheide RH, Schultze JL, Anderson KS, et al. Equivalent induction of telomerase-specific cytotoxic T lymphocytes from tumor-bearing patients and healthy individuals. Cancer Res. 2001;61:8366–70. [PubMed] [Google Scholar]
- 11.Su Z, Dannull J, Heiser A, et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63:2127–33. [PubMed] [Google Scholar]
- 12.Vonderheide RH, Domchek SM, Schultze JL, et al. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin Cancer Res. 2004;10:828–39. doi: 10.1158/1078-0432.ccr-0620-3. [DOI] [PubMed] [Google Scholar]
- 13.Maecker B, von Bergwelt-Baildon MS, Sherr DH, Nadler LM, Schultze JL. Identification of a new HLA-A*0201-restricted cryptic epitope from CYP1B1. Int J Cancer. 2005;115:333–6. doi: 10.1002/ijc.20906. [DOI] [PubMed] [Google Scholar]
- 14.Murray GI, Taylor MC, McFadyen MC, et al. Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Res. 1997;57:3026–31. [PubMed] [Google Scholar]
- 15.Wen YJ, Min R, Tricot G, Barlogie B, Yi Q. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002;99:3280–5. doi: 10.1182/blood.v99.9.3280. [DOI] [PubMed] [Google Scholar]
- 16.Dhodapkar MV, Krasovsky J, Olson K. T cells from the tumor microenvironment of patients with progressive myeloma can generate strong, tumor-specific cytolytic responses to autologous, tumor-loaded dendritic cells. Proc Natl Acad Sci USA. 2002;99:13009–13. doi: 10.1073/pnas.202491499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maecker B, Anderson KS, von Bergwelt-Baildon MS, et al. Viral antigen-specific CD8+ T-cell responses are impaired in multiple myeloma. Br J Haematol. 2003;121:842–8. doi: 10.1046/j.1365-2141.2003.04375.x. [DOI] [PubMed] [Google Scholar]
- 18.Steven NM, Annels NE, Kumar A, Leese AM, Kurilla MG, Rickinson AB. Immediate early and early lytic cycle proteins are frequent targets of the Epstein—Barr virus-induced cytotoxic T cell response. J Exp Med. 1997;185:1605–17. doi: 10.1084/jem.185.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman JD, Moss PAH, Goulder PJR, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. [PubMed] [Google Scholar]
- 20.Pittet MJ, Valmori D, Dunbar PR, et al. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med. 1999;190:705–15. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jager E, Nagata Y, Gnjatic S, et al. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci USA. 2000;97:4760–5. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masutomi K, Kaneko S, Yasukawa M, Arai K, Murakami S, Kobayashi K. Identification of serum anti-human telomerase reverse transcriptase (hTERT) auto-antibodies during progression to hepatocellular carcinoma. Oncogene. 2002;21:5946–50. doi: 10.1038/sj.onc.1205788. [DOI] [PubMed] [Google Scholar]
- 23.Su Z, Dannull J, Yang BK, et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 24.Lee PP, Yee C, Savage PA, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]