Abstract
Alloreactive T cells may be activated via a direct or an indirect antigen presentation pathway. We questioned whether the frequency of interferon (IFN)-γ producing cells determined by enzyme-linked immunospot (ELISPOT) assay is an effective tool to monitor the direct and/or indirect presentation pathway. Secondly, we wondered whether early and late acute rejection (AR) are associated with both pathways. Before (n = 15), during (n = 18) and after (n = 16) a period of AR, peripheral blood mononuclear cell (PBMC) samples were tested from 13 heart transplant recipients. The direct presentation pathway was always present. The number of IFN-γ producing cells reactive to this pathway increased significantly (P = 0·04) during AR and the number decreased (P = 0·005) after AR therapy. In contrast, the indirect allogeneic presentation pathway was present in only eight of 18 AR samples. When the indirect presentation pathway was detectable, it increased significantly during AR. Five of eight of these AR occurred more than 6 months after transplantation. The ELISPOT assay, enumerating alloreactive IFN-γ producing cells, is a valuable tool to determine the reactivity via both the direct and the indirect presentation pathway. The direct presentation pathway always plays a role in AR, while the indirect pathway contributes especially to late AR.
Keywords: heart transplantation, host-versus-graft disease, human studies, T cells
Introduction
The recognition of allo-major histocompatibility complex (MHC) antigens by T cells is the central event that initiates acute rejection (AR). This alloantigen recognition occurs via two mechanisms: the direct and the indirect allorecognition pathway [1]. Direct recognition refers to recognition by T cells of determinant peptides on intact donor MHC molecules displayed on the surface of transplanted cells. The T cell precursor frequency involved in the direct recognition pathway is extremely high [2], and can be driven by antigenic mimicry [3]. Indirect recognition refers to the stimulation of antigen-presenting cells (APCs) of the recipient by peptides of donor proteins presented by self-MHC antigens.
On the basis of experimental models, it seems that the direct presentation pathway is most active during the first weeks after grafting, but becomes less potent when the donor-derived APCs have left the graft [4,5]. Early clinical AR may also be mediated predominantly via this pathway, because at that time grafts contain many donor-derived passenger APCs expressing a high density of allo-MHC molecules. In renal transplant recipients with stable graft function the direct pathway was not different from that of healthy volunteers, whereas these patients showed no response to antigens presented via the indirect pathway [6]. After heart and renal transplantation, the direct donor-specific recognition increased during AR [7], while more than 1 year after transplantation donor-specific T cell hyporesponsiveness via the direct pathway was observed [8,9].
In human studies, the indirect pathway of allorecognition after organ transplantation has also been analysed. Several groups have shown a relation with proliferative responses to specific donor HLA peptides via this pathway and chronic rejection in renal [9,10], heart [11] and lung [12–14] transplant recipients. Some investigators also found this correlation after stimulating peripheral blood mononuclear cells (PBMC) from heart or kidney transplant recipients with fragmented donor cells [15] or with allogeneic PBMC that had been depleted of APCs [6,16]. The advantage of using fragmented donor cells is that the T cells that react to donor cells via the indirect pathway may respond to the full repertoire of donor HLA epitopes, in contrast to the limited number of synthetic peptides used by most groups.
In healthy volunteers, it has been demonstrated that the frequency of T cells engaged in the indirect pathway of allorecognition is about 100-fold lower than that of T cells participating in the direct recognition of native HLA-DR antigen [17].
The role of the indirect presentation pathway in AR is less clear. The group of Suciu-Foca reported reactivity to synthetic peptides corresponding to the hypervariable regions of the mismatched HLA-DR antigens of the donor during AR after heart and liver transplantation [18,19]. In a small pilot study, it has been suggested that the presence of both the direct and indirect pathway is predictive for AR after kidney transplantation [6,16], but there is no consensus on the reproducibility of the test used for measuring the indirect allorecognition pathway [20] and more data are necessary to support this hypothesis.
In the present study we questioned, first, whether the enzyme-linked immunospot (ELISPOT) assay, enumerating alloreactive interferon (IFN)-γ producing cells (pc) is an adequate tool to determine the frequency of T cells responding via the direct and indirect allogeneic presentation pathway. We used fragmented donor cells to test the indirect presentation pathway. From literature [21] and from our centre [22], it is known that most AR occur in the first 6 months after heart transplantation. Thereafter, fewer AR episodes occur, but still exist. Therefore, we questioned whether these late AR differ from early AR, and correlated early (0–6 months after transplantation) AR and late (> 6 months after transplantation) with the direct and the indirect presentation pathway.
Materials and methods
Patients
We made a selection of PBMC available from patients who experienced early and/or late AR episodes. Patient characteristics are depicted in Table 1. Before (n = 15), during (n = 18) and after (n = 16) periods of AR, PBMC samples from 13 patients were tested in the first 15 months after heart transplantation. Twenty-two of these 49 PBMC samples were taken more than 6 months after transplantation (before AR, n = 7; during AR, n = 8; after AR, n = 7). Blood sampling was approved by the local medical ethical committee on human research. All patients gave informed consent before blood sampling.
Table 1. Patient characteristics of heart transplant recipients during the period of an acute rejection (AR) period.
| Patient ID | Time after transplantation of AR | Immunosuppressive load* (dose per day) |
|---|---|---|
| Ka | 1 week | 650 mg CsA +40 mg Pred |
| Po | 6 months | 200 mg CsA +100 mg AZA +10 mg Pred |
| Fl | 7·4 months | 300 mg CsA +10 mg Pred |
| Ru | 3·1 weeks | 550 mg CsA +20 mg Pred |
| 3·7 months | 500 mg CsA +10 mg Pred | |
| Ho | 7·2 months | 300 mg CsA +150 mg AZA +10 mg Pred |
| 8·8 months | 250 mg CsA +100 mg AZA +10 mg Pred | |
| Cl | 13·3 months | 250 mg CsA +150 mg AZA +10 mg Pred |
| Th | 12·8 months | 350 mg CsA +3000 mg MMF +10 Pred |
| Ri | 4 weeks | 350 mg CsA +2000 mg MMF +15 Pred |
| 6·6 weeks | 400 mg CsA +2000 mg MMF +10 Pred | |
| He | 4·9 weeks | 450 mg CsA +3000 mg MMF +17·5 mg Pred |
| 12·9 weeks | 350 mg CsA +1000 mg MMF +10 mg Pred | |
| Jo | 8·1 weeks | 450 mg CsA +3000 mg MMF +10 mg Pred |
| Sc | 9·8 months | 350 mg CsA +3000 mg MMF +10 mg Pred |
| 11·2 months | 300 mg CsA +10 mg Pred | |
| Co | 10·3 weeks | 250 mg CsA +1500 mg MMF +10 mg Pred |
| Sh | 3 weeks | 350 mg CsA +2000 mg MMF +20 mg Pred |
CsA: cyclosporin A, MMF: mycophenolate mofetil, AZA: azathioprine, Pred: prednisone.
Only patients with biopsy proven ISHLT grade ≥ 3A episodes [23] were considered to experience acute cellular rejection.
Peripheral blood mononuclear cells (PBMC) and spleen cells
PBMC were isolated from heparinized blood by density gradient centrifugation using Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) as described previously [24] and stored at −140°C until use.
Spleen cells were obtained by mechanical dissociation of small pieces of spleen derived from the organ donor [24]. Subsequently, the cell suspension was filtrated through a 40 µm cell strainer (Falcon, Franklin Lakes, NJ, USA) and washed. Thereafter, the cells were centrifuged over a Ficoll-Paque (Amersham Pharmacia Biotech) density gradient, collected, washed and stored at −140°C.
To test the direct presentation pathway, intact spleen cells were used.
To test the indirect presentation pathway, cells were subjected to three cycles of 5 min in liquid N2 and rapidly thawed in a water bath of 37°C.
Enzyme linked immunospot (ELISPOT) assay
To test the direct presentation pathway, 100 µl with 1 × 105 patients’ PBMC in complete culture medium [RPMI-1640 D M (Gibco BRL, Scotland, UK) supplemented with 2 ml-glutamine (BioWhittaker, Verviers, Belgium), 100 IU/ml penicillin (BioWhittaker), 100 µg/ml streptomycin (BioWhittaker) and 10% pooled heat-inactivated and filtered (0·20 µm sterile syringe filter, Corning Incorporated, Corning, NY, USA) human serum, that was tested for adequate cell growth support in mixed lymphocyte cultures] was added to (a) culture medium in round-bottomed wells (sixfold) of a 96-well plate (Nunc, Roskilde, Denmark); (b) 100 µl 1 × 105 irradiated (40 Gy) spleen cells derived from the donor; or (c) 100 µl 1 × 105 irradiated (40 Gy) spleen cells derived from a third party, which did not share HLA antigens with the donor and patient. For each patient the same third-party cells were used in all experiments.
To test the indirect presentation pathway, 100 µl with 2 × 105 patients’ PBMC in complete culture medium was added to (a) 100 µl 2 × 105 fragmented spleen cells derived from the donor or (b) tetanus toxoid (TET: RIVM, Bilthoven, the Netherlands) at 30 lf/ml final concentration as a positive control of this presentation pathway.
After 40 h of incubation, the non-adherent cells were harvested and transferred in triplicate to a flat-bottomed 96-well ELISPOT plate (U-CyTech biosciences, Utrecht, the Netherlands) precoated with a mouse antihuman IFN-γ monoclonal antibody (mAb) and postcoated with phosphate buffered saline (PBS) containing 1% bovine serum albumin (BSA). Cells were incubated in the ELISPOT plate for 5 h to allow spot formation. Subsequently, the bulk of cells was flicked off and 200 µl/well ice-cold milli-Q water was pipetted, and the plate was placed for 10 min on melting ice. Thereafter, the wells were washed 10 times with PBST (PBS containing 0·05% Tween-20). Then, 100 µl/well of an appropriately diluted biotinylated anti-human IFN-γ detector antibody preparation was added and incubated overnight at 4°C. After six cycles of washing with PBST, the wells were incubated with 50 ml Φ-labelled goat-antibiotine antibody for 1 h at 37°C. After another washing step (sixfold) with PBST, 30 µl/well of reagent (activator I +II) was added that activated Φ. After 20–30 min incubation at room temperature in the dark spots became microscopically visible. The reaction was then stopped by discarding the solution and rinsing the plate with milli-Q water.
The spots were counted automatically using a Bioreader 3000 ELISPOT reader (BioSys GmbH, Karben, Germany).
Proliferation assay
At the same time that the ELISPOT assay was performed, the phytohaemagglutinin (PHA) proliferation assay [25] was performed to control viability of the PBMC. Only ELISPOT data of viable cells, according to this stimulation assay (stimulation index: ratio of the counts per minute obtained in the presence of PHA to the counts per minute in the absence of PHA ≥ 50), were analysed in the described results.
Statistical analysis
The paired Wilcoxon signed-rank test was used to test the difference between before, during and after AR. Two-sided P-values ≤ 0·05 were considered significant.
Results
We used the ELISPOT assay to evaluate the frequency of IFN-γ alloreactive cells before, during and after a period of AR. PBMC were activated via the direct or indirect allorecognition pathway.
Direct allorecognition pathway
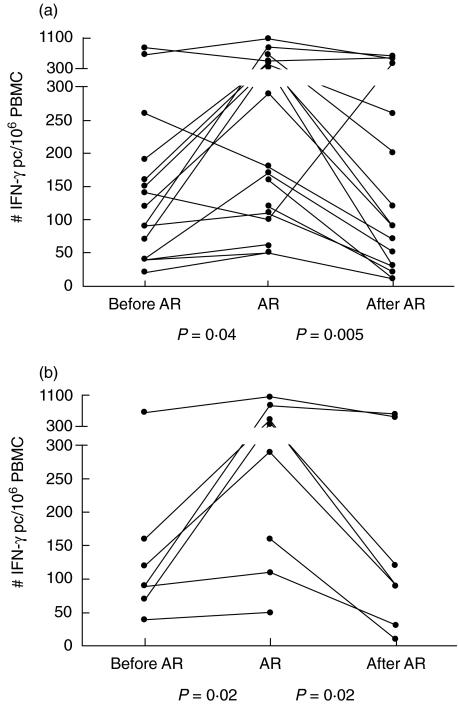
The T cell response directed against donor antigens measured via the direct presentation pathway was always readily detectable (Fig. 1). This response increased during an episode of AR from median 120 (range: 20–830) to 235 (50–1070) IFN-γ producing cells (pc)/106 PBMC (50–1070) (P = 0·04). After successful treatment the response decreased to 90 (10–610) IFN-γ pc/106 PBMC (P = 0·005).
Fig. 1.
Number of interferon (IFN)-γ producing cells (pc) reactive to donor cells via the direct presentation pathway (a) determined from peripheral blood mononuclear cells (PBMC) of heart transplant patients before, during and after a period of acute rejection (AR). (b) PBMC samples taken more than 6 months after transplantation.
When we analysed the PBMC samples only around a late period of AR (> 6 months after transplantation), the response increased significantly from 90 IFN-γ pc/106 PBMC (range: 40–660) to 340 IFN-γ pc/106 PBMC (range: 50–1070) during AR (P = 0·02). The response decreased again after successful AR therapy to 90 IFN-γ pc/106 PBMC (range: 10–610) (P = 0·02).
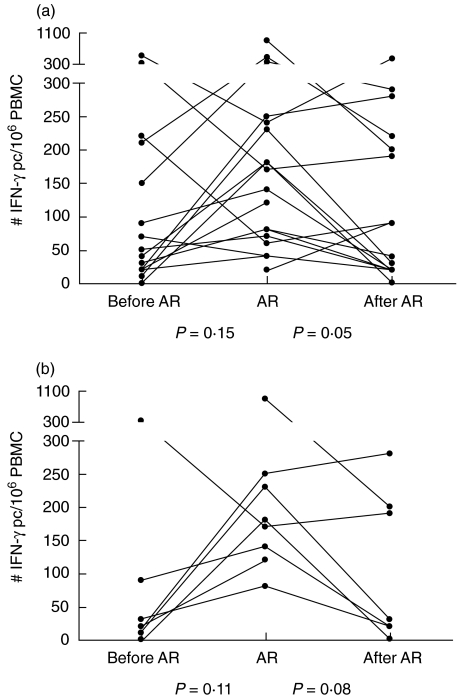
The third-party reactivity via the direct pathway was not significantly different when we compared the AR episode with the period before AR (Fig. 2). However, the response decreased after AR treatment from 155 IFN-γ pc/106 (range: 20–900) to 65 IFN-γ pc/106 PBMC (range: 0–440) (P = 0·05).
Fig. 2.
Number of interferon (IFN)-γ producing cells (pc) reactive to third-party cells via the direct presentation pathway (a) determined from peripheral blood mononuclear cells (PBMC) of heart transplant patients before, during and after a period of acute rejection (AR). (b) PBMC samples taken more than 6 months after transplantation.
Indirect allorecognition pathway
No intact cells were observed by microscopic examination of the fragmented donor spleen cells, and no spots were generated in response to PHA. Also, no spots were generated when fragmented spleen cells were stimulated with fragmented spleen cells from another donor.
Response via the indirect allogeneic pathway was present in eight of 27 (29·6%) PBMC samples taken in the first 6 months after transplantation, and in 15 of 22 (68·2%) samples taken more than 6 months after transplantation (Fisher's exact test: P = 0·01). The number of PBMC reactive to donor antigens via the indirect pathway was significantly lower than the reactivity via the direct pathway both in the first 6 months (eight of 27 versus 27 of 27, P < 0·0001) and more than 6 months (15 of 22 versus 22 of 22, P = 0·009) after transplantation.
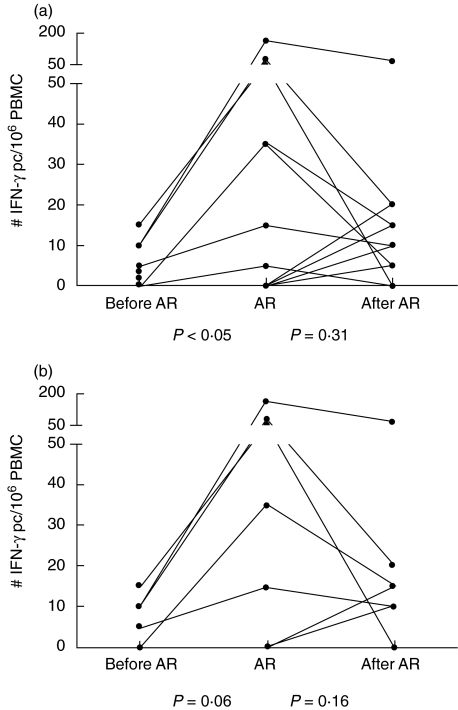
Reactivity via the indirect pathway was present in only eight of 18 samples during AR, in only three of 10 samples from early AR and in five of eight samples taken during late AR (Fig. 3). When the reactivity was detectable during AR (median: 35 IFN-γ pc/106 PBMC, range 5–165), it was significantly lower before AR (median: 3 IFN-γ pc/106 PBMC, range 0–15) (P = 0·008), but not after AR. In particular, we found an increased response via the indirect presentation pathway during late AR (median 65 IFN-γ pc/106 PBMC, range 15–165) compared to before AR (median: 10 IFN-γ pc/106 PBMC, range 0–15) (P = 0·06).
Fig. 3.
Number of interferon (IFN)-γ producing cells (pc) reactive to fragmented donor cells via the indirect presentation pathway (a) determined from peripheral blood mononuclear cells (PBMC) of heart transplant patients before, during and after a period of acute rejection (AR). (b) PBMC samples taken more than 6 months after transplantation.
Tetanus toxoid (TET) reactivity was present in a comparable number of patients in the early (12 of 27) and late (14 of 22) period after transplantation.
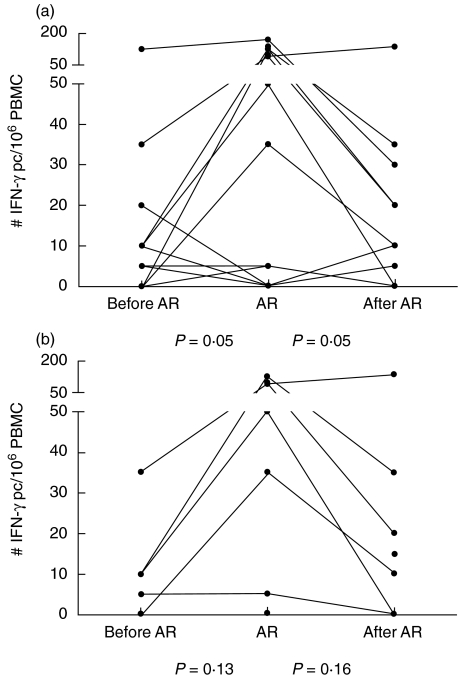
The number of IFN-γ pc reactive to TET increased during AR (median: 5 IFN-γ pc/106 PBMC, range 0–170) (P = 0·05) compared to before AR (median: 5 IFN-γ pc/106 PBMC, range 0–125) and decreased again after AR therapy (median: 3 IFN-γ pc/106 PBMC, range 0–135) (P = 0·05) (Fig. 4). The number of IFN-γ pc responsive to TET was comparable before, during late AR and after therapy.
Fig. 4.
Number of interferon (IFN)-γ producing cells (pc) reactive to tetanus toxoid (TET) via the indirect presentation pathway (a) determined from peripheral blood mononuclear cells (PBMC) of heart transplant patients before, during and after a period of acute rejection (AR). (b) PBMC samples taken more than 6 months after transplantation.
Discussion
T cells that recognize alloantigens are present in the circulation of transplant recipients before and during the onset of AR. These cells migrate into the graft and play a key role in initiating and sustaining AR by secreting cytokines and growth factors required for the and maturation proliferation of cytotoxic T cells. The ELISPOT technique represents a sensitive method for the detection of individual cytokine producing T cells allowing the quantification of antigen-specific responses of in vivo activated peripheral blood cells [26,27]. The ELISPOT assay determines the number of reactive cells and not the overall amount of cytokines produced, as in enzyme linked immunosorbent assay (ELISA). In the present study, we investigated whether the frequency of IFN-γ pc determined by ELISPOT assay is an effective tool to monitor contribution of the direct and indirect presentation pathways in relation to AR.
We demonstrated that the donor-specific T cell response via the direct presentation pathway is always detectable using the IFN-γ ELISPOT assay. During an AR episode this response increases significantly. Lechler's group also used the ELISPOT assay to determine alloreactivity via the direct presentation pathway. Long after transplantation, they demonstrated donor-specific hyporesponsiveness via the direct presentation pathway in 50% of the patients after heart transplantation [8] and in 77% of the patients after renal transplantation [9]. Hyporesponsiveness was defined as an anti-donor response lower than the third-party response. In the present study, we found that the anti-donor response in most patients via this pathway was higher than the third-party response (before AR in 80% of the patients, during AR in 72% and after AR in 69% of the patients); moreover, the anti-donor response was always present. The difference may be explained by the time after transplantation: we determined T cell reactivity in the first year after transplantation, while the other group [8,9] performed their tests 1–7 years after heart transplantation and 8–28 years after kidney transplantation. Heeger's group [28,29] also performed the IFN-γ ELISPOT assay to determine the reactivity to donor cells via the direct presentation pathway. They showed that the mean frequency of IFN-γ pc measured during the first 6 months after kidney transplantation was higher in patients with AR than in those without AR [29], but they did not distinguish early from late AR.
Donor APCs prime T cells through the direct pathway. Direct T cell priming is time-limited, as the donor APCs are destroyed. This may imply that the direct pathway might only mediate rejection early post-transplantation. However, initial high-frequency priming through the direct pathway may result in a residual population of donor-reactive memory T cells [30]. These memory T cells can be reactive, even in the absence of new direct priming, and may mediate late AR detected via direct allorecognition. This phenomenon could explain that we found a correlation with the direct allorecognition pathway not only during early AR, but also during late AR. It should be kept in mind that T cells in vivo primed via indirect presented alloantigens can induce memory T cells that can be reactive via the direct pathway in vitro.
In the present study, we have demonstrated that the ELISPOT assay is a useful tool to determine T cell alloreactivity not only via the direct but also via the indirect presentation pathway. We have shown that the number of PBMCs responding to donor antigens via the indirect pathway is significantly lower than the reactivity via the direct pathway. We also demonstrated that when alloreactivity via the indirect presentation pathway is present, this reactivity increases during AR. Other studies have also used the ELISPOT assay to determine the indirect pathway [31,32]. These groups used synthetic HLA-DR peptides that were matched for donor HLA-DR to monitor indirect alloreactivity among transplant recipients. The advantage of the present study is the use of whole splenocytes as a source of donor antigen; this results in the full repertoire of donor HLA epitopes, in contrast to the synthetic HLA-DR peptides used by most groups.
Some studies have demonstrated the presence of peptide immunodominance [10,11,32], with a shift of T cell responses toward different allopeptides with time. Our results could not be influenced by this process, termed ‘epitope shifting’ or ‘spreading’, because all different allopeptides are present in the donor splenocytes used.
Studies about indirect allorecognition are published mainly in relation to chronic rejection. The incidence of chronic rejection after heart transplantation was significantly higher in patients who continued to respond against donor HLA-DR peptides late after transplantation [11]. Lechler's group showed that donor reactivity, determined by the helper T lymphocyte frequency, via the indirect presentation pathway was low (< 12/106 IFN-γ pc/106 PBMC) in patients with chronic rejection after heart transplantation. This reactivity against frozen/thawed donor cells was always higher than that to third-party cells [15]. The same group demonstrated that after renal transplantation higher donor responses via the indirect pathway were found in patients with chronic allograft nephropathy than those without chronic allograft nephropathy [9]. Another group showed that when the direct and indirect pathways after kidney transplantation were detected, a correlation with AR and chronic rejection was found [6,16]. These investigators found a correlation with the presence of these pathways in two patients who developed AR in the first 2 months after transplantation and two patients who had late AR (20 and 36 months after transplantation). In the present study, a cohort of 18 AR periods were investigated. In patients with early AR only three of 10 (30%) PBMC samples showed a donor response via both the direct and indirect presentation pathway, while during late AR five of eight (62·5%) PBMC samples were reactive via both pathways.
In future, we will use our assay to monitor both the direct and indirect allogeneic presentation pathways in PBMC from patients with and without signs of chronic rejection after organ transplantation. T cells remaining in the circulation after treatment for AR that are responsive to donor antigens, presented via the indirect pathway, could be responsible for the development of chronic rejection in transplant recipients.
In conclusion, the ELISPOT assay is a valuable tool to determine the reactivity via both the direct and the indirect presentation pathways. The direct presentation pathway correlated with early and late AR, while the indirect pathway is associated especially with late AR.
References
- 1.Auchincloss H, Jr, Sultan H. Antigen processing and presentation in transplantation. Curr Opin Immunol. 1996;8:681–7. doi: 10.1016/s0952-7915(96)80086-0. [DOI] [PubMed] [Google Scholar]
- 2.Matis LA, Sorger SB, McElligott DL, Fink PJ, Hedrick SM. The molecular basis of alloreactivity in antigen-specific, major histocompatibility complex-restricted T cell clones. Cell. 1987;51:59–69. doi: 10.1016/0092-8674(87)90010-9. [DOI] [PubMed] [Google Scholar]
- 3.Lechler RI, Heaton T, Barber L, Bal V, Batchelor JR, Lombardi G. Molecular mimicry by major histocompatibility complex molecules and peptides accounts for some alloresponses. Immunol Lett. 1992;34:63–9. doi: 10.1016/0165-2478(92)90028-m. [DOI] [PubMed] [Google Scholar]
- 4.Lechler RI, Batchelor JR. Immunogenicity of retransplanted rat kidney allografts. Effect of inducing chimerism in the first recipient and quantitative studies on immunosuppression of the second recipient. J Exp Med. 1982;156:1835–41. doi: 10.1084/jem.156.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muluk SC, Clerici M, Via CS, Weir MR, Kimmel PL, Shearer GM. Correlation of in vitro CD4+ T helper cell function with clinical graft status in immunosuppressed kidney transplant recipients. Transplantation. 1991;52:284–91. doi: 10.1097/00007890-199108000-00019. [DOI] [PubMed] [Google Scholar]
- 7.van Besouw NM, Vaessen LMB, Knoop CJ, et al. Evidence that cyclosporin A prevents clinical cardiac allograft rejection by blocking both direct and indirect antigen presentation pathways. Transplant Int. 1996;9(Suppl. 1):S345–7. doi: 10.1007/978-3-662-00818-8_85. [DOI] [PubMed] [Google Scholar]
- 8.Hornick PI, Mason PD, Yacoub MH, Rose ML, Batchelor R, Lechler RI. Assessment of the contribution that direct allorecognition makes to the progression of chronic cardiac transplant rejection in humans. Circulation. 1998;97:1257–63. doi: 10.1161/01.cir.97.13.1257. [DOI] [PubMed] [Google Scholar]
- 9.Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Cook HT, Lechler RI. Loss of direct and maintenance of indirect alloresponses in renal allograft recipients: implications for the pathogenesis of chronic allograft nephropathy. J Immunol. 2001;167:7199–206. doi: 10.4049/jimmunol.167.12.7199. [DOI] [PubMed] [Google Scholar]
- 10.Vella JP, Spadafora-Ferreira M, Murphy B, et al. Indirect allorecognition of major histocompatibility complex allopeptides in human renal transplant recipients with chronic graft dysfunction. Transplantation. 1997;64:795–800. doi: 10.1097/00007890-199709270-00001. [DOI] [PubMed] [Google Scholar]
- 11.Ciubotariu R, Liu Z, Colovai AI, et al. Persistent allopeptide reactivity and epitope spreading in chronic rejection of organ allografts. J Clin Invest. 1998;101:398–405. doi: 10.1172/JCI1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SivaSai KS, Smith MA, Poindexter NJ, et al. Indirect recognition of donor HLA class I peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Transplantation. 1999;67:1094–8. doi: 10.1097/00007890-199904270-00002. [DOI] [PubMed] [Google Scholar]
- 13.Reznik SI, Jaramillo A, SivaSai KS, et al. Indirect allorecognition of mismatched donor HLA class II peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Am J Transplant. 2001;1:228–35. doi: 10.1034/j.1600-6143.2001.001003228.x. [DOI] [PubMed] [Google Scholar]
- 14.Stanford RE, Ahmed S, Hodson M, Banner NR, Rose ML. A role for indirect allorecognition in lung transplant recipients with obliterative bronchiolitis. Am J Transplant. 2003;3:736–42. doi: 10.1034/j.1600-6143.2003.00142.x. [DOI] [PubMed] [Google Scholar]
- 15.Hornick PI, Mason PD, Baker RJ, et al. Significant frequencies of T cells with indirect anti-donor specificity in heart graft recipients with chronic rejection. Circulation. 2000;101:2405–10. doi: 10.1161/01.cir.101.20.2405. [DOI] [PubMed] [Google Scholar]
- 16.Schulick RD, Weir MB, Miller MW, Cohen DJ, Bermas BL, Shearer GM. Longitudinal study of in vitro CD4+ T helper cell function in recently transplanted renal allograft patients undergoing tapering of their immunosuppressive drugs. Transplantation. 1993;56:590–6. [PubMed] [Google Scholar]
- 17.Liu Z, Sun YK, Xi YP, et al. Contribution of direct and indirect recognition pathways to T cell alloreactivity. J Exp Med. 1993;177:1643–50. doi: 10.1084/jem.177.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Colovai AI, Tugulea S, et al. Indirect recognition of donor HLA-DR peptides in organ allograft rejection. J Clin Invest. 1996;98:1150–7. doi: 10.1172/JCI118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molajoni ER, Cinti P, Orlandini A, et al. Mechanism of liver allograft rejection: the indirect recognition pathway. Hum Immunol. 1997;53:57–63. doi: 10.1016/S0198-8859(97)00029-3. [DOI] [PubMed] [Google Scholar]
- 20.van Besouw NM, Vaessen LM, Daane CR, et al. Peripheral monitoring of direct and indirect alloantigen presentation pathways in clinical heart transplant recipients. Transplantation. 1996;61:165–7. doi: 10.1097/00007890-199601150-00033. [DOI] [PubMed] [Google Scholar]
- 21.Meiser BM, Groetzner J, Kaczmarek I, et al. Tacrolimus or cyclosporine: which is the better partner for mycophenolate mofetil in heart transplant recipients? Transplantation. 2004;78:591–8. doi: 10.1097/01.tp.0000129814.52456.25. [DOI] [PubMed] [Google Scholar]
- 22.Balk AH, Meeter K, Simoons ML, et al. Polyclonal versus monoclonal rejection prophylaxis after heart transplantation: a randomised study. Transplant Int. 1992;5(Suppl. 1):S476–9. doi: 10.1007/978-3-642-77423-2_139. [DOI] [PubMed] [Google Scholar]
- 23.Billingham ME, Cary NR, Hammond ME, et al. The International Society for Heart Transplantation. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. J Heart Transplant. 1990;9:587–93. [PubMed] [Google Scholar]
- 24.van Besouw NM, van der Mast BJ, de Kuiper P, et al. Donor-specific T cell reactivity identifies kidney transplant patients in whom immunosuppressive therapy can be safely reduced. Transplantation. 2000;70:136–43. [PubMed] [Google Scholar]
- 25.van Besouw NM, Vaessen LMB, Zuijderwijk J, et al. The frequency of IFN-γ producing cells reflects alloreactivity against minor histocompatibility antigens. Transplantation. 2003;75:1400–4. doi: 10.1097/01.TP.0000064376.78084.50. [DOI] [PubMed] [Google Scholar]
- 26.Heeger PS, Greenspan NS, Kuhlenschmidt S, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–75. [PubMed] [Google Scholar]
- 27.Tary-Lehmann M, Hricik DE, Justice AC, Potter NS, Heeger PS. Enzyme-linked immunosorbent assay spot detection of interferon-gamma and interleukin 5-producing cells as a predictive marker for renal allograft failure. Transplantation. 1998;66:219–24. doi: 10.1097/00007890-199807270-00014. [DOI] [PubMed] [Google Scholar]
- 28.Gebauer BS, Hricik DE, Atallah A, et al. Evolution of the enzyme-linked immunosorbent spot assay for post-transplant alloreactivity as a potentially useful immune monitoring tool. Am J Transplant. 2002;2:857–66. doi: 10.1034/j.1600-6143.2002.20908.x. [DOI] [PubMed] [Google Scholar]
- 29.Hricik DE, Rodriguez V, Riley J, et al. Enzyme linked immunosorbent spot (ELISPOT) assay for interferon-gamma independently predicts renal function in kidney transplant recipients. Am J Transplant. 2003;3:878–84. doi: 10.1034/j.1600-6143.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169:4667–73. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 31.Salama AD, Najafian N, Clarkson MR, Harmon WE, Sayegh MH. Regulatory CD25+ T cells in human kidney transplant recipients. J Am Soc Nephrol. 2003;14:1643–51. doi: 10.1097/01.asn.0000057540.98231.c1. [DOI] [PubMed] [Google Scholar]
- 32.Najafian N, Salama AD, Fedoseyeva EV, Benichou G, Sayegh MH. Enzyme-linked immunosorbent spot assay analysis of peripheral blood lymphocyte reactivity to donor HLA-DR peptides: potential novel assay for prediction of outcomes for renal transplant recipients. J Am Soc Nephrol. 2002;13:252–9. doi: 10.1681/ASN.V131252. [DOI] [PubMed] [Google Scholar]