Abstract
Human immunodeficiency virus (HIV)-1 infection influences natural killer (NK) cell expression of inhibitory NK receptors and activating natural cytotoxicity receptors. It is unknown whether expression of the co-stimulatory NK cell receptor 2B4 (CD244) on NK cells and CD3+ CD8+ cells are affected by highly active antiretroviral therapy (HAART), low-level viraemia, proviral-DNA or immune activation in HIV-1 infected patients. A total of 101 HAART-treated HIV-1 infected patients with ≤ 200 HIV-RNA copies/ml were followed prospectively for 24 months. HIV-RNA was investigated 3-monthly and 2B4 expression on CD3− CD16+ NK cells and CD3+ CD8+ cells, proviral-DNA and plasma soluble tumour necrosis factor receptor (sTNFr)-II were investigated 6-monthly. For comparison, 2B4 expression was investigated in 20 healthy individuals. The concentration of 2B4+ NK cells was initially reduced in HIV-1 infected patients (P < 0·001) but increased to a normal level during the 24 months’ follow-up. The concentration of CD3+ CD8+ 2B4+ cells in HIV-1 infected patients was normal and did not change during follow-up. The relative fluorescence intensity (RFI) of 2B4 increased on both NK cells and CD3+ CD8+ cells during follow-up (both P < 0·001). Higher levels of proviral-DNA carrying cells and plasma sTNFrII were associated with reductions in the concentration of 2B4+ NK cells (all P < 0·05). HIV-RNA had no effect on 2B4 expression on NK cells or CD3+ CD8+ cells. These findings demonstrate that the concentration of 2B4+ NK cells normalizes during long-term HAART in HIV-1 infected patients. The finding that proviral-DNA and sTNFrII were associated negatively with the concentration of 2B4+ NK cells suggests that immune activation in HIV-1 infected patients receiving HAART influences the target cell recognition by NK cells.
Keywords: 2B4, CTL, HAART, HIV-1, NK cells
Introduction
Many viruses, including human immunodeficiency virus (HIV), interfere with major histocompatibility complex (MHC)-I expression to avoid MHC-restricted cytotoxicity [1,2]. Natural killer (NK) cells recognize and kill cells with an absent or insufficient MHC-I expression through opposite signals delivered by MHC-I-specific inhibitory NK receptors (iNKRs) and activating natural cytotoxicity receptors (NCRs) [3,4]. It has also been suggested that molecules expressed by ‘stressed’ cells (e.g. virus infected cells) may serve as ligands for certain NCRs [3].
HIV-1 infection is characterized by profound and inappropriate immune activation and progressive immunodeficiency [5–11], which includes impaired function of NK cells [1,12] and CD8 cells [13]. Untreated HIV-1 infection leads to increased NK cell expression of iNKRs [14–16], decreased NK cell expression of NCRs [14,17] and a pronounced expansion of activated CD8+ T cells (CD8 cells) [5, 8, 9, 18].
Although highly active antiretroviral therapy (HAART) normalizes some of the perturbations within the NK cell subset [14–16] and reduces the level of activated CD8 cells [19], the level of activated CD8 cells remains above normal despite years of virological suppression [20,21].
The 2B4 antigen (CD244, p38, C1·7) is expressed by all NK cells, by half of the CD8 cells [22], by γδ T cells, monocytes, basophils, thymocytes and by a minor proportion of CD4+ T cells (CD4 cells) [23–25]. In NK cells, 2B4 function as a co-receptor that enhances synergistically NCR-mediated cytotoxicity [26]. In CD8 cells, the function of 2B4 is less well understood [23–25] but 2B4 may be an activation antigen, induced by cell stimulation and coinciding with CD29 expression [27]. Furthermore, 2B4 seems to be involved in non-MHC restricted cytotoxicity [22]. In accordance with 2B4 being an activation antigen on CD8 cells, the level of 2B4+ CD8 cells is increased in HIV-1 infected patients and 2B4+ CD8 cells correlate negatively with CD4 count [27]. Furthermore, high levels of 2B4+ CD8 cells are associated with faster clinical HIV disease progression [27].
Immune activation and HIV disease progression are associated intimately, as the main driver of CD4 cell depletion probably is immune activation [10,11]. It has been demonstrated that immune activation impedes CD4 gain in patients receiving HAART [28,29], but it is not known whether immune activation affects 2B4 expression on NK cells and CD8 cells in HIV-1 infected patients receiving HAART.
The rationale behind the present study was to investigate 2B4 expression on NK cells and CD8 cells in HIV-1 infected patients followed prospectively during HAART. To assess the association between immune activation and 2B4 expression, it was investigated whether a higher level of HIV-RNA, proviral-DNA or plasma soluble tumour necrosis factor receptor (sTNFrII)-II was associated with changes in the concentration or expression level of 2B4 on NK cells and CD8 cells. The study was initiated in 1997 from the expectation that a substantial proportion of the patients would experience treatment failure and it was hypothesized that 2B4 expression would differ between patients with or without viraemia. However, as most patients maintained good virological control during follow-up, the data obtained from this cohort were used to investigate the effect of low-level viraemia on 2B4 expression in patients receiving HAART.
Finally, 2B4 expression on NK cells and CD8 cells in HIV-1 infected patients followed prospectively during HAART was compared with that in healthy individuals.
Materials and methods
Study population
The present study was carried out at the Department of Infectious Diseases, Rigshospitalet, Copenhagen, Denmark as described previously [30]. In the period September 1997—August 1998, 101 patients with reproducible plasma HIV-RNA ≤ 200 copies/ml were included and followed prospectively for 24 months (Table 1). Plasma HIV-RNA, CD4 count and CD8 count were analysed 3-monthly, whereas 2B4 expression on NK cells and CD8 cells, proviral-DNA and plasma sTNFrII were analysed 6-monthly. Baseline data and antiretroviral treatment history were extracted from patient files. Written informed consent was obtained from all subjects and the study was approved by the Local Ethical Committee for Copenhagen and Frederiksberg Communities, Denmark (project no: 01–192/97). For comparison, 20 age- and gender-matched HIV-seronegative healthy individuals were included [18 males/two females, median age 43 years (range 31–59)]. Data from the cohort have been published previously [29,30].
Table 1. Baseline and preinclusion characteristics of all 101 HIV-1 infected patients and of patients stratified according to HIV-RNA in the study period.
| Patients stratified according to HIV-RNA in the study period | ||||
|---|---|---|---|---|
| All patients (n = 101) | uVL-patientsa (n = 33) | dVL-patientsb (n = 68) | P-valuec | |
| Patient characteristics at inclusion in the study | ||||
| Age (years)d | 44 (25–67) | 44 (24–65) | 45 (29–67) | n.s |
| Gender (M/F) | 93/8 | 30/3 | 63/5 | n.s |
| AIDS-diagnoses | 25 (25%) | 8 (24%) | 17 (25%) | n.s |
| Months HAARTd | 14 (4–32) | 14 (4–31) | 14 (5–32) | n.s. |
| Months HIV-RNA ≤ 200 copies/mld | 9 (0–24) | 9 (0–24) | 6 (0–18) | n.s |
| CD4 count (cells/µl)d | 330 (55–880) | 330 (89–880) | 335 (55–810) | n.s |
| CD8 count (cells/µl)d | 920 (110–2500) | 800 (110–2100) | 1000 (200–2500) | 0·023 |
| Patient characteristics at initiation of HAART prior to inclusion in the study | ||||
| Years HIV-1 antibody positived | 6 (0–16) | 9 (0–13) | 6 (0–16) | n.s |
| Pre-HAART CD4 count (cells/µl)d | 170 (1–480) | 180 (1–480) | 170 (1–440) | n.s. |
| Pre-HAART CD8 count (cells/µl)d | 710 (110–3800) | 580 (110–1700) | 740 (110–3800) | 0·048 |
| Pre-HAART HIV-RNA (copies/ml)d | 65 722 (≤ 20–1, 632, 104) | 22 764 (≤ 20–1, 293, 627) | 95 458 (≤ 20–1, 632, 104) | n.s |
a–b
The patients were stratified into two groups according to the HIV-RNA level during the 24 months follow-up: 33 patients had undetectable HIV-RNA at all time-points (uVL patients)
whereas 68 patients had ≥ 1 episode with detectable viraemia (dVL patients)
The patient groups were compared with two-sample t-test or χ2/Fisher's exact test. P-values < 0·05 are shown in bold type and P-values > 0·15 are not shown.
Median (range).
Number of patients (percentage) within the present group. n.s., non-significant. M, male. F, female.
Plasma HIV-1 RNA and proviral-DNA/106 peripheral blood mononuclear cells (PBMC)
Plasma HIV-1 RNA (HIV-RNA) was quantified by a standardized reverse transcriptase-polymerase chain reaction assay (RT-PCR) (Amplicor HIV-1 Monitor, Roche Diagnostic Systems Inc., Branchburg, NJ, USA). Some baseline samples were analysed with a standard assay [lower limit of detection (LLD) 200 copies/ml], whereas all later samples were analysed with an ultrasensitive assay (LLD 20 copies/ml). Samples analysed by the standard assay were re-analysed by the ultrasensitive assay. Samples yielding a signal ≥ twice that of the mean of numerous negative controls were recorded as detectable but non-quantifiable (D-NQ) while samples with weaker signals were recorded as undetectable (UD). For statistical analysis, D-NQ and UD samples were given values of 20 and 19 copies/ml, respectively [30]. Proviral HIV DNA copies/106 PBMC (proviral-DNA) were quantified with a prototype assay (Amplicor HIV DNA assay, Roche Diagnostic Systems Inc., Branchburg, NJ, USA) according to the manufacturer's recommendations, as described previously [30].
Plasma concentration of sTNFrII
The plasma concentration (pg/ml) of sTNFrII (type-a, 75 kDa) was measured in duplicate by a commercially available double-sandwich enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA). The LLD, intra-assay and interassay coefficient of variation were <1·0 pg/ml, 2·2% and 4·1%, respectively.
Flow cytometric analysis
In the patients, the total concentration of CD4 cells and CD8 cells ( × 106/l) were quantified by BD Tritest™ CD4/CD8/CD3 TruCount Tubes (Becton Dickinson, Franklin Lakes, NJ, USA). In patients and healthy individuals, 2B4 expression on NK cells and CD8 cells was analysed on fresh whole blood by direct cell surface immunofluorescent staining using: anti-CD45, -CD14, -CD3, -CD8, -C1·7 (IM 1607, anti2B4), IgG1 (all Beckman Coulter Inc., Fullerton, CA, USA) and anti-CD16 and IgG1 (all Dako, Glostrup, Denmark). Labelled cells were analysed by an Epics XL-MCL flow cytometer (Beckman Coulter) and the subsequent computer analyses were performed with winlist 4·0 (Verity Software House, Inc., Topsham, ME, USA). The combination of labelled antibodies used was (fluorescein isothiocyanate/phycoerythrin/R-phycoerythrin-cyanin-5·1/phycoerythrin-texas red) CD45/CD14/−/− and CD16/2B4/CD8/CD3 in the patients and fluorescein isothiocyanate/phycoerythrin/R-phycoerythrin-cyanin-5·1) CD45/CD14/−, CD16/2B4/CD3 and CD8/2B4/CD3 in the healthy individuals.
Lymphocyte gates in forward/right-angle light scatter with <3% CD14+ monocytes were used in all the analyses. The absolute cell concentration (106/l) was calculated by multiplying the proportion with the concentration of lymphocytes, obtained from haematology analysis. The relative fluorescence intensity (RFI) of 2B4 expression on CD3−CD16+ 2B4+ NK cells and CD3+ CD8+ 2B4+ T cells was calculated as a ratio of the mean intensity of 2B4 on the cells divided by the mean intensity of the negative control antibody.
Statistics
Repeated-measures analyses for each investigated variable were performed using a means model (sas proc mixed), with the time-scale being months from initiation of HAART (Fig. 1). A group- and time × group effect was included in the model to investigate differences in longitudinal changes between patients with or without detectable HIV-RNA during follow-up. Goodness-of-fit of the means model was assessed by investigating the residuals. Due to the low number of subjects outside the period 12–36 months after initiation of HAART, the longitudinal changes in NK cells, CD8 cells, proviral-DNA and plasma sTNFrII were investigated only in this period. Specific changes over time were analysed by Bonferroni adjusted post hoc paired t-tests. Baseline variables in patients with or without detectable HIV-RNA during follow-up were compared by two-sample t-test or χ2/Fisher's exact test (Table 1).
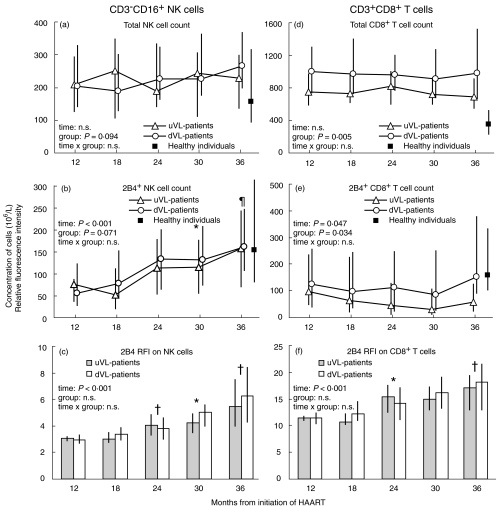
Fig. 1.
The total concentration of CD3−CD16+ natural killer (NK) cells and CD3+ CD8+ T cells and the concentration and expression level of 2B4 on NK cells and CD8 cells in healthy individuals (black squares) and in HIV-1 infected patients stratified according to plasma HIV-RNA in the study period: 33 patients had HIV-RNA ≤ 20 copies/ml at all visits (uVL patients, triangles, grey bars) whereas 68 patients had HIV-RNA > 20 copies/ml at ≥ 1 visit (dVL patients, circles, white bars). Data are shown for the period 12–36 months after initiation of highly active antiretroviral therapy (HAART) due to the low number of subjects contributing to the analysis outside this period. The relative fluorescence intensity (RFI) of 2B4 was calculated as a ratio of the mean intensity of 2B4 on the cells divided by the mean intensity of the negative control. The median concentration (106 cells/l) and RFI are shown with interquartile ranges for (a) total concentration of CD3−CD16+ NK cells, (b) concentration of CD3−CD16+ 2B4+ NK cells, (c) 2B4 RFI on CD3−CD16+ NK cells (d) total concentration of CD3+ CD8+ T cells, (e) concentration of CD3+ CD8+ 2B4+ T cells and (f) 2B4 RFI on CD3+ CD8+ T cells. P-values from time-, group- and time × group effect in the mixed repeated models are shown. Significant differences between time-point 12 months and the following time-points were analysed for all patients (combining uVL and dVL patients as time × group effects were non-significant) by Bonferroni adjusted post hoc paired t-test if the time-effect was significant: *P < 0·05, ¶P < 0·01, †P < 0·001.
A random effects model (sas proc mixed) assuming a variance component covariance structure and with random effects between subjects levels [31] was used to investigate the statistical association between the level of an explanatory variable and the dependent variable during follow-up. From each patient all five time-points, including missing values, contributed to the analysis. By this model it was investigated whether a higher level of HIV-RNA, proviral-DNA or plasma sTNFrII during follow-up was associated with changes in 2B4 expression on NK cells and CD8 cells during follow-up (Table 2). Results are presented as the mean relative change in the concentration or expression-level of 2B4 on the investigated cells during follow-up associated with 10-fold higher HIV-RNA or twofold higher proviral-DNA or plasma sTNFrII. All results are presented with 95% confidence intervals (CI). Goodness-of-fit was assessed through residual plots against predicted values, simultaneously for all data as well as separately for each subject.
Table 2. Associations between a higher level of HIV-RNA, proviral-DNA or plasma sTNFrII and the concentration and expression level of 2B4 on CD3−CD16+ NK cells and CD3+ CD8+ T cells in 101 HAART-treated HIV-1 infected patients followed prospectively for 24 months.
| Higher HIV-RNAa | Higher proviral-DNAb | Higher plasma sTNFrIIc | ||||
|---|---|---|---|---|---|---|
| Mean (95% CI)d | Pe | Mean (95% CI)d | Pe | Mean (95% CI)d | Pe | |
| NK cells | ||||||
| Total NK cellsf | 0·92 (0·83–1·02) | 0·110 | 0·98 (0·93–1·03) | n.s. | 0·85 (0·74–0·98) | 0·029 |
| 2B4+ NK cellsf | 1·01 (0·84–1·22) | n.s. | 0·91 (0·84–1·00) | 0·041 | 0·63 (0·49–0·80) | <0·001 |
| 2B4 RFI on NK cellsg | 1·04 (0·99–1·11) | 0·132 | 0·99 (0·96–1·01) | n.s. | 0·94 (0·88–1·00) | 0·063 |
| CD8 cells | ||||||
| Total CD8 cellsf | 0·94 (0·89–0·98) | 0·010 | 1·03 (1·00–1·06) | 0·023 | 1·02 (0·94–1·10) | n.s. |
| 2B4+ CD8 cellsf | 1·09 (0·84–1·41) | n.s. | 0·98 (0·86–1·10) | n.s. | 0·90 (0·65–1·25) | n.s. |
| 2B4 RFI on CD8 cellsg | 1·05 (0·99–1·11) | 0·095 | 0·99 (0·97–1·02) | n.s. | 0·95 (0·89–1·01) | 0·112 |
A random effects model (sas proc mixed) was used to estimate the statistical association between a higher level of HIV-RNA (10-fold higher)
proviral-DNA (twofold higher)
or plasma sTNFrII (twofold higher)
during follow-up and the mean change in the concentration or expression level of 2B4 on NK cells and CD8 cells during follow-up.
Results from the model are presented as the mean relative change [95% confidence intervals (CI)] in the concentration
or relative fluorescence intensity (RFI)
a–c of 2B4 on the investigated cells during follow-up associated with a higher level of HIV-RNA, proviral-DNA or plasma sTNFrII. The interpretation of a relative change <1, >1 or = 1 is a relative reduction, increase or unchanged, respectively, mean change in the investigated variables during follow-up.
P-values <0·05 are shown in bold type and P-values >0·15 are not shown (n.s., non-significant).
2B4 expression on NK cells and CD8 cells in HIV-1 infected patients at 12 months after initiation of HAART was compared with that in healthy individuals by two-sample t-test. If 2B4 expression differed significantly at 12 months, 2B4 expression in the patients at 36 months after initiation of HAART was compared with that in healthy individuals by two-sample t-test.
Data are presented as medians with ranges or interquartile ranges (IQR). P < 0·05 is considered significant. Statistical calculations were performed using sas 8·2 (SAS Institute Inc., Cary, NC, USA).
Results
HIV-1 infected patients
A total of 101 HAART-treated patients who both fulfilled the HIV-RNA inclusion criteria and had available flow cytometric data were included (Table 1), as described previously [29,30]. The patients entered the study varying times from initiation of HAART (median 14 months (range 4–32)) (Table 1), due to the HIV-RNA inclusion criteria. The number of patient samples at time-points 6, 12, 18, 24, 30, 36, 42, 48 and 54 months after initiation of HAART was 15, 65, 90, 93, 97, 82, 32, 10 and 6 [29].
The patients were stratified according to HIV-RNA during follow-up: 33 patients had HIV-RNA ≤ 20 copies/ml at all visits (uVL patients) whereas 68 patients had HIV-RNA > 20 copies/ml at ≥ 1 visit (dVL-patients) (Table 1). The median increase in HIV-RNA at time-points with virological rebound in dVL patients was 81 (IQR 37–480) HIV-RNA copies/ml.
Except from a higher CD8 count in dVL patients at initiation of HAART and at inclusion in the study (Table 1), the study groups had comparable changes in CD4 count and CD8 count the first 36 months after initiation of HAART [29].
The initial antiretroviral therapy prior to HAART consisted of one, two, three or four drugs in 38, 24, 34 and five patients [30]. At inclusion, most patients received protease inhibitor (PI)-based therapy with three (n = 82) or four drugs (n = 18) and six patients also received a non-nucleoside reverse transcriptase inhibitor (NNRTI). No patients discontinued HAART during follow-up but 37 patients had their antiretroviral treatment modified, either to another NRTI or PI combination or to include a NNRTI. In the vast majority of patients, the modification of therapy was due to adverse effects rather than treatment failure. The patients still contributed to the statistical analyses after modification of HAART. The uVL patients and dVL patients had comparable treatment history and treatment regimens during follow-up [29].
2B4 expression on NK cells and CD8 cells
NK cells
The total concentration of CD3−CD16+ NK cells did not change in the patients during follow-up (Fig. 1a), whereas the concentration of 2B4+ NK cells and 2B4 RFI on NK cells increased (Fig. 1b, c).
The total concentration of NK cells did not differ between patients and healthy individuals (Fig. 1a), whereas the concentration of 2B4+ NK cells was reduced in the patients at 12 months after initiation of HAART (P < 0·001) but increased to a level comparable to that in healthy individuals at 36 months after initiation of HAART (Fig. 1b). In addition, 2B4 RFI was reduced markedly in the patients (Fig. 1c) compared with that in healthy individuals [median 48 (IQR 38–70) RFI, P < 0·001].
Neither the concentration of NK cells nor 2B4 RFI on NK cells differed between uVL patients and dVL patients (Fig. 1a–c).
CD8 cells
The total concentration of CD8 cells and 2B4+ CD8 cells did not change in the study period but were higher in dVL patients (Fig. 1d, e). Despite an unchanged concentration, 2B4 RFI on CD8 cells increased in both uVL patients and dVL patients (Fig. 1f).
The total concentration of CD8 cells was increased in the patients (P < 0·001) (Fig. 1d), whereas the concentration of 2B4+ CD8 cells was comparable to that in healthy individuals (Fig. 1e). 2B4 RFI on CD8 cells was reduced markedly in the patients (Fig. 1f) compared with that in healthy individuals [median 32 (IQR 23–41) RFI, P < 0·001].
HIV-RNA, proviral-DNA or plasma sTNFrII and 2B4 expression on NK cells and CD8 cells
In the study period, HIV-RNA and proviral-DNA were higher in dVL patients (group effect P < 0·001 and P = 0·003, respectively) and HIV-RNA increased in dVL patients (P < 0·001), whereas proviral-DNA was unchanged in both groups [29]. A higher HIV-RNA during follow-up was not associated with changes in the concentration of 2B4+ cells but it was associated with a reduction in the concentration of total CD8 cells (Table 2). In contrast, a higher proviral-DNA was associated with a reduction in the concentration of 2B4+ NK cells and an increase in the total concentration of CD8 cells (Table 2). Neither HIV-RNA nor proviral-DNA was associated with changes in 2B4 RFI on NK cells or CD8 cells (Table 2).
The plasma level of sTNFrII was comparable in the groups and it did not change in the study period (data not shown). A higher plasma sTNFrII was associated with reductions in the total concentration of CD3−CD16+ NK cells and 2B4+ NK cells and with marginally reductions in 2B4 RFI on NK cells (Table 2). Plasma sTNFrII was not associated with changes in the CD8 cell subset (Table 2).
Discussion
This study investigated 2B4 expression on NK cells and CD8 cells in 101 HIV-1 infected patients followed prospectively for 24 months during HAART. The main finding was that the concentration of 2B4+ NK cells was reduced in HIV-1 infected patients at 12 months after initiation of HAART but that it increased to a level comparable to that in healthy individuals after 36 months of HAART. The concentration of 2B4+ CD8 cells did not change from 12 to 36 months after initiation of HAART and was comparable to that in healthy individuals. Finally, higher levels of proviral-DNA and plasma sTNFrII in HIV-1 infected patients were associated with reductions in the concentration of 2B4+ NK cells whereas higher HIV-RNA, proviral-DNA or plasma sTNFrII had no effect on the concentration or expression level of 2B4 on CD8 cells.
In the present study, the concentration and expression level of 2B4 on NK cells was reduced in HIV-1 infected patients at 12 months after initiation of HAART. In the period from 12 to 36 months after initiation of HAART, 2B4 expression on NK cells, however, increased in the patients, resulting in a normalization of the 2B4+ NK cell concentration at 36 months. The increase in concentration and expression level of 2B4 on NK cells was comparable in patients with or without detectable low-level viraemia and a higher level of HIV-RNA was not associated with changes in 2B4 expression on NK cells. As most patients with detectable viraemia had intermittent low-level viraemia, these data indicate that low-level viraemia in HAART-treated HIV-1 infected patients has no detectable effect on 2B4 expression on NK cells.
It has been demonstrated recently that the proportion of 2B4+ NK cells was comparable in healthy donors, untreated viraemic and HAART-treated aviraemic HIV-1 infected patients [14,15]. Because the aviremic HIV-1 infected patients had received HAART for at least 24 months in the study by Mavilio et al. [14], the finding of a normalized 2B4 expression on NK cells during years of prospective follow-up of HAART-treated HIV-1 infected patients in our study is in accordance with the findings in the former study [14].
In vitro stimulation of 2B4 on NK cells with an anti-2B4 antibody induces Ca2+ signalling and polyphosphoinositol turnover and stimulates cytokine production [22]. Various cells express the ligand for 2B4, CD48 [23–25] and CD48 expression is up-regulated by viral infections and interferons probably to enhance NK cell cytotoxicity upon target cell recognition [25]. As 2B4 expression seems important for NK cell function [22, 25, 26], it is tempting to speculate that our finding of an increased 2B4 expression on NK cells in patients receiving HAART may be associated with improved NK cell cytotoxicity.
The concentration of 2B4+ CD8 cells in HIV-1 infected patients was comparable to that in healthy individuals and did not change during follow-up. In contrast, 2B4 RFI on CD8 cells was reduced in the patients, and despite an increase during follow-up it did not reach a level comparable to that in healthy individuals. It should be noted that as 2B4 RFI on NK cells and CD8 cells was analysed by four- and three-colour immunofluorescent staining in patients and healthy individuals, respectively, these might be difficult to compare. It is, however, notable that 2B4 RFI on NK cells was higher than 2B4 RFI on CD8 cells in healthy individuals whereas 2B4 RFI on NK cells was lower than 2B4 RFI on CD8 cells in HIV-1 infected patients.
The concentration of 2B4+ CD8 cells (and the total concentration of CD8 cells) was higher in patients with detectable viraemia, although a higher level of HIV-RNA was associated with a reduction in the total CD8 count. It has been demonstrated recently that some immune functions differ between time-points with or without detectable viraemia in patients with intermittent low-level viraemia [32]. Thus, the divergent findings between viraemia and CD8 count could be attributed to a disparate association between the total CD8 count and time-points with detectable viraemia (modelled by the random effects model) and periods with intermittent low-level viraemia (modelled by the repeated means model).
It has been demonstrated that the level of viraemia in HIV-1 infected patients correlates positively with the level of iNKR and CCR5-expressing NK cells [15] and negatively with NK cell production of CC-chemokines [33]. In addition to viraemia, immune activation may also influence NK cell function and phenotype [15].
In this study, higher levels of proviral-DNA and plasma sTNFrII were both associated with reductions in the concentration of 2B4+ NK cells.
Cell-associated proviral-HIV-DNA comprises integrated and unintegrated genomes, of which the majority represent defective genomes [34]. One previous study has detected proviral-DNA in purified NK cells from aviraemic HAART-treated patients [35]. Consequently, preferential infection of 2B4− NK cell subsets or down-regulation of 2B4 in HIV-1 infected NK cells could, hypothetically, explain the association between high proviral-DNA and 2B4− NK cells. A later study, however, failed to confirm the presence of proviral-DNA in NK cells [14]. Consequently, expression of HIV-1 products by proviral-DNA-carrying cells could alternatively influence 2B4 expression on NK cells indirectly through induction of immune activation. This notion is in line with the association between plasma sTNFrII and 2B4− NK cells.
The circulating level of sTNFrII originates mainly from activated T cells, B cells and neutrophils [36]. As sTNFrII shedding is induced by cellular activation and by many proinflammatory cytokines, high circulating sTNFrII levels reflect non-specific immune activation [36]. The finding of an association between high plasma sTNFrII and 2B4− NK cells thus suggests that the non-specific immune activation that characterizes HIV-1 infection may influence 2B4 expression on NK cells and, theoretically, the function of NK cells. Consequently, it is tempting to speculate that the decline in plasma sTNFrII (and thus non-specific immune activation) in HIV-1 infected patients initiating HAART [37] may explain in part the concomitant increase in the concentration of 2B4+ NK cells.
In contrast to 2B4 expression on NK cells, proviral-DNA and plasma sTNFrII was not associated with 2B4 expression on CD8 cells. The finding that a higher level of proviral-DNA was associated with an increase in the total CD8 count could indicate that expression of HIV-1 products by proviral-DNA carrying cells induces activation and expansion of CD8 cells.
In summary, this study found that the concentration of 2B4+ NK cells was reduced in HIV-1 infected patients at 12 months after initiation of HAART but increased to a level comparable to that in healthy individuals after 36 months of HAART. Higher levels of proviral-DNA and plasma sTNFrII were both associated with reductions in the concentration of 2B4+ NK cells suggesting that proviral-DNA carrying cells and non-specific immune activation influence the target cell recognition by NK cells in patients receiving HAART.
Acknowledgments
We thank the patients for their participation in this study. Thomas Scheike MSc, PhD, DSci, Department of Biostatistics, University of Copenhagen, Denmark, is thanked for his invaluable help with the statistical models. Gitte Grauert, laboratory technician, is acknowledged for help with the flow cytometric analyses. The Danish AIDS Foundation provided grant support to the study.
References
- 1.Scott-Algara D, Paul P. NK cells and HIV infection: lessons from other viruses. Curr Mol Med. 2002;2:757–68. doi: 10.2174/1566524023361781. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–42. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 3.Moretta A, Bottino C, Mingari MC, Biassoni R, Moretta L. What is a natural killer cell? Nat Immunol. 2002;3:6–8. doi: 10.1046/j.1365-3083.2002.01055.x. [DOI] [PubMed] [Google Scholar]
- 4.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–9. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miedema F. Immunological abnormalities in the natural history of HIV infection: mechanisms and clinical relevance. Immunodefic Rev. 1992;3:173–93. [PubMed] [Google Scholar]
- 6.Fahey JL, Taylor JM, Detels R, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–72. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 7.Fahey JL, Taylor JM, Manna B, et al. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS. 1998;12:1581–90. doi: 10.1097/00002030-199813000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Haynes BF, Pantaleo G, Fauci AS. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–8. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson M. HIV-1 pathogenesis. Nat Med. 2003;9:853–60. doi: 10.1038/nm0703-853. [DOI] [PubMed] [Google Scholar]
- 10.Grossman Z, Meier-Schellersheim M, Sousa AE, Victorino RM, Paul WE. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8:319–23. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- 11.Deeks SG, Walker BD. The immune response to AIDS virus infection: good, bad, or both? J Clin Invest. 2004;113:808–10. doi: 10.1172/JCI21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ullum H, Gøtzsche PC, Victor J, Dickmeiss E, Skinhoj P, Pedersen BK. Defective natural immunity. an early manifestation of human immune defiency virus infection. J Exp Med. 1995;182:789–99. doi: 10.1084/jem.182.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieberman J, Shankar P, Manjunath N, Andersson J. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood. 2001;98:1667–77. doi: 10.1182/blood.v98.6.1667. [DOI] [PubMed] [Google Scholar]
- 14.Mavilio D, Benjamin J, Daucher M, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viraemia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci USA. 2003;100:15011–6. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kottilil S, Shin K, Planta M, et al. Expression of chemokine and inhibitory receptors on natural killer cells: effect of immune activation and HIV viraemia. J Infect Dis. 2004;189:1193–8. doi: 10.1086/382090. [DOI] [PubMed] [Google Scholar]
- 16.Parato KG, Kumar A, Badley AD, et al. Normalization of natural killer cell function and phenotype with effective anti-HIV therapy and the role of IL-10. AIDS. 2002;16:1251–6. doi: 10.1097/00002030-200206140-00007. [DOI] [PubMed] [Google Scholar]
- 17.De Maria A, Fogli M, Costa P, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–8. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 20.Valdez H, Connick E, Smith KY, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–66. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 21.Arno A, Ruiz L, Juan M, et al. Impact on the immune system of undetectable plasma HIV-1 RNA for more than 2 years. AIDS. 1998;12:697–704. doi: 10.1097/00002030-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Valiante NM, Trinchieri G. Identification of a novel signal transduction surface molecule on human cytotoxic lymphocytes. J Exp Med. 1993;178:1397–406. doi: 10.1084/jem.178.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moretta A, Bottino C, Vitale M, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. 197–223. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima H, Colonna M. 2B4: an NK cell activating receptor with unique specificity and signal transduction mechanism. Hum Immunol. 2000;61:39–43. doi: 10.1016/s0198-8859(99)00170-6. [DOI] [PubMed] [Google Scholar]
- 25.Boles KS, Stepp SE, Bennett M, Kumar V, Mathew PA. 2B4 (CD244) and CS1: novel members of the CD2 subset of the immunoglobulin superfamily molecules expressed on natural killer cells and other leukocytes. Immunol Rev. 2001;181:234–49. doi: 10.1034/j.1600-065x.2001.1810120.x. [DOI] [PubMed] [Google Scholar]
- 26.Sivori S, Parolini S, Falco M, et al. 2B4 functions as a co-receptor in human NK cell activation. Eur J Immunol. 2000;30:787–93. doi: 10.1002/1521-4141(200003)30:3<787::AID-IMMU787>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 27.Peritt D, Sesok-Pizzini DA, Schretzenmair R, et al. C1.7 antigen expression on CD8+ T cells is activation dependent: increased proportion of C1.7+CD8+ T cells in HIV-1 infected patients with progressing disease. J Immunol. 1999;162:7563–8. [PubMed] [Google Scholar]
- 28.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 29.Ostrowski SR, Katzenstein TL, Thim PT, Pedersen BK, Gerstoft J, Ullum H. Low-level viremia and proviral DNA impede immune reconstitution in HIV-1 infected patients receiving highly active antiretroviral therapy. J Infect Dis. 2005;191:348–57. doi: 10.1086/427340. [DOI] [PubMed] [Google Scholar]
- 30.Katzenstein TL, Ullum H, Roge BT, et al. Virological and immunological profiles among patients with undetectable viral load followed prospectively for 24 months. HIV Med. 2003;4:53–61. doi: 10.1046/j.1468-1293.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 31.Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 1998;76:1216–31. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- 32.Kottilil S, Chun TW, Moir S, et al. Innate immunity in human immunodeficiency virus infection: effect of viraemia on natural killer cell function. J Infect Dis. 2003;187:1038–45. doi: 10.1086/368222. [DOI] [PubMed] [Google Scholar]
- 33.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 34.Valentin A, Rosati M, Patenaude DJ, et al. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2002;99:7015–20. doi: 10.1073/pnas.102672999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aderka D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine Growth Factor Rev. 1996;7:231–40. doi: 10.1016/s1359-6101(96)00026-3. [DOI] [PubMed] [Google Scholar]
- 36.Ostrowski SR, Katzenstein TL, Piironen T, Gerstoft J, Pedersen BK, Ullum H. Soluble urokinase receptor levels in plasma during 5 years of highly active antiretroviral therapy in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2004;35:337–42. doi: 10.1097/00126334-200404010-00002. [DOI] [PubMed] [Google Scholar]
- 37.Karlsson AC, Younger SR, Martin JN, et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viraemia. AIDS. 2004;18:981–9. doi: 10.1097/00002030-200404300-00005. [DOI] [PubMed] [Google Scholar]