Abstract
In the early development of type 1 diabetes macrophages and dendritic cells accumulate around the islets of Langerhans at sites of fibronectin expression. It is thought that these macrophages and dendritic cells are derived from blood monocytes. Previously, we showed an increased serum level of MRP8/14 in type 1 diabetes patients that induced healthy monocytes to adhere more strongly to fibronectin (FN). Here we show that MRP8/14 is expressed and produced at a higher level by type 1 diabetes monocytes, particularly after adhesion to FN, creating a positive feedback mechanism for a high fibronectin-adhesive capacity. Also adhesion to endothelial cells was increased in type 1 diabetes monocytes. Despite this increased adhesion the transendothelial migration of monocytes of type 1 diabetes patients was decreased towards the proinflammatory chemokines CCL2 and CCL3. Because non-obese diabetic (NOD) mouse monocytes show a similar defective proinflammatory migration, we argue that an impaired monocyte migration towards proinflammatory chemokines might be a hallmark of autoimmune diabetes. This hampered monocyte response to proinflammatory chemokines questions whether the early macrophage and dendritic cell accumulation in the diabetic pancreas originates from an inflammatory-driven influx of monocytes. We also show that the migration of type 1 diabetes monocytes towards the lymphoid tissue-related CCL19 was increased and correlated with an increased CCR7 surface expression on the monocytes. Because NOD mice show a high expression of these lymphoid tissue-related chemokines in the early pancreas it is more likely that the early macrophage and dendritic cell accumulation in the diabetic pancreas is related to an aberrant high expression of lymphoid tissue-related chemokines in the pancreas.
Keywords: chemokines, diabetes, migration, monocytes, MRP8/14
Introduction
Macrophages (MØ) and dendritic cells (DC) play an important role in the development of type 1 diabetes (DM1). Prior to the infiltration of autoreactive T cells around and into the islets, a peri- and para-islet accumulation of MØ and DC can be detected in animal models of DM1 [1–3]. These MØ and DC act as antigen-presenting cells (APC), pick up autoantigens and migrate subsequently to the draining lymph nodes, where they present the autoantigens to naive T cells and B cells to initiate the specific autoimmunization process. After autoimmunization, T cells, MØ and DC infiltrate the islets, and the MØ in particular are capable of assisting the T cells in the destruction of the β cells [1,2].
Monocytes form an important precursor population for MØ and DC. We have reported previously on a raised serum level of the myeloid-related protein (MRP)8/14 in the serum of DM1 patients [4]. MRP8 and MRP14 are calcium-binding proteins of the S100 family. These proteins form heterodimeric complexes in a calcium-dependent manner and are expressed specifically by recently transmigrated monocytes and granulocytes [5]. MRP8- and MRP14-expressing cells dominate inflammatory reactions. Stimulation of monocytes with granulocyte-macrophage colony stimulating factor, interleukin (IL)-1β or lipopolysaccharide (LPS) induces the expression and secretion of the MRP8/14 heterodimer [6]. When monocytes are stimulated with MRP14 or MRP8/14, a rapid increase in their CD11b surface expression is observed [4,7]. Although the precise function of the MRPs is still unclear, they are thought to be involved in leucocyte extravasation in inflammation [7,8]. Here we have extended our former studies and aimed, first, to investigate the actual expression and secretion of MRP8/14 by monocytes of DM1 patients.
In our previous study we also showed that the serum-borne MRP8/14 in DM1 patients was capable of inducing monocytes of healthy individuals to adhere more to the extracellular matrix (ECM) component fibronectin (FN) [4]. FN is abundantly present at the vasoductular poles of the islets of Langerhans and is probably of importance in the early peri- and para-islet localization of the MØ and DC in DM1 [9]. Besides adhesion to endothelium and to components of the ECM, migration also plays an important role in the accumulation of monocyte-derived cells at specific tissue sites. Chemokines direct such migration. Chemokines form a large superfamily of small (8–10 kDa) secreted proteins [10,11]. About 40–50 members are distributed into four structural families according to the relative positions of cysteine residues. In general, chemokines are divided functionally into inflammatory and constitutive chemokines, although several chemokines show a dual function [10,11]. Chemokines exert their biological functions through interaction with seven-transmembrane G protein-coupled specific receptors that are expressed differentially on leucocyte populations [12]. The second aim of this study was to investigate the migratory and chemotactic properties of monocytes of DM1 patients. We report on an increased production of the proinflammatory chemokines CCL2 and CCL3 by DM1 monocytes, particularly after FN-adhesion, yet these monocytes had a poor migratory response to these chemokines. Interestingly, the chemotactic response of the monocytes to CCL19, a chemokine expressed constitutively in lymphoid tissue, was enhanced.
Materials and methods
Patients and controls
Heparinized blood was drawn from patients with type 1 diabetes (DM1 patients) and age- and gender-matched healthy control subjects. In addition, blood was drawn from type 2 diabetic patients (DM2 patients) as disease controls. Patients with obvious vascular complications and/or recent surgical interventions were excluded from this study. Patients’ characteristics are summarized in Table 1. Monocytes were isolated via Ficoll density (Pharmacia, Uppsala, Sweden; density 1·077 g/ml) gradient centrifugation, followed by Percoll density (Pharmacia; density 1·063 g/ml) gradient centrifugation and a final purity of 85–95% was reached. This study was approved by the ethics committee of the Erasmus MC, University Medical Center in Rotterdam and all subjects gave written informed consent.
Table 1. Patients’ characteristics.
| DM1 patients | Healthy control subjects | DM2 patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |||||||
| Age | 41·7 ± 2·5 | 35·0 ± 3·1 | 38·8 ± 3·4 | 41·0 ± 4·6 | 52·9 ± 4·0 | 56·9 ± 3·6 | ||||||
| HbA1c† | 8·59 ± 0·4 | 8·45 ± 0·5 | n.d.‡ | n.d. | 7·5 ± 0·3 | 8·0 ± 0·4 | ||||||
| Duration of diabetes | 22·7 ± 3·2 | 15·0 ± 2·9 | n.a.§ | n.a. | n.d. | n.d. | ||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| DRB1*15/DRB1*16 | 0 | (0%) | 1 | (8%) | 4 | (36%) | 5 | (38%) | n.d. | – | n.d. | – |
| DRB1*03/DRB1*04 | 18 | (82%) | 10 | (77%) | 6 | (55%) | 6 | (46%) | n.d. | – | n.d. | – |
| Other DR | 4 | (18%) | 2 | (15%) | 1 | (9%) | 2 | (15%) | n.d. | – | n.d. | – |
| n.d. | 8 | 9 | 1 | 1 | 8 | 14 | ||||||
| Total n | 30 | 22 | 12 | 14 | 8 | 14 | ||||||
% in serum
not determined
not applicable. DM1, diabetes mellitus type 1; DM2, diabetes mellitus type 2.
Chemotaxis
The in vitro migration towards CCL2, CCL3, CCL4, CCL19, CXCL12 (all from PeproTech, Rocky Hill, NJ, USA) and fMLP of monocytes was evaluated using a Boyden chemotaxis chamber (Neuroprobe, Gaithersburg, MD, USA) and polycarbonate membranes (5 µm pore size; Whatman, Clifton, NJ, USA), as described previously [13]. Monocyte (1·5 × 106/ml) migration was determined after 90 min and expressed as a migration index (chemokine-migrated cells divided by the medium-migrated cells). Each experiment was performed in triplicate and cells were counted in five high-power fields (1000× magnification).
Adhesion
Human umbilical vein endothelial cells (HUVEC; kindly provided by Dr W. Sluiter) were grown to confluence in flat-bottomed 96-well plates in Ham's F-12, supplemented with 100 units/ml penicillin, 100 µg/ml streptomycin (Cambrex, Verviers, Belgium), 20% fetal bovine serum (FBS), 50 µg/ml endothelial cell growth supplement (ECGS: BD Pharmingen, Alphen aan den Rijn, the Netherlands) and 100 µg/ml heparin (Sigma, St Louis, MO, USA). Monocytes were labelled with Na251CrO4 (Amersham BioSciences, Uppsala, Sweden) and co-incubated with the endothelial monolayer for 60 min at 37°C. Non-adherent cells were washed away and adherent cells were lysed with 0·1 ml of 1% sodium dodecyl sulphate (SDS), 0·05% NaOH. Radioactivity of non-adherent and adherent cells was measured and results are expressed as the percentage of adherent cells.
Transmigration
HUVEC were grown to confluence on Transwell filters (5-µm pore; Costar, Acton, MA, USA) that were precoated with 10 µg/ml fibronectin (Sigma) and stimulated for 24 h with or without IL-1β (R&D Systems, Minneapolis, MN, USA). In the lower compartment, the chemokines CCL2, CCL3 (both of PeproTech) or medium were added. In the upper compartment, a total of 1 × 105 51Cr-labelled monocytes was seeded and co-incubated with HUVEC monolayers for 1 h at 37°C. The upper compartment was gently washed and together with the filter and the lower compartment measured for radioactivity. Transmigration was calculated as the radioactivity measured in the lower compartment and the downside of the filter, divided by the total radioactivity. Transmigration is shown relative to the migration towards medium.
Flow cytometry
The expression of chemokines receptors on monocytes was studied using the following antibodies PE-CCR1, PE-CCR2, CyChrome-CCR5, FITC-CCR6, PE-CCR7 and FITC-CXCR4 (all from R&D Systems except for CyChrome-CCR5, obtained from BD Pharmingen). MRP8/14 on monocytes was studied using a biotinylated antibody against MRP8/14 (27E10, BMA Biomedicals, Augst, Switzerland) followed by APC—streptavidin (BD Pharmingen). Monocytes were typified by flow cytometry using CD14 [fluorescein isothiocyanate (FITC) or APC labelled] and CD16 (FITC or PE labelled; both CD14 and CD16 antibodies were obtained from BD Pharmingen) as CD14++CD16− and CD14+CD16+ cells, within a peripheral blood mononuclear cell (PBMC) fraction obtained after Ficoll density gradient purification. All flow cytometric analysis was performed using a FACSCalibur (Becton Dickinson, San Jose, CA, USA) and CellQuest software (BD Pharmingen).
Calcium mobilization
The mobilization of intracellular calcium upon receptor activation was determined by flow cytometric measurement of calcium-bound Indo1-AM (Molecular Probes, Eugene, OR, USA). Briefly, PBMC were labelled with Indo1-AM in calcium-free medium, followed by washing and incubation with APC-labelled CD14 and phycoerythrin (PE)-labelled CD16 (BD Pharmingen) in calcium-containing medium. After equilibrating to 37°C, the cells were analysed on a FACSDiva (Becton Dickinson) and during measurement different stimuli were added.
Enzyme-linked immunosorbent assay (ELISA)
The production of MRP8/14 (Bachem, Heidelberg, Germany), CCL2, CCL3 and CCL19 (R&D Systems) by monocytes (1 × 105), on fibronectin or unstimulated, was measured in the supernatant after 24 h by commercially available ELISA according to the manufacturer's protocol.
HLA-DRB1 typing
Genomic DNA was extracted from PBMC using a commercially available kit (QIAamp DNA blood isolation kit; Qiagen, Hilden, Germany). Subsequently, HLA-DRB1 typing was performed at the two-digit level using a commercially available typing system in which exon 2 of the HLA-DRB1 gene is amplified and analysed with allele-specific probes in a line probe assay (INNO-LiPA, Innogenetics, Ghent, Belgium), as described previously [14].
Statistical analyses
Data were analysed using Student's t-test. All data were tested for two-tailed significance. A P-value below 0·05 was considered to be statistically significant.
Results
MRP8/14 expression and adhesion to fibronectin
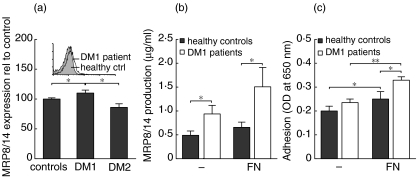
We studied the surface expression of MRP8/14 on circulating monocytes in DM1. As shown in Fig. 1a, the expression of MRP8/14 on monocytes was slightly but significantly increased in DM1 patients in comparison to healthy controls and DM2 patients: healthy controls 7·57 ± 1·14 [median fluorescence intensity (MFI) ± s.d.], DM1 patients 9·14 ± 1·31 and DM2 patients 6·28 ± 0·81 (control IgG typically < 5 in all groups). We also determined the production of MRP8/14 by monocytes of DM1 patients and healthy control subjects. As shown in Fig. 1b, monocytes of DM1 patients produced increased amounts of MRP8/14 in comparison to healthy control monocytes. MRP8/14 induces monocytes to adhere to fibronectin (FN) [4,11] and monocytes of DM1 patients indeed showed an increased adhesion to FN (Fig. 1c) [4,15]. When the monocytes were allowed to adhere to FN, a stimulus that is known to induce MRP8/14 monocyte surface expression [16], the monocytes of DM1 patients showed increased MRP8/14 production (Fig. 1b) compared to healthy control monocytes.
Fig. 1.
Adhesion and MRP8/14 expression of monocytes in diabetes mellitus type 1 (DM1). (a) Monocytes of DM1 patients (n = 12) showed increased surface expression of MRP8/14 compared to healthy controls (n = 13) and DM2 patients (n = 3). The MRP8/14 expression is shown relative to that observed in healthy control subjects. The inlay shows representative histograms of monocytes of a DM1 patient and a healthy control; IgG controls and a DM2 patient are not shown to avoid overcrowding the figure. (b) The production of MRP8/14 by monocytes after 24 h was increased in DM1 patients (n = 9) in comparison to healthy controls (n = 9), both with and without fibronectin (FN) stimulation. (c) Monocytes of DM1 patients showed increased adhesion to FN in comparison to monocytes of healthy control subjects. Adhesion after 24 h is shown of nine DM1 patients and nine healthy control subjects. Data are presented as average ± s.e.m., *P < 0·05 as determined with unpaired two-tailed Students’t-test.
Production of proinflammatory CCL2 and CCL3
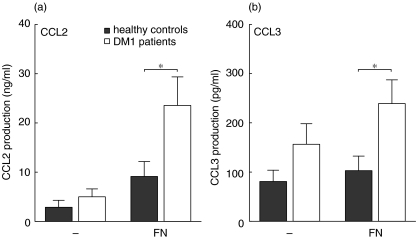
Apart from MRP8/14, a molecule instrumental in the adhesion of monocytes to endothelium and extracellular matrix components, we also studied the production of the proinflammatory chemokines CCL2 (MCP-1) and CCL3 (MIP-1α) by DM1 monocytes, as these chemokines are thought to play an important role in the inflammatory accumulation of monocytes and monocyte-derived cells in tissues. The production of CCL2 and CCL3 by monocytes of DM1 patients was slightly, but not significantly, increased compared to monocytes of healthy control subjects (Fig. 2a,b). Exposure to FN increased the production of CCL2 and CCL3 and monocytes of DM1 patients showed a significantly increased production of these chemokines in comparison to monocytes of healthy controls (Fig. 2a,b).
Fig. 2.
Expression of proinflammatory cytokines by monocytes under fibronectin (FN)-adherent condition. (a) When stimulated with fibronectin, monocytes of diabetes mellitus type 1 (DM1) patients showed an increased production of CCL2 compared to monocytes of healthy control subjects. (b) The production of CCL3 was also increased by monocytes of DM1 patients after FN stimulation. Data are presented as average ± s.e.m., n = 9, *P < 0·05, **P < 0·01 as determined with unpaired two-tailed Students’t-test.
Chemokine-directed migratory responses
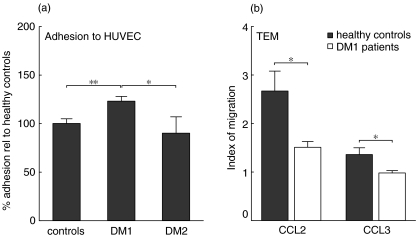
Previously we have demonstrated that an increased level of MRP8/14 in the serum of DM1 patients was able to induce increased adhesion of monocytes to FN and increased CD11b/CD18 expression [4]. This increased adhesion and expression of CD11b/CD18 is also involved in inflammation by participating in the transendothelial migration (TEM) of monocytes [5,7]. Therefore, we studied the adhesive and migratory capacity of monocytes of DM1 patients in further detail. We allowed radiolabelled monocytes to adhere to a monolayer of HUVEC). Monocytes of DM1 patients showed an increased potential to adhere to the endothelial monolayer compared to healthy control subjects and DM2 patients (Fig. 3a).
Fig. 3.
Monocyte adhesion and transmigration across human umbilical vein endothelial cells (HUVEC). (a) Monocytes of type 1 diabetic patients (n = 7) showed increased adhesion to HUVEC compared to monocytes of healthy subjects (n = 7) and type 2 diabetes patients (n = 4). (b) Transmigration in response to CCL2 and CCL3 of type 1 diabetes monocytes (n = 12) was strongly decreased compared to healthy control monocytes (n = 12). Adhesion is shown relative to that observed with monocytes of healthy control subjects. Data are presented as average ± s.e.m., *P < 0·05, **P < 0·01 as determined with unpaired two-tailed Students’t-test.
To study the actual TEM, transwell membranes were coated with HUVEC and the migration of radiolabelled monocytes towards the proinflammatory chemokines CCL2 and CCL3 studied. As shown in Fig. 3b, monocytes of DM1 patients showed a decreased TEM towards CCL2 and hardly any transmigration in response to CCL3 in comparison to monocytes of healthy control subjects. When the HUVEC were stimulated for 24 h with IL-1β, which induces an up-regulation of adhesion molecules, the transmigration of monocytes to these chemokines remained decreased in DM1 patients in comparison to healthy controls (data not shown).
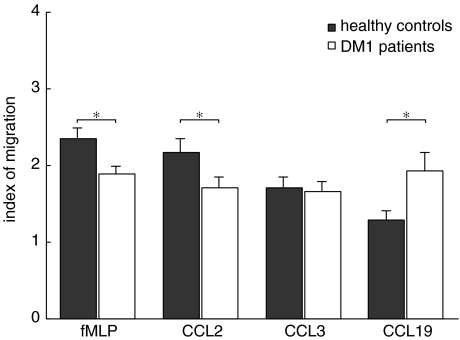
Because the transwell assay is a laborious process chemotaxis was also studied using the classical Boyden chamber, allowing us to test a larger array of monocyte-attracting chemokines. The proinflammatory chemokines CCL2, CCL3 and CCL4 (MIP-1β) and fMLP were used as chemoattractants in this assay, as were the constitutive chemokines CXCL12 (SDF-1) and CCL19 (MIP-3β). Migration of monocytes towards CCL3, CCL4 and CXCL12 proved to be of similar magnitude in DM1 patients and healthy control subjects, but the response to fMLP, CCL2 and CCL19 was not (Fig. 4 and data not shown). Monocytes of DM1 patients showed a decreased chemotactic response towards the proinflammatory compounds fMLP and to CCL2 but − surprisingly − the migratory response of DM1 monocytes to the constitutive chemokine CCL19 was increased in comparison to monocytes of healthy control subjects (Fig. 4). In this respect it is of interest to note that we could not detect a significant production of the lymphoid tissue-related CCL19 either by monocytes of DM 1 patients or of healthy controls (data not shown). To exclude the possibility that the monocytes of DM1 patients showed altered chemotaxis due to alterations of the cells by exposure to high glucose levels in the blood, we performed a chemotaxis assay of healthy control monocytes towards CCL2 in the presence of increasing glucose levels. No significant effects of glucose were observed on the chemotactic response towards CCL2 of healthy control monocytes (data not shown).
Fig. 4.
Disturbed chemotactic response of monocytes of type 1 diabetes patients. Monocytes of type 1 diabetes patients displayed a decreased chemotactic response to fMLP and CCL2, while in contrast the response towards CCL19 was increased. Data are presented as average ± s.e.m., n = 12, *P < 0·05, **P < 0·01 as determined with unpaired two-tailed Students’t-test.
Chemokine receptor expression
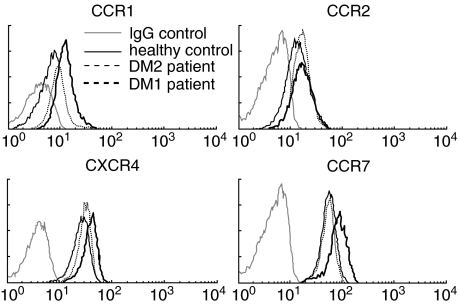
To relate the decreased chemotactic response of the monocytes to proinflammatory chemokines to a lower expression level of receptors for such chemokines, we studied the surface expression of the chemokine receptors CCR2 (for CCL2) and CCR1 (for CCL3) on DM1 monocytes using flow cytometry. As shown in Fig. 5 and summarized in Table 2, reduced expression of CCR2 could not be detected on DM1 monocytes compared to DM2 and healthy control monocytes. The surface expression of CCR1 was even increased. Because we observed decreased TEM and chemotaxis of the monocytes of DM1 patients towards CCL2, but no differences in the expression of CCR2, we studied the ability of the CCR2 to mobilize intracellular calcium upon CCL2 stimulation as a different functionality. No differences in the ability to mobilize calcium upon stimulation with CCL2 could be found between DM1 patients and healthy controls (data not shown).
Fig. 5.
Chemokine receptor expression on monocytes. Representative histograms of CCR1, CCR2, CXCR4 and CCR7 expression of 16 type 1 diabetes patients, nine type 2 diabetes patients and 11 healthy control subjects show that the expression of CCR1, CXCR4 and CCR7 was increased in type 1 diabetes patients.
Table 2. Surface expression of chemokine receptors on monocytes.
| Healthy controls (n = 11) | DM1 patients (n = 16) | DM2 patients (n = 9) | |
|---|---|---|---|
| CCR1 | 10·53 ± 0·65 | 12·90 ± 0·65†‡ | 10·06 ± 1·31 |
| CCR2 | 17·79 ± 1·13 | 17·96 ± 0·80 | 18·46 ± 2·06 |
| CCR7 | 50·38 ± 7·04 | 65·16 ± 2·87†‡ | 52·85 ± 3·72 |
| CXCR4 | 27·49 ± 3·77 | 39·20 ± 1·94†‡ | 31·58 ± 2·81 |
P < 0·05 to healthy control monocytes
P < 0·05 to DM2 patients’ monocytes as determined with Student's t-test. Mean fluorescent intensities are shown ± s.e.m. DM1: diabetes mellitus type 1.
Because we found an increased responsiveness of the DM1 monocytes to the constitutive chemokine CCL19, we also studied the surface expression of receptors for constitutive chemokines on DM1 monocytes and found CCR7 (for CCL19 and CCL21) and CXCR4 (for SDF-1) increased on monocytes of DM1 patients in comparison to healthy controls and DM2 patients (Fig. 5 and Table 2).
As it is known that the CD14+CD16+ subpopulation of monocytes shows a different expression of chemokine receptors [17], we also determined the chemokine receptor expression on the CD14+CD16+ monocytes. No significant differences in chemokine receptor expression were observed on the CD14+CD16+ monocyte populations between DM1, DM2 patients and healthy controls, nor did we find differences in the percentage of CD14+CD16+ monocytes (data not shown).
Association with HLA-DRB1 genotype
To investigate whether the aberrant adhesion to FN and the aberrant chemotactic migratory capacity of the circulating DM1 monocytes were associated with the HLA-DRB1 genotype of the patients (see Table 1), we searched for possible associations between HLA-DRB1 genotypes and the outcome of the different experiments. We were unable to find any statistically significant correlations.
Discussion
MRP8/14, a product secreted by infiltrating monocytes, is known to increase the number of monocytes adhering to FN [4]. Increased levels of MRP14 and MRP8/14 are present in the circulation of DM1 patients and indeed circulating monocytes show an increased adhesion to FN [4,15]. In this report we show that ex vivo circulating monocytes of DM1 patients express and secrete more of MRP8/14 compared to monocytes of healthy control subjects, particularly after adhesion to a coating of FN. Our finding thus provides evidence for an enhanced positive feed-back mechanism in type 1 diabetes regarding the adhesion of monocytes to FN: type 1 diabetic monocytes are able to express and secrete more MRP8/14 as compared to healthy control monocytes inducing the cells to adhere more vigorously to FN compared to healthy monocytes, which leads to an even larger expression and secretion of MRP8/14 compared to healthy monocytes.
In non-obese diabetic (NOD) mice extensive deposits of FN are present at the vasoductular pole of the islets, the location of the MØ and DC accumulating prior to the extensive para- and peri-lymphocytic infiltration. Especially at preweaning age these deposits are larger compared to control mice [9]. Also NOD monocytes [18] and NOD DC (Bouma et al. submitted) show an increased adhesion to FN − analogous to human monocytes − yet whether MRP8/14 is involved in this increased adhesion has not been studied. Nevertheless, the parallel between NOD mice and humans regarding this enhanced FN adhesive capability of monocytes points to an important mechanism most probably involved in the (early) accumulation of monocyte-derived cells, i.e. MØ and DC in the ductovascular poles in the pancreas.
The actual importance of such enhanced adhesive capability of monocytes to FN at such para-islet location for the development of islet autoimmunity remains to be identified. Homeostatic trafficking of DC is thought to be important for the maintenance of peripheral tolerance [19–21]. However, little is known about the kinetics of trafficking of monocytes, MØ and DC in the prediabetic pancreas. When APC are retained in the tissue by either increased adhesion, decreased emigration to the draining lymph node or both, they may acquire an immunocompetent rather than a tolerogenic phenotype. Indeed, contact of DC with ECM induces maturation of the cells [22,23]. Therefore a prolonged contact with ECM may induce complete maturation of the DC in NOD mice resulting in the induction of an immune response in the draining lymph node rather than the induction or maintenance of tolerance.
The found enhanced adhesive phenotype of the circulating DM1 monocytes to FN suggested to us that the cells would also adhere more strongly to endothelial cells, resulting in an increased TEM [7,8]. We show here that monocytes of DM1 patients did exhibit a stronger adhesion to HUVEC, but when we studied the migratory response of monocytes towards proinflammatory chemokines CCL2 and CCL3 (both important in the pathogenic process of autoimmune insulitis [24,25]), monocytes of DM1 patients showed a decreased TEM towards these chemokines as well as a decreased migration in the classical Boyden chamber assay towards CCL2. Interestingly, comparable defects in monocyte migration to CCL2 have been described in a mouse model of autoimmune insulitis [24]. In this mouse model CCL2 was transgenically expressed in islets and did attract monocytes to the islets. However, when CCL2 is additionally expressed systemically, such islet infiltration is disrupted and the investigators explained the phenomenon by assuming that such high systemic levels of CCL2 physically disrupted the chemoattractant gradient of CCL2 originating from the CCL2-expressing islets [24]. Could it be that the raised production of CCL2 and CCL3 by circulating monocytes (particularly after adhesion) and the consequent high level of CCL2 and CCL3 around DM1 monocytes under such circumstances desensitizes the receptors [26] and prevents the cells from responding to the CCL2 and CCL3 gradient in the in vitro assays?
Further, it is important to note that the impaired chemotactic response of DM1 monocytes appeared to be selective for the proinflammatory chemokines: we observed an increased migration of monocytes of DM1 patients towards the constitutively expressed chemokine CCL19 that was paralleled by an increased expression on DM1 monocytes of the receptor for CCL19, i.e. CCR7.
As is the case for the aberrant FN adhesion, there also exists an interesting parallel between the DM1 patient and the NOD mouse regarding the aberrant migratory response of monocytes to inflammatory and constitutive chemokines. Also in the NOD mouse the chemotactic response of monocytes and DC towards proinflammatory chemokines is strongly reduced both in vitro and in vivo (questioning whether the early MØ and DC accumulation in the diabetic pancreas originates from an inflammatory-driven influx of monocytes), while there are signs of a role for constitutive chemokines in the diabetic process ([13] and Bouma et al. submitted). At the time of the early MØ and DC accumulation close to and around the islets of Langerhans and prior to the lymphocyte infiltration, CCL19 and CCL21 are expressed at a higher level in the NOD pancreas compared to control mice, while proinflammatory chemokines only came to a noteworthy expression later in the process (Bouma et al. submitted). We therefore assume that the early increased expression of CCL19 and CCL21 in the NOD pancreas plays a role in the early peri- and para-islet MØ and DC accumulation and perhaps disease induction. Indeed, transgenic expression of CCL21 in mouse islets is sufficient to attract large numbers of immune cells to the islets and to induce the proliferation of islet-specific T cells, which in lymphopenic hosts led to the development of autoimmune diabetes [27].
Because the high adhesive potential of monocytes to FN and the poor chemotactic properties to proinflammatory chemokines are present in the NOD mouse prior to the MØ and DC accumulation (5–7 weeks of age) and as the patients studied here all had longstanding type 1 diabetes (hence the acute islet inflammation must have been subsided), we assume that the here-described aberrant adhesive and migratory properties of the monocytes are intrinsic to cells of individuals prone to develop autoimmune insulitis and not the result of the inflammatory process. A decreased migration of T1D monocytes to proinflammatory stimuli has been described previously using zymosan-activated culture fluid [28] and C5a [29]. Moreover, in other autoimmune diseases such as rheumatoid arthritis [30] and systemic lupus erythematosus [31] an impaired monocyte migration has been observed and patients with the Wiskott—Aldrich syndrome (WAS) show a defective monocyte migration due to an inherited defect in the cytoskeletal-associated WAS protein [32] and are prone to develop autoimmune disorders [33]. These findings suggest a possible relation between migratory potential of monocytes and the proneness to develop autoimmunity, such as autoimmune diabetes. However, to be sure of that, the migratory properties of monocytes of autoantibody-positive non-diabetic (prediabetic) individuals should be studied. Unfortunately, we did not have access to such individuals and their cells. Interestingly, monocytes of autoantibody-positive non-diabetic individuals (and of recent-onset DM1 patients) show a high prostaglandin synthase 2 (PGS2) expression [34,35]. This aberrance can be linked theoretically to the decreased migration towards proinflammatory chemokines. High activity of PGS2 results in abundant expression of prostaglandins, including prostaglandin E2 (PGE2) [36]. In monocytes, PGE2 induces a down-regulation of the proinflammatory chemokine receptor CCR5 [37]. Furthermore, PGE2 is involved in migration of DC towards CCL19 and CCL21 [38,39]. It is possible that the constitutive active PGS2 in monocytes of DM1 patients yields high PGE2 levels that down-regulate the responsiveness of monocytes to proinflammatory chemokines and up-regulate CCR7 expression and responsiveness.
Apart from being an intrinsic abnormality of DM1 monocytes, high glucose levels might have played a role in the aberrant chemotactic responses. It has been reported that high glucose can induce the expression of CCL2 by and of CCR2 on monocytes [40]. From monocytes of patients that may have episodes of hypoglycaemia, an aberrant migration towards CCL2 might be expected. However, we observed a decreased response to CCL2. Monocytes of recent onset hyperglycaemic DM1 patients before and after adequate insulin treatment should be studied to rule out clearly an effect of hyperglycaemia on the here-described aberrant chemotaxis. However, it must be noted that with regard to the CCR2 expression, we controlled our experiments with monocytes of DM2 patients (with similarly slightly raised HbA1c levels) and we observed neither altered expression of CCR2 on monocytes of DM1 nor on those of DM2 patients.
In conclusion, we show here increased expression and secretion of MRP8/14 of DM1 monocytes that is linked closely to their enhanced adhesive capability to FN. In addition, monocytes of DM1 patients exhibited a decreased migration towards proinflammatory chemokines, but an increased migration to the constitutively expressed chemokine CCL19 in lymphoid tissue. Given the fact that similar aberrances have been found in the NOD mouse prior to disease development, it is likely that these aberrations are associated with the development of islet autoimmunity.
Acknowledgments
This study was supported by research grants from the European Union (QLRT-1999–00276-MONODIAB), the Dutch Diabetes Research Foundation (96·606) and a travel grant from the Vereniging Trustfonds Erasmus University Rotterdam.
References
- 1.Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA. Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and beta-cell destruction in NOD mice. Diabetes. 1994;43:667–75. doi: 10.2337/diab.43.5.667. [DOI] [PubMed] [Google Scholar]
- 2.Rosmalen JG, Homo-Delarche F, Durant S, Kap M, Leenen PJ, Drexhage HA. Islet abnormalities associated with an early influx of dendritic cells and macrophages in NOD and NODscid mice. Lab Invest. 2000;80:769–77. doi: 10.1038/labinvest.3780080. [DOI] [PubMed] [Google Scholar]
- 3.Voorbij HA, Jeucken PH, Kabel PJ, De Haan M, Drexhage HA. Dendritic cells and scavenger macrophages in pancreatic islets of prediabetic BB rats. Diabetes. 1989;38:1623–9. doi: 10.2337/diab.38.12.1623. [DOI] [PubMed] [Google Scholar]
- 4.Bouma G, Lam-Tse WK, Wierenga-Wolf AF, Drexhage HA, Versnel MA. Increased serum levels of MRP-8/14 in type 1 diabetes induce an increased expression of CD11b and an enhanced adhesion of circulating monocytes to fibronectin. Diabetes. 2004;53:1979–86. doi: 10.2337/diabetes.53.8.1979. [DOI] [PubMed] [Google Scholar]
- 5.Hogg N, Allen C, Edgeworth J. Monoclonal antibody 5.5 reacts with p8,14, a myeloid molecule associated with some vascular endothelium. Eur J Immunol. 1989;19:1053–61. doi: 10.1002/eji.1830190615. [DOI] [PubMed] [Google Scholar]
- 6.Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem. 1997;272:9496–502. doi: 10.1074/jbc.272.14.9496. [DOI] [PubMed] [Google Scholar]
- 7.Eue I, Pietz B, Storck J, Klempt M, Sorg C. Transendothelial migration of 27E10+ human monocytes. Int Immunol. 2000;12:1593–604. doi: 10.1093/intimm/12.11.1593. [DOI] [PubMed] [Google Scholar]
- 8.Kerkhoff C, Eue I, Sorg C. The regulatory role of MRP8 (S100A8) and MRP14 (S100A9) in the transendothelial migration of human leukocytes. Pathobiology. 1999;67:230–2. doi: 10.1159/000028098. [DOI] [PubMed] [Google Scholar]
- 9.Geutskens SB, Homo-Delarche F, Pleau JM, Durant S, Drexhage HA, Savino W. Extracellular matrix distribution and islet morphology in the early postnatal pancreas: anomalies in the non-obese diabetic mouse. Cell Tissue Res. 2004;318:579–89. doi: 10.1007/s00441-004-0989-0. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today. 1999;20:254–7. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- 11.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Locati M, Otero K, Schioppa T, et al. The chemokine system: tuning and shaping by regulation of receptor expression and coupling in polarized responses. Allergy. 2002;57:972–82. doi: 10.1034/j.1398-9995.2002.02166.x. [DOI] [PubMed] [Google Scholar]
- 13.Bouma G, Nikolic T, Coppens JM, et al. NOD mice have a severely impaired ability to recruit leukocytes into sites of inflammation. Eur J Immunol. 2005;35:225–35. doi: 10.1002/eji.200425513. [DOI] [PubMed] [Google Scholar]
- 14.Thonnard J, Deldime F, Heusterspreute M, et al. HLA class II genotyping: two assay systems compared. Clin Chem. 1995;41:553–6. [PubMed] [Google Scholar]
- 15.Setiadi H, Wautier JL, Courillon-Mallet A, Passa P, Caen J. Increased adhesion to fibronectin and Mo-1 expression by diabetic monocytes. J Immunol. 1987;138:3230–4. [PubMed] [Google Scholar]
- 16.Mahnke K, Bhardwaj R, Sorg C. Heterodimers of the calcium-binding proteins MRP8 and MRP14 are expressed on the surface of human monocytes upon adherence to fibronectin and collagen. Relation to TNF-alpha, IL-6, and superoxide production. J Leukoc Biol. 1995;57:63–71. doi: 10.1002/jlb.57.1.63. [DOI] [PubMed] [Google Scholar]
- 17.Weber C, Belge KU, von Hundelshausen P, et al. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol. 2000;67:699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- 18.Nikolic T, Bouma G, Drexhage HA, Leenen PJ. Diabetes-prone NOD mice show an expanded subpopulation of mature circulating monocytes, which preferentially develop into macrophage-like cells in vitro. J Leukoc Biol. 2005;78:70–9. doi: 10.1189/jlb.1104662. [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 20.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 21.Flores-Romo L. In vivo maturation and migration of dendritic cells. Immunology. 2001;102:255–62. doi: 10.1046/j.1365-2567.2001.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brand U, Bellinghausen I, Enk AH, et al. Influence of extracellular matrix proteins on the development of cultured human dendritic cells. Eur J Immunol. 1998;28:1673–80. doi: 10.1002/(SICI)1521-4141(199805)28:05<1673::AID-IMMU1673>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Mahnke K, Bhardwaj RS, Luger TA, Schwarz T, Grabbe S. Interaction of murine dendritic cells with collagen up-regulates allostimulatory capacity, surface expression of heat stable antigen, and release of cytokines. J Leukoc Biol. 1996;60:465–72. doi: 10.1002/jlb.60.4.465. [DOI] [PubMed] [Google Scholar]
- 24.Grewal IS, Rutledge BJ, Fiorillo JA, et al. Transgenic monocyte chemoattractant protein-1 (MCP-1) in pancreatic islets produces monocyte-rich insulitis without diabetes: abrogation by a second transgene expressing systemic MCP-1. J Immunol. 1997;159:401–8. [PubMed] [Google Scholar]
- 25.Camacho SA, Heath WR, Carbone FR, et al. A key role for ICAM-1 in generating effector cells mediating inflammatory responses. Nat Immunol. 2001;2:523–9. doi: 10.1038/88720. [DOI] [PubMed] [Google Scholar]
- 26.Aragay AM, Mellado M, Frade JM, et al. Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2. Proc Natl Acad Sci USA. 1998;95:2985–90. doi: 10.1073/pnas.95.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ploix C, Lo D, Carson MJ. A ligand for the chemokine receptor CCR7 can influence the homeostatic proliferation of CD4 T cells and progression of autoimmunity. J Immunol. 2001;167:6724–30. doi: 10.4049/jimmunol.167.12.6724. [DOI] [PubMed] [Google Scholar]
- 28.Hill HR, Augustine NH, Rallison ML, Santos JI. Defective monocyte chemotactic responses in diabetes mellitus. J Clin Immunol. 1983;3:70–7. doi: 10.1007/BF00919141. [DOI] [PubMed] [Google Scholar]
- 29.Josefsen K, Nielsen H, Lorentzen S, Damsbo P, Buschard K. Circulating monocytes are activated in newly diagnosed type 1 diabetes mellitus patients. Clin Exp Immunol. 1994;98:489–93. doi: 10.1111/j.1365-2249.1994.tb05517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wandall JH. Leucocyte function in patients with rheumatoid arthritis: quantitative in-vivo leucocyte mobilisation and in-vitro functions of blood and exudate leucocytes. Ann Rheum Dis. 1985;44:694–700. doi: 10.1136/ard.44.10.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukacs K, Kavai M, Sipca S, Sonkoly I, Szabo G, Szegedi G. Defective monocyte chemotaxis in systemic lupus erythematosus. Acta Med Acad Sci Hung. 1981;38:49. [Google Scholar]
- 32.Badolato R, Sozzani S, Malacarne F, et al. Monocytes from Wiskott—Aldrich patients display reduced chemotaxis and lack of cell polarization in response to monocyte chemoattractant protein-1 and formyl-methionyl-leucyl-phenylalanine. J Immunol. 1998;161:1026–33. [PubMed] [Google Scholar]
- 33.Dupuis-Girod S, Medioni J, Haddad E, et al. Autoimmunity in Wiskott—Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics. 2003;111:e622–7. doi: 10.1542/peds.111.5.e622. [DOI] [PubMed] [Google Scholar]
- 34.Litherland SA, She JX, Schatz D, et al. Aberrant monocyte prostaglandin synthase 2 (PGS2) expression in type 1 diabetes before and after disease onset. Pediatr Diabetes. 2003;4:10–8. doi: 10.1034/j.1399-5448.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 35.Litherland SA, Xie XT, Hutson AD, et al. Aberrant prostaglandin synthase 2 expression defines an antigen-presenting cell defect for insulin-dependent diabetes mellitus. J Clin Invest. 1999;104:515–23. doi: 10.1172/JCI4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hempel SL, Monick MM, Hunninghake GW. Lipopolysaccharide induces prostaglandin H synthase-2 protein and mRNA in human alveolar macrophages and blood monocytes. J Clin Invest. 1994;93:391–6. doi: 10.1172/JCI116971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thivierge M, Le Gouill C, Tremblay MJ, Stankova J, Rola-Pleszczynski M. Prostaglandin E2 induces resistance to human immunodeficiency virus-1 infection in monocyte-derived macrophages: downregulation of CCR5 expression by cyclic adenosine monophosphate. Blood. 1998;92:40–5. [PubMed] [Google Scholar]
- 38.Luft T, Jefford M, Luetjens P, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–72. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 39.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–61. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 40.Shanmugam N, Reddy MA, Guha M, Natarajan R. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes. 2003;52:1256–64. doi: 10.2337/diabetes.52.5.1256. [DOI] [PubMed] [Google Scholar]