Abstract
Human immunodeficiency virus (HIV) codes for a protein, Rev, that mediates the viral RNA export from the nucleus to the cytoplasm. Recently, it has been found that Sam68, the substrate of Src associated in mitosis, is a functional homologue of Rev, and a synergistic activator of Rev activity. Thus, it has been suggested that Sam68 may play an important role in the post-transcriptional regulation of HIV. Sam68 contains an RNA binding motif named KH [homology to the nuclear ribonucleoprotein (hnRNP) K]. Tyrosine phosphorylation of Sam68 and binding to SH3 domains have been found to negatively regulate its RNA binding capacity. Besides, tyrosine phosphorylation of Sam68 allows the formation of signalling complexes with other proteins containing SH2 and SH3 domains, suggesting a role in signal transduction of different systems in human lymphocytes, such as the T cell receptor, and leptin receptor, or the insulin receptor in other cell types. In the present work, we have found that Sam68 is tyrosine phosphorylated in peripheral blood mononuclear cells (PBMC) from HIV infected subjects, leading to the formation of signalling complexes with p85 the regulatory subunit of PI3K, GAP and STAT-3, and decreasing its RNA binding capacity. In contrast, PBMC from HIV infected subjects have lower expression levels of Sam68 compared with controls. These results suggest that Sam68 may play some role in the immune function of lymphocytes in HIV infection.
Keywords: HIV, PBMC, Sam68, signalling, tyrosine phosphorylation
Introduction
Complex retroviruses such as the human immunodeficiency virus (HIV) have a common strategy for the transport of viral RNA, both the messenger and the intron-containing genomic RNA, from the nucleus to the cytoplasm [1]. HIV codes for a protein, Rev, which contain a leucine-rich, nuclear export sequence (NES) and binds to a viral response element (RRE) and the nuclear export receptor CRM1, functioning as an adaptor between the viral RNA and the export machinery [2,3]. Rev is synthesized from processed RNA and is localized in the nucleus because of the nuclear localization signal, which interact with the import receptor, importin β[4]. In the nucleus, Rev interact with unspliced viral mRNAs to induce the sequence-specific nuclear export. The translation of the viral transcrpits results in the production of structural and accessory proteins. Rev is therefore acting as a regulator of the switch from the early to the late viral gene expression.
Recently, it has been found that Sam68, the substrate of Src associated in mitosis [5,6], is a functional homologue of Rev, and a synergistic activator of Rev activity [7]. Moreover, deletion of Sam68 C-terminal part, or single mutation in the nuclear localization domain of Sam68, produce a dominant negative mutant, which may inhibit Rev activity and even HIV replication [7,8]. Thus, it has been suggested that Sam68 may play an important role in the post-transcriptional regulation of retroviruses, including HIV [9].
Sam68 contains an RNA binding motif named KH because of its homology to the nuclear ribonucleoprotein (hnRNP) K, within a larger conserved domain called GSG or STAR, which may mediate protein localization and cell cycle progression [9,10]. Sam68 also contains a region similar to RGG (a domain with various Arg-Gly-Gly motives). Both KH domain and RGG box mediates Sam68 binding to RNA. Besides, Sam68 may bind single strand nucleic acids and polymeric RNA in vitro. A splice variant lacking the KH domain has a lower capacity to bind RNA and may antagonize cell cycle [11]. In addition, tyrosine phosphorylation of Sam68 by p59fyn or BRK/sik and binding to SH3 domains may negatively regulate its nucleic acid binding capacity [10,12,13]. Tyrosine phosphorylated Sam68 also leads to the interaction with SH2 domain-containing proteins [5,14], including kinases of the Src family [5,6,13,15], Sik/BRK [12], as well as the kinases of the Itk/Tec family [16,17]. Moreover, tyrosine phosphorylated Sam68 can also interact with SH2 containing adaptor proteins and signalling enzymes such as Grb2 [18,19], Grap [19], Nck [20], PLCγ-1 [18], Ras-GAP [18,21,22] and p85α-PI3K [15,23].
Sam68 also has six proline-rich motifs, which can interact with the SH3 domains of Src [5,6,24], and other kinases of this family, such as Sik or BRK [12], p59fyn [18] or Itk/Tec/BTK [16,17]. This interaction of Sam68 with SH3 domains enables these kinases to tyrosine phosphorylate the substrate [18,24]. Sam68 can also interact with SH3 domains of various signalling enzymes and adaptor proteins. Thus, Sam68 has been found to bind the SH3 domains of p85 PI3K [15], PLC-γ-1 [18,25], protein arginine methyltransferase (PRMT) [26], Grb-2, Grap [19] and Nck [20]. The presence of these consensus sequences to interact with different domains allows this protein to participate in signal transduction pathways triggered by tyrosine kinases. Thus, Sam68 participate in the signalling of T cell receptors (TCR), being tyrosine phosphorylated and recruited to specific signalling complexes [27–29].
Recently, we have found that leptin stimulation of peripheral blood mononuclear cells (PBMC) induces the tyrosine phosphorylation of Sam68, promoting the association with p85, the regulatory subunit of PI3K. Moreover, leptin also increased the association of tyrosine phosphorylated Sam68 with tyrosine phosphorylated STAT-3, which then translocate from the cytoplasm to the nucleus, whereas the RNA binding activity of tyrosine phosphorylated Sam68 was decreased [30–32]. Thus, leptin seems to play a role in the activation of lymphocytes [32]. In this context, we have found that leptin receptor is overexpressed in PBMC from HIV infected subjects and tyrosine phosphorylated in a similar way to the in vitro stimulation with PHA or ConA [33], suggesting a possible role of leptin in the activation of lymphocytes in HIV infection. Because we had previously found the participation of Sam68 in leptin receptor signalling of PBMC [32,34], which is overexpressed in HIV patients [34], and Sam68 has been also found to be recruited to the TCR signalling, which is activated in HIV infection [35], we sought to study the Sam68 signalling in the PBMC from HIV infected subjects, by studying the tyrosine phosphorylation of Sam68, the formation of signalling complexes, the RNA binding activity and the expression level of Sam68.
Materials and methods
Materials
Antibodies against Sam68, STAT-3 and GAP were purchased from Santa Cruz (Santa Cruz, CA, USA). Antibody against p85-PI3K was from Upstate Biotechnology (Lake Placid, NY, USA). Monoclonal antibodies to phosphotyrosine (α-PY) were purchased from Transduction Laboratories (Lexington, KY, USA).
Patients
HIV infected patients were from the Internal Medicine Department (AIDS Unit) and were selected by their similar clinical characteristics, low viral load and intermediate number of CD4+ T cells (between 200 and 600 per microlitre). Clinical characteristics of subjects are shown in Table 1. Informed consent was obtained from the patients and the studies had the approval from the ethical committee of the Virgen Macarena University Hospital.
Table 1. Clinical features of HIV infected patients.
| Age (mean) | 30 |
| Years since diagnosis (median) | 7 |
| Male/female gender | 7/4 |
| Risk category | |
| Parenteral drug users | 4 |
| Homosexual men | 2 |
| Heterosexual contact | 4 |
| AIDS | 4 |
| Highly active antiretroviral therapy | 7 |
| Protease inhibitor | 2 |
| Non-nucleoside transcriptase inhibitor | 5 |
| Undetectable viral load (< 50 copies/ml) | 6 |
| Viral load log* (median, range) | 5·2 |
| CD4 cell count/mm3 (median) | 408 |
| Co-infection | |
| HCV | 5 |
| HBV | 0 |
In those with detectable viral load.
Cell preparation and culture
PBMC obtained from normal donors and HIV infected patients were isolated from heparinized venous blood by density-gradient sedimentation over Ficoll-Hypaque (Seromed Biochrom KG, Berlin, Germany), as described previously [36,37]. Cells were then washed twice in phosphate buffered saline (PBS) and solubilized for 30 min at 4°C in lysis buffer containing 20 m M Tris, pH 8, 1% nonidet P-40, 137 mM NaCl, 1 m M MgCl2, 1 m M CaCl2, 1 m M dithiothreitol (DTT), 10% glycerol, 1 m M phenylmethyl-sulphonyl fluoride and 0·4 m M sodium orthovanadate [21,23,30,31]. After centrifugation, protein concentration was determined by a kit from Bio-Rad (Richmond, CA, USA), using bovine serum albumin as a standard.
Immunoprecipitation and Western blotting analysis
Soluble cellular lystes (0·5 mg of protein) were precleared with 50 ml of protein A-Sepharose (Pharmacia, Uppsala, Sweden) for 2 h at 4°C by end-over-end rotation. The precleared cellular lysates were incubated with appropriate antibodies for 2 h at 4°C[21,23,30,31]. Next, 50 µl of protein A-Sepharose was added to immunocomplexes and incubation was continued for 1 h at 4°C. The immunoprecipitates were washed three times with lysis buffer and 40 µl of sodium dodecyl sulphate (SDS)-stop buffer containing 100 mmol/l DTT added. The immunoprecipitates samples were boiled for 5 min and the resultant products resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically onto nitrocellulose membranes. The blots were then analysed with the appropriate antibody as previously described [21,23,30,31] using a high sensitive chemiluminescence system (SuperSignal, Pierce, Rockford, IL, USA). The bands obtained were scanned and analysed by the pcbas 2·0 program. Student's t-test was used for comparisons, with differences being considered significant at P < 0·05.
Sam68 mRNA detection by reverse transcription-polymerase chain reaction (RT-PCR) and protein detection by specific immunoblot
Total RNA from PBMC (1 × 106 cells) was extracted using the QuickPrep Total RNA extraction kit (Amersham Pharmacia Biotech, Barcelona, Spain). First-strand cDNA synthesis was performed using an oligo-dT primer (Kit from Roche Molecular Biochemicals, Barcelona, Spain) and this was then used for detection of Sam68 mRNA by RT-PCR as described previously [11]. The sequences of primers and hybridization probes for Sam68, encompassing nucleotides 511–534 and 1101–1125 of Sam68cDNA located within the KH domain have been used previously for the detection of Sam68 expression [11]. β-actin mRNA expression was used as an internal control. The PCR products were analysed by 1% agarose gel with ethidium bromide staining. The bands obtained were scanned and analysed by the pcbas 2·0 program [30,31]. Values are expressed as means ± s.e.m. Student's t-test was used for comparisons, with differences being considered significant at P < 0·05.
Cell lysates for Sam68 protein detection was obtained as described above, denatured in SDS-stop buffer containing 100 mmol/l DTT by boiling for 5 min. Samples were then analysed by Western blot as described above. The bands obtained were scanned and analysed by the pcbas 2·0 program. Student's test was used for comparisons, with differences being considered significant at P < 0·05.
Results
Sam68 is tyrosine phosphorylated decreasing its RNA binding capacity in PBMC from HIV infected subjects
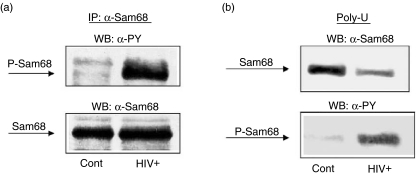
It has been shown that Sam68 is tyrosine phosphorylated after lymphocyte stimulation and then associates with various SH2 domain-containing proteins [13,18,27,28]. More recently, we have found that PBMC from HIV+ patients contain activated leptin receptor, whereas PBMC from control subjects lack such activation [32]. Moreover, we have found that Sam68 is tyrosine phosphorylated upon human leptin stimulation in PBMC human cells [30,34]. On the basis of these previous data, we sought to check the possible tyrosine phosphorylation of Sam68 in PBMC from HIV+ patients. We analysed the phosphorylation of immunoprecipitated Sam68 proteins by Western blot using antiphosphotyrosine antibody. Immunoprecipitation was controlled by immunoblotting with the same immunoprecipitating antibody. As shown in Fig. 1a, Sam68 tyrosine phosphorylation is increased significantly in PBMC from HIV+ patients compared with Sam68 in PBMC from control subjects. As tyrosine phosphorylation of Sam68 by BRK and leptin signalling has been shown previously to inhibit RNA binding activity [12,33], we checked the association of Sam68 of PBMC from HIV infected subjects to immobilized poly(U). As shown in Fig. 1b, there is a decreased association of Sam68 with poly(U) in PBMC from HIV infected subjects compared with controls. The lower amount of Sam68 from HIV samples bound to poly(U) shows higher phosphorylation level than the higher amount of Sam68 associated with poly(U) in samples from control subjects. Similar results have been found in activated PBMC in vitro[33].
Fig. 1.
Sam68 is tyrosine phosphorylated in peripheral blood mononuclear cells (PBMC) from HIV infected patients, decreasing the RNA binding capacity of Sam68. Cells (PBMC) from HIV infected and control subjects were homogenized in lysis buffer and soluble supernatant immunoprecipitated with anti-Sam68 antibodies or precipitated with Sepharose-coupled poly(U) beads. Immunoprecipitation samples were then analysed by immunoblot with antiphosphotyrosine or anti-Sam68 antibodies. Representative results are shown.
Tyrosine phosphorylation of Sam68 in PBMC from HIV infected subjects promotes the interaction with signalling complexes
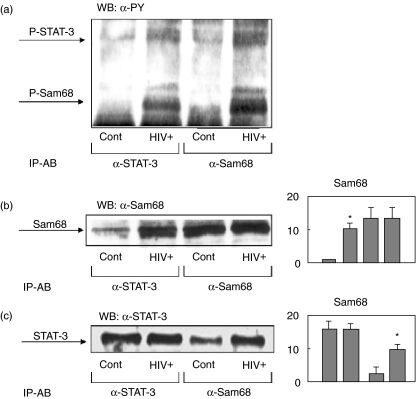
Because we had found previously that tyrosine phosphorylated Sam68 associates with phosphorylated STAT-3 in activated lymphocytes [30], we next wanted to assess this association in PBMC from HIV infected subjects. As shown in Fig. 2, cell lysates from controls and HIV infected subjects where immunoprecipitated with anti-STAT-3 and anti-Sam68 antibodies. Immunoblot analysis with antiphosphotyrosine antibodies showed the same pattern of bands (Fig. 2a), i.e. two major bands of 68 and 92 Kda, corresponding to the expected size of Sam68 and STAT-3 [30]. The immunoprecipitates were also analysed by immunoblot using anti-Sam68 antibodies, demonstrating the association of Sam68 to STAT-3 in PBMC from HIV infected subjects (Fig. 2b). The Western blot with anti-STAT-3 antibodies showed similar results, confirming the increased association of both proteins in PBMC from HIV infected subjects (Fig. 2c).
Fig. 2.
Sam68 is tyrosine phosphorylated in peripheral blood mononuclear cells (PBMC) from HIV infected patients, mediating the formation of signalling complexes with STAT-3. Cells (PBMC) from HIV infected and control subjects were homogenized in lysis buffer and soluble supernatant immunoprecipitated with anti-Sam68 or anti-STAT-3 antibodies. Immunoprecipitation samples were then analysed by immunoblot with antiphosphotyrosine (a), anti-Sam68 (b) and anti-STAT-3 antibodies (c). Representative results are shown. Panels show the means ± s.e.m. of the quantitative data. *P < 0·001 versus control.
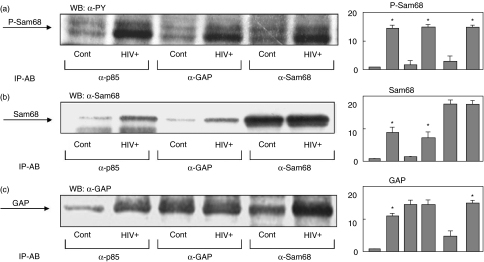
Tyrosine phosphorylated Sam68 has also been shown to associate with other SH2 domain containing proteins in different systems [18,20–22], as well as in activated T lymphocytes [13]. Therefore, we wanted to assess the physical association of the phosphorylated Sam68 with the SH2 domain containing proteins p85 PI3K and p120ras-GAP in PBMC from HIV infected subjects. In fact, the phosphotyrosine immunoblot of antip85, anti-GAP and anti-Sam68 showed the corresponding band of phosphorylated Sam68 in HIV samples (Fig. 3a). The physical association of Sam68 with p85 and GAP was confirmed by Western blot analysis of the same immunoprecipitates with anti-Sam68 antibodies. As shown in Fig. 3b, PBMC from HIV infected subjects showed an increase in the association of Sam68 to p85 PI3K and GAP compared with control samples. Similar results were obtained by immunoblotting of the same immunoprecipitates with anti-GAP antibodies, showing an increase in GAP association to the Sam68 and p85 complexes in the PBMC from HIV infected subjects.
Fig. 3.
Tyrosine phosphorylation of Sam68 in peripheral blood mononuclear cells (PBMC) from HIV infected patients mediates the formation of signalling complexes with p85-PI3K and GAP. Cells were homogenized in lysis buffer and soluble supernatant immunoprecipitated with anti-Sam68, anti-GAP or antip85 antibodies. Immunoprecipitation samples were then analysed by immunoblot with antiphosphotyrosine (a), anti-Sam68 (b) and anti-GAP (c) antibodies. Representative results are shown. Panels show the means ± s.e.m. of the quantitative data. *P < 0·001 versus control.
Sam68 mRNA and Sam68 protein levels are decreased in PBMC from HIV infected subjects
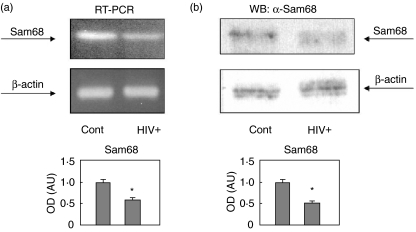
Because Sam68 seems to play a role in HIV replication and transcription [2,4,7,8,38,39], and we have found that Sam68 is tyrosine phosphorylated and recruited to signalling complexes in PBMC from HIV infected patients (Figs 1–3), we next wanted to study the possible effect of HIV infection in the expression of Sam68 from PBMC compared to control samples. We have found that the expression level of Sam68 is decreased in PBMC from HIV samples. As shown in Fig. 4a, PBMC from HIV infected subjects express about half the mRNA expressed by control subjects. Similar results were obtained by analysing the amount of Sam68 by specific immunoblot. As shown in Fig. 4b, the amount of Sam68 in PBMC from HIV infected subjects was about half the amount observed in control samples.
Fig. 4.
Sam68 expression is decreased in peripheral blood mononuclear cells (PBMC) from HIV infected patients. (a) Total RNA was isolated from control and patient PBMC. RNA was retrotranscribed and amplified by reverse transcription-polymerase chain reaction (RT-PCR) using primers that amplify KH region. Twenty cycles of PCR allowed the evidence for differences in the RNA levels. β-actin was amplified as a control of total RNA amount. The lower panel shows the means ± s.e.m. of the quantitative data. *P < 0·01 versus control. (b) Cells were homogenized in lysis buffer and soluble supernatants were normalized by protein concentration and analysed by Western blot using anti-Sam68 antibodies. Anti-β-actin immunoblot was employed to check the general amount of protein in the blots. The lower panel shows the means ± s.e.m. of the quantitative data. *P < 0·01 versus control.
Discussion
Sam68 has been found to be a cellular RNA binding protein that is able to substitute for the HIV protein Rev in RNA export function [2,4,7,8], although via different mechanisms [7]. Besides, Sam68 and other KH containing proteins may modulate HIV replication by post-transcriptional regulation [40]. In fact, it has been found that that down-modulation of constitutive Sam68 expression markedly inhibits HIV-1 production [41]. On the other hand, there is growing evidence of the role of Sam68 in signalling of different systems [9,34]. Thus, binding of the ligands to CD16, CD32, TCR, leptin receptor and insulin receptor increase the tyrosine phosphorylation level of Sam68 [13,23,28,31,42]. In this context, we have found previously that leptin receptor expression is up-regulated, and tyrosine phosphorylated in HIV infected patients, in a similar way to other cytokine receptors [34]. Therefore the tyrosine phosphorylation of Sam68 in HIV+ subjects that we have found in the present work was not striking. Moreover, other groups have found that Sam68 can be tyrosine phosphorylated by TCR signalling [13,27,28], which is known to be activated in lymphocytes from HIV infected patients [35,43]. Thus, our results showing an increase in the tyrosine phosphorylation level of Sam68 in PBMC from HIV+ samples should actually be expected. However, we do not know which tyrosine kinase is responsible for this increased tyrosine phosphorylation level of Sam68 in these subjects, although possible candidates should include those reported previously to have Sam68 as a substrate, such as Lck, Fyn [13,28], ZAP-70 [44], or some kinase downstream of cytokine receptors belonging to the JAK family [33,34]. In this context, we have found that leptin stimulation activates JAK-2 and JAK-3, which could mediate the leptin-stimulated tyrosine phosphorylation of Sam68. Moreover, JAK/STAT pathway has been found previously to be activated in HIV infection [45].
Tyrosine phosphorylation of Sam68 by the Src family kinase p59fyn has been shown previously to regulate negatively its association with RNA [46] and poly(U) [47]. Moreover, leptin stimulation of peripheral blood mononuclear cells dose-dependently increased tyrosine phosphorylation level of Sam68 [31], inhibiting the binding efficiency of Sam68 to poly(U) [31]. Similarly, we have found that tyrosine phosphorylated Sam68 has a decreased capacity of binding to poly(U) in PBMC from HIV infected patients. The modulation by phosphorylation of the RNA binding capacity of Sam68 may be involved in the post-transcriptional modulation of RNA. In this way, Sam68 has been proposed to give means for a rapid pathway to regulate protein expression by modifying the mRNA stability and/or mRNA translation. Moreover, Sam68 has been shown to interact with the splicing-associated factor YT521-B in nuclear dots and this interaction is regulated by Tyr-phosphorylation [46]. Thus, Tyr-phosphorylation of Sam68 by leptin stimulation could modulate its association with the splicing machinery in a similar way to that described for p59fyn, and in this way it could influence splice site selection. We do not know, however, the functional significance of this modification of RNA binding ability of Sam68 in HIV infection.
The amino acid sequence of Sam68 presents different domains that mediate the interaction with different signalling proteins. In fact, the modular structure of Sam68 containing protein—protein interaction motifs led to the concept that consider Sam68 as an adaptor protein with a putative role in signal transduction [13,18,34]. Thus tyrosine phosphorylated Sam68 has been found to interact with the SH2 domains of p85, the regulatory subunit of PI3K [15,23,29]. Thus, we have found recently that tyrosine phosphorylated Sam68 associates with p85 PI3K upon activation of PBMC with leptin stimulation [31]. PI3K is involved in TCR and CD28 signal in T cell activation [48]. In this context, we have found that tyrosine phosphorylated Sam68 is associated with p85 PI3K in PBMC from HIV infected subjects. Activation of TCR has been found previously to promote the formation of signalling complexes containing tyrosine phosphorylated Sam68 and p85 PI3K [49]. Therefore, the association of phosphorylated Sam68 with p85 PI3K that we have found in PBMC from HIV samples may be explained by the activation state of T cells. Similarly, tyrosine phosphorylated Sam68 has also been found to associate with GAP in T cells upon T cell activation [49]. In fact, it has been shown that Sam68 tyrosine phosphorylated by TCR activation associates with both PI3K and GAP in T cells [49], in a similar way to the signalling complexes observed upon lymphocyte activation with leptin [29–32]. In this way, Sam68 may provide a link between the PI3K and Ras pathway in the signalling of T cells in a similar manner to that observed in other systems, such as the insulin receptor [21,23,29].
Even though Sam68 seems to be recruited to signalling in activated lymphocytes in HIV infection, we have found that Sam68 expression in decreased in PBMC from HIV infected subjects. Thus, mRNA levels of Sam68 as well as protein levels are decreased compared with controls. At this stage, we do not know whether this effect is due to the HIV infection or whether it may be caused by the activation of lymphocytes. More importantly, we do not know what may be the pathophysiological consequences of this effect in lymphocytes, although it can be speculated that the proliferation response of T cells may be compromised as Sam68 is known to have a role controlling cell proliferation [9,11].
In summary, we have found that Sam68 is tyrosine phosphorylated in PBMC from HIV infected subjects, decreasing the RNA binding capacity of Sam68, decreasing Sam68 mRNA levels, and being recruited to p85 PI3K and p120 GAP, leading to the engagement of PI3K and GAP-Ras signalling pathways. In conclusion, these data suggest that Sam68 may play a role in the immune function of lymphocytes in HIV infection.
Acknowledgments
This work was supported by the Consejería de Salud, Junta de Andalucia, Spain (grant 05/02 and 76/04). Souad Najib is the recipient of a fellowship from Virgen Macarena University Hospital (Beca Asociacion Sanitaria Virgen Macarena).
References
- 1.Cullen BR. Retroviruses as model systems for the study of nuclear RNA export pathways. Virology. 1998;249:203–10. doi: 10.1006/viro.1998.9331. [DOI] [PubMed] [Google Scholar]
- 2.Cochrane AW, Chen C-H, Rosen CA. Specific interaction of the HIV Rev transactivator protein with a structured region in the env mRNA. Proc Natl Acad Sci USA. 1990;87:1198–201. doi: 10.1073/pnas.87.3.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fornerod M, Ohno M, Yoshida M, Mattaj I. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–60. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 4.Malim MH, Bohnlein S, Hauber J, Cullen BR. The HIV-1 rev trans-activator-derivation of a trans-dominant repressor of rev function. Cell. 1989;58:205–15. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- 5.Fumagalli S, Totty N, Hsuan J, Courtneidge S. A target for Src in mitosis. Nature. 1994;368:871–4. doi: 10.1038/368871a0. [DOI] [PubMed] [Google Scholar]
- 6.Taylor SJ, Shalloway D. An RNA-binding protein associated with Src through its SH2 and SH3 domains in mitosis. Nature. 1994;368:867–71. doi: 10.1038/368867a0. [DOI] [PubMed] [Google Scholar]
- 7.Reddy TR, Xu W, Mau J, et al. Inhibition of HIV replication by dominant negative mutants of Sam68, a functional homolog of HIV. Nat Med. 1999;5:635–42. doi: 10.1038/9479. [DOI] [PubMed] [Google Scholar]
- 8.Reddy TR. A single point mutation in the nuclear localization domain of Sam68 blocks the Rev/REE-mediated transactivation. Oncogene. 2000;19:3110–4. doi: 10.1038/sj.onc.1203637. [DOI] [PubMed] [Google Scholar]
- 9.Lukong KE, Richard S. Sam68, the KH domain-containing superStar. Biochim Biophys Acta. 2003;1653:73–86. doi: 10.1016/j.bbcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Chen T, Damaj BB, Herrera C, Lasko P, Richard S. Self association of the single KH-domain family members Sam68, GRP33, GLD-1 and QK1: role of the KH domain. Mol Cell Biol. 1997;17:5707–18. doi: 10.1128/mcb.17.10.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barlat I, Maurier F, Duchesne M, Guitard E, Tocque B, Schweighoffer F. A role for Sam68 in cell cycle progression antagonized by a spliced variant within the KH domain. J Biol Chem. 1997;272:3129–32. doi: 10.1074/jbc.272.6.3129. [DOI] [PubMed] [Google Scholar]
- 12.Derry JJ, Richard S, Valderrama Carvajal H, et al. Sik (BRK) phosphorylates Sam68 in the nucleus and negatively regulates its RNA binding ability. Mol Cell Biol. 2000;20:6114–26. doi: 10.1128/mcb.20.16.6114-6126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fusaki N, Iwamatsu A, Iwashima M, Fujisawa JI. Interaction between Sam68 and src family tyrosine kinases Fyn and Lck, in T cell receptor signalling. J Biol Chem. 1997;272:6214–19. doi: 10.1074/jbc.272.10.6214. [DOI] [PubMed] [Google Scholar]
- 14.Pawson T, Gish GD, Nash P. SH2 domains, interaction modules and cellular wiring. Trends Cell Biol. 2001;11:504–11. doi: 10.1016/s0962-8924(01)02154-7. [DOI] [PubMed] [Google Scholar]
- 15.Taylor SJ, Anafi M, Pawson T, Shalloway D. Functional interaction between c-Src and its mitotic target Sam68. J Biol Chem. 1995;270:10120–4. doi: 10.1074/jbc.270.17.10120. [DOI] [PubMed] [Google Scholar]
- 16.Andreotti AH, Bunnell SC, Feng S, Berg LJ, Schreiber SL. Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature. 1997;38:93–7. doi: 10.1038/385093a0. [DOI] [PubMed] [Google Scholar]
- 17.Bunnell SC, Henry PA, Kolluri R, Kirchhausen T, Rickles RJ, Berg LJ. Identification of Itk/Tsk Src homology 3 domain ligands. J Biol Chem. 1996;271:25646–56. doi: 10.1074/jbc.271.41.25646. [DOI] [PubMed] [Google Scholar]
- 18.Richard S, Yu D, Blumer KJ, et al. Association of p62, a multifunctional SH2- and SH3- binding protein, with src-family tyrosine kinaes, Grb2, and phospholipase Cγ-1. Mol Cell Biol. 1995;15:186–97. doi: 10.1128/mcb.15.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trub T, Frantz JD, Miyazaki M, Band H, Shoelson SE. The role of a lymphoid-restricted, Grb2-like SH3-SH2-SH3 protein in T cell receptor signalling. J Biol Chem 1997. 1997;272:894–902. doi: 10.1074/jbc.272.2.894. [DOI] [PubMed] [Google Scholar]
- 20.Lawe DC, Hahn C, Wong AJ. The Nck SH2/SH3 adaptor protein is present in the nucleus and associates with the nuclear protein SAM68. Oncogene. 1997;14:223–31. doi: 10.1038/sj.onc.1200821. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Margalet V, Najib S. Sam68 is a docking protein linking GAP and PI3K in insulin receptor signalling. Mol Cell Endocrinol. 2001;183:113–21. doi: 10.1016/s0303-7207(01)00587-1. [DOI] [PubMed] [Google Scholar]
- 22.Guitard E, Barlat I, Maurier F, Schweighoffer F, Tocque B. Sam68 is a Ras-GAP- associated protein in mitosis. Biochem Biophys Res Commun. 1998;245:562–6. doi: 10.1006/bbrc.1998.8374. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Margalet V, Najib S. p68 Sam is a substrate of the insulin receptor and associates with the SH2 domains of p85 PI3K. FEBS Lett. 1999;455:307–10. doi: 10.1016/s0014-5793(99)00887-x. [DOI] [PubMed] [Google Scholar]
- 24.Weng Z, Thomas SM, Rickles RJ, et al. Identification of Src, Fyn, and Lyn SH3-binding proteins: implications for a function of SH3 domains. Mol Cell Biol. 1994;14:4509–21. doi: 10.1128/mcb.14.7.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maa MC, Leu TH, Trandel BJ, Chang JH, Parsons SJ. A protein that is highly related to GTPase-activating protein-associated p62 complexes with phospholipase C gamma. Mol Cell Biol. 1994;14:5466–73. doi: 10.1128/mcb.14.8.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Côté J, Boisvert FM, Boulanger MC, Bedford MT, Richard S. Sam68 RNA binding protein is an in vivo substrate for protein arginine N-methyltransferase 1. Mol Cell Biol. 2003;14:274–87. doi: 10.1091/mbc.E02-08-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jabado N, Pallier A, Le Deist F, Bernard F, Fischer A, Hivroz C. CD4 ligands inhibit the formation of multifunctional transduction complexes involved in T cell activation. J Immunol. 1997;158:94–103. [PubMed] [Google Scholar]
- 28.Jabado N, Jauliac S, Pallier A, Bernard F, Fischer A, Hivroz C. Sam68 association with p120GAP in CD4+ T cells is dependent on CD4 molecule expression. J Immunol. 1998;161:2798–803. [PubMed] [Google Scholar]
- 29.Najib S, Martín-Romero C, González-Yanes C, Sánchez-Margalet V. Role of sam68 as an adaptor protein in signal transduction. Cell Mol Life Sci. 2005;62:36–43. doi: 10.1007/s00018-004-4309-3. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Margalet V, Martin-Romero C. Human leptin signalling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cell Immunol. 2001;211:30–6. doi: 10.1006/cimm.2001.1815. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Romero C, Sanchez-Margalet V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: possible role of Sam68. Cell Immunol. 2001;212:83–91. doi: 10.1006/cimm.2001.1851. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez-Margalet V, Martin-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003;133:11–19. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez-Margalet V, Martin-Romero C, Gonzalez-Yanes C, Goberna R, Rodriguez-Bano J, Muniain MA. Leptin receptor (Ob-R) expression is induced in peripheral blood mononuclear cells by in vitro activation and in vivo in HIV-infected patients. Clin Exp Immunol. 2002;129:119–24. doi: 10.1046/j.1365-2249.2002.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Najib S, Martin-Romero C, Gonzalez-Yanes C, Sanchez-Margalet V. Role of Sam68 as an adaptor protein in signal transduction. Cell Mol Life Sci. 2005;62:37–44. doi: 10.1007/s00018-004-4309-3. [DOI] [PubMed] [Google Scholar]
- 35.Abbate I, Dianzani F, Capobianchi MR. Activation of signal transduction and apoptosis in healthy lymphomonocytes exposed to bystander HIV-1-infected cells. Clin Exp Immunol. 2000;122:374–80. doi: 10.1046/j.1365-2249.2000.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest. 1968;97:77–89. [PubMed] [Google Scholar]
- 37.Lucas M, Sanchez-Margalet V, Sanz A, Solano F. Protein kinase C activation promotes cell survival in mature lymphocytes prone to apoptosis. Biochem Pharmacol. 1994;47:667–72. doi: 10.1016/0006-2952(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 38.Soros VB, Carvajal HV, Richard S, Cochrane AW. Inhibition of human immunodeficiency virus type 1 Rev function by a dominant-negative mutant of Sam68 through sequestration of unspliced RNA at perinuclear bundles. J Virol. 2001;75:8203–15. doi: 10.1128/JVI.75.17.8203-8215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLaren M, Asai K, Cochrane A. A novel function for Sam68: enhancement of HIV-1 RNA-3′ end processing. RNA. 2004;10:1119–29. doi: 10.1261/rna.5263904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddy TR, Suhasini M, Xu W, et al. A role for KH domain proteins (Sam68-like mammalian proteins and Quaking roteins) in the post-transcriptional regulation of HIV replication. J Biol Chem. 2002;277:5778–84. doi: 10.1074/jbc.M106836200. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Liu Y, Kim BO, He JJ. Direct participation of Sam68, the 68-Kilodalton Src-associated protein in mitosis, in the CRM1-mediated Rev nuclear export pathway. J Virol. 2002;76:8374–82. doi: 10.1128/JVI.76.16.8374-8382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilbert C, Barabe F, Rollet-Labelle E, et al. Evidence for a role for SAM68 in the responses of human neutrophils to ligation of CD32 and to monosodium urate crystals. J Immunol. 2001;166:4664–71. doi: 10.4049/jimmunol.166.7.4664. [DOI] [PubMed] [Google Scholar]
- 43.Schmid-Antomarchi H, Benkirane M, Breittmayer V, et al. HIV induces activation of phosphatidylinositol 4-kinase and mitogen-activated protein kinase by interacting with T cell CD4 surface molecules. Eur J Immunol. 1996;26:717–20. doi: 10.1002/eji.1830260331. [DOI] [PubMed] [Google Scholar]
- 44.Lang V, Mege D, Semichon M, Gary-Gouy H, Bismouth G. A dual participation of ZAP-70 and src protein tyrosine kinases is required for TCR-induced tyrosine phosphorylation of Sam68 in Jurkat T cells. Eur J Immunol. 1997;27:3360–7. doi: 10.1002/eji.1830271235. [DOI] [PubMed] [Google Scholar]
- 45.Bovolenta C, Camorali L, Lorini AL, et al. Constitutive activation of STATs upon in vivo human immunodeficiency virus infection. Blood. 1999;94:4202–9. [PubMed] [Google Scholar]
- 46.Hartmann AM, Nayler O, Schwaiger FW, Obermeier A, Stamm S. The interaction and colocalization of Sam68 with the splicing-associated factor YT521-B in nuclear dots is regulated by the Src family kinase p59 (fyn) Mol Biol Cell. 1999;10:3909–26. doi: 10.1091/mbc.10.11.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang LL, Richard S, Shaw AS. P62 association with RNA is regulated by tyrosine phosphorylation. J Biol Chem. 1995;270:2010–3. doi: 10.1074/jbc.270.5.2010. [DOI] [PubMed] [Google Scholar]
- 48.Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PI3P. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 49.Jauliac S, Mazerolles F, Jabado N, et al. Ligands of CD4 inhibit the association of phospholipase Cgamma1 with phosphoinositide 3 kinase in T cells: regulation of this association by the phosphoinositide 3 kinase activity. Eur J Immunol. 1998;28:3183–91. doi: 10.1002/(SICI)1521-4141(199810)28:10<3183::AID-IMMU3183>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]