Abstract
We assessed the effect of exposure to Mycobacterium avium on the development of immune responses and the pathogenesis of disease observed following Mycobacterium bovis challenge. A degree of protection against M. bovis was observed in calves which were pre-exposed to M. avium as assessed by the extent of lesions and bacterial load compared to the M. bovis alone group. The immune response following M. bovis challenge in cattle previously inoculated with M. avium was biased towards antigens (PPD) present in M. avium, whereas the response following M. bovis alone was biased towards antigens present in M. bovis, indicating an imprinting of memory to avian antigens on T lymphocytes. A consequence of the memory to M. avium antigens was failure to diagnose M. bovis infection by the skin test or the IFNγ assay in some of the animals which had lesions of tuberculosis at necropsy. The use of M. bovis specific antigens ESAT-6 and CFP-10 increased IFNγ test specificity in animals previously exposed to M. avium but the responses to these antigens were lower than those observed in animals exposed to M. bovis alone. The implication is that responses to M. avium, although providing some immunity, may mask diagnosis of M. bovis infection, even when specific antigens are employed, potentially contributing to disease transmission in the field.
Keywords: Mycobacterium avium, Mycobacterium bovis, interferon γ, T lymphocytes, dendritic cells
Introduction
Mycobacterium bovis is the causative agent of bovine tuberculosis which is increasing in cattle herds in developed countries such as the UK, New Zealand and in the USA, possibly as a result of infection from wildlife reservoirs. Persistence of M. bovis in other parts of the world may account for up to 10% of cases of human tuberculosis [1]. Thus, an increase in M. bovis infections poses an increased human health risk and is also a major economic problem. In the UK, the introduction of the skin test and slaughter control policy in the 1950s significantly reduced the incidence of the disease. However, it is now clear that more effective control or vaccination strategies are required to control bovine tuberculosis.
The bacillus Calmette Guerin (BCG) vaccine was developed as a human tuberculosis vaccine in the 1920s but has variable efficacy in both humans and cattle [2–4]. Interference or masking of protection by previous exposure to environmental mycobacteria [3,5] or the presence of inappropriately biased immune responses [6,7] have been suggested as possible reasons for the variation in efficacy. However, there is also evidence that exposure to environmental mycobacteria may give a low level of protection against Mycobacterium tuberculosis that cannot be improved upon by vaccination with BCG [7–10].
TB in cattle has a similar pathogenesis to that in humans and immune responses post vaccination and post challenge can be easily measured [11]. Thus, M. bovis infections in cattle provide a model from which parallels in human disease can be suggested. Exposure to environmental mycobacteria occurs early following birth, with the majority of calves showing responses to avian PPD (PPD-A) within 6 weeks [12]. Studies of cattle with existing responses to M. avium revealed that following M. bovis challenge both skin test and IFNγ diagnostic tests were compromised [13] resulting in a failure to diagnose TB despite the presence of tuberculous lesions. In addition, BCG vaccination of adult cattle with pre-existing responses to environmental mycobacteria gave a significantly lower degree of protection against M. bovis challenge than that seen in animals without pre-existing responses [4, 14, 15]. As shown in human studies [16,17], neonatal vaccination of calves with BCG affords effective protection against M. bovis infections [12,18], providing a strategy to appropriately prime immune responses before environmental exposure.
Previously, we demonstrated that prior exposure to Mycobacterium avium affected the response of calves to BCG such that the PPD-B specific response to vaccination was masked by a strong response to PPD-A [19]. Here we demonstrate that M. avium confers a degree of protection from M. bovis infection and that diagnosis by both the comparative skin test and IFNγ test are compromised. Although inclusion of the specific antigens ESAT-6 and CFP-10 slightly improved the sensitivity of the IFNγ test in M. avium presensitized calves, responses to these antigens were significantly lower than those observed in the M. bovis alone group. These results highlight the importance of alternative diagnostic tests or vaccination strategies to circumvent the problem of environmental mycobacterial exposure.
Materials and methods
Inoculation with M. avium and M. bovis challenge
Cattle were British Holstein-Friesian calves (Bos taurus) bred at the Institute for Animal Health. The IAH herd has been confirmed free from bovine TB for more than 10 years. Animals were aged 4–6 months at time zero.
Ten calves were inoculated subcutaneously with 1·6 × 106 cfu M. avium strain D4ER [19] and nine with 7H9 control medium. After 12 weeks, five M. avium exposed and five control calves were challenged intranasally with 1 × 104 cfu virulent M. bovis[18]. All animals were necropsied 12 weeks later. The four calf groups were thus: M. avium alone; M. avium—M. bovis; M. bovis alone; mock control. The experiments were approved by the local ethics committee according to national UK guidelines.
Post-mortem examination and bacteriology
Lymph nodes of the head (retropharyngeal, submandibular, parotid) and thorax (mediastinal and four bronchus associated), tonsils, nasal and tracheal mucosa and the seven pulmonary lobes were examined for lesions. Gross lesions, and microscopic lesions in fixed tissue, were scored as previously described [18]. Tissue samples were frozen at −70 °C and the bacterial load was determined by titration of homogenates on modified 7H11 agar [20]. Colonies were counted after four weeks and suspect isolates examined after Ziehl-Neelsen staining.
Antigens
Purified protein derivatives from M. avium (PPD-A) and M. bovis (PPD-B) were obtained from the Tuberculin production unit at Veterinary Laboratories Agency (VLA), Weybridge, UK. Recombinant ESAT-6 and CFP-10 [21] were provided by the TB Research Group, VLA.
Tuberculin skin tests
The single comparative intradermal tuberculin test was performed, using 0·1 ml of PPD-A and PPD-B and reactions were read 72 h after injection. Results were recorded as increase in skin thickness at 72 h compared to thickness pre-injection and interpreted according to the standard protocol (European Communities Commission regulation number 1226/2002 [22]).
Immunological assays
Blood was collected into heparin (10 U/ml). Four ml of blood was incubated at 37 °C for 24 h with 200 µl PPD-A or PPD-B diluted in RPMI + 50 µg/ml gentamicin (final concentration 20 µg per ml). ESAT-6 and CFP-10 were used at a final concentration of 5 µg/ml. An equivalent volume of RPMI + gentamicin was used as control. The supernatant was stored at −20 °C. IFNγ was assayed by ELISA as described [23]. For analysis PPD-B specific IFNγ secretion was considered positive when antigen-induced IFNγ was >250 pg/ml above that induced by medium alone. This ‘cut off’ value was determined as the mean + 2 SD of PPD-B specific IFNγ induced in a group of 40 naive animals.
Generation of bovine monocyte derived-dendritic cells (DC)
Bovine monocytes were cultured with GMCSF and IL-4 to derive DC [24]. Peripheral blood mononuclear cells (PBMC) were incubated with anti-human CD14-labelled super-paramagnetic particles (Miltenyi-Biotech, Bergisch Gladbach, Germany), and cells were isolated from a Midimacs column (Miltenyi-Biotech). Cells were adjusted to 8 × 105 per ml in RPMI-1640 medium containing Glutamax-1 (Life Technologies, Paisley, UK), 10% heat inactivated FCS, 5 × 10−5 M 2-ME, 50 µg/ml gentamycin (tissue culture medium; TCM), 200 U/ml bovine rIL-4 and 0·2 U/ml bovine rGMCSF [24] and 3 ml of this suspension was added per well of 6-well plates. On day 3 of culture DC were harvested, washed, resuspended in TCM without gentamicin and cultured overnight with 1 cfu per cell of M. bovis or M. avium or an equivalent volume of TCM alone. Dendritic cells were then washed extensively to remove extracellular bacteria.
Measurement of intracellular IFN-γ
PBMC (105 per well) were incubated with 104M. avium or M. bovis infected or control DC for 5 days at 37 °C. PMA (50 ng/ml), ionomycin (1 µg/ml) and brefeldin-A (10 µg/ml) were added for the final 5 h of culture. Cells were fixed with 1% paraformaldehyde and then permeabilized (permeabilization solution; Becton Dickinson, Oxford, UK). Expression of IFNγ by CD4 and CD8 subsets was detected following staining with mAb CC30 (anti-bovine CD4, IgG1) plus 6H5 (anti-bovine IFNγ, IgG2a) or CC63 (anti-bovine CD8, IgG2a) plus CC302 (anti-bovine IFNγ, IgG1) [24]. Bound antibody was detected with FITC or PE labelled anti-mouse isotype specific reagents (Southern Biotechnology Associates, Birmingham, AL, USA). Immunofluorescent staining was analysed using FCS express software (De Novo Software, Ontario, Canada).
Statistical analysis
Analyses were performed using MINITAB. Differences in immunological responses, degree of pathology and bacterial colonization were compared using Mann—Whitney nonparametric test. Correlations were assessed using Spearman rank correlations.
Results
Exposure to M. avium induces partial protection against M. bovis
One group of calves inoculated with M. avium and a control group were challenged intranasally 12 weeks later with M. bovis. The effect of prior M. avium exposure on the development of disease was assessed by post mortem examinations performed 12 weeks post challenge (Table 1). Macroscopic lesions typical of TB were observed in 4 of 5 animals that were exposed to M. avium followed by M. bovis and in 4 of 5 control calves exposed only to M. bovis. Lesions were confined largely to the head lymph nodes. The extent of lesions varied considerably between animals and although the M. avium presensitized animals had a reduced degree of pathology (median lesion scores M. avium-M. bovis: 4; M. bovis alone: 9), these differences were not significant (P = 0·29). No lesions were found in calves exposed only to M. avium or in the mock control group of calves.
Table 1. Lesions at necropsy 12 weeks post M. bovis challenge in M. avium exposed and control calves.
| Animal number | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. avium-M. bovis | M. bovis alone | |||||||||||
| 270 | 291 | 356 | 349 | 282 | Median | 336 | 286 | 277 | 353 | 343 | Median | |
| Tissue | ||||||||||||
| ″Parotid R | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 3 | 2 | ||
| ″Parotid L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | ||
| ″Submandibular R | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 3 | 3 | 1 | ||
| ″Submandibular L | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 2 | ||
| ″Retropharyngeal R | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 3 | 2 | 4 | ||
| ″Retropharyngeal L | 0 | 0 | 1 | 3 | 1 | 0 | 0 | 3 | 4 | 3 | ||
| ″Mediastinal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| ″Bronchial 1 | 0 | 0 | 0 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | ||
| Total tissues affected | 0 | 1 | 2 | 2 | 5 | 1·5 | 0 | 2 | 3 | 4 | 6 | 3 |
| Total lesion score | 0 | 1 | 4 | 6 | 9 | 4 | 0 | 5 | 9 | 12 | 15 | 9 |
No lesions were evident in tonsils, diaphragmatic, apical, medial or intermediate lobes of the lung, or in 3 other bronchus associated lymph nodes. L and R, left and right. Twenty-two tissues were examined for each animal. Score 0–4 indicates increasing severity of lesions.
M. bovis was present in numbers up to 5·2 × 104 cfu/g from the majority of tissues with gross lesions (17/23 tissues; Table 2), with a significant correlation between the number of bacteria and lesion score (P < 0·01). Fewer bacteria were present in tissues from the M. avium—M. bovis group compared to the M. bovis alone group (P = 0·045).
Table 2. M. bovis viable counts in tissues taken postmortem.
| Animal number | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. avium-M. bovis | M. bovis alone | |||||||||||
| 270 | 291 | 356 | 349 | 282 | 336 | 286 | 277 | 353 | 343 | |||
| Tissue | ||||||||||||
| ″Parotid R | 0† | 0 | 0 | 0 | 0 | 0 | 4·6 | 0 | 3·61 | 3·32 | ||
| ″Parotid L | 0 | 0 | 0 | 0 | 2·98 | 0 | 1·69 | 0 | 0 | 3·86 | ||
| ″Submandibular R | 0 | 0 | 2·78 | 4·15 | 0 | 0 | 0 | 4·19 | 4·08 | 2·65 | ||
| ″Submandibular L | 0 | 0 | 0 | 0 | 4·48 | 0 | 0 | 4·22 | 4·23 | 3·51 | ||
| ″Retropharyngeal R | 0 | 0 | 3·7 | 3·52 | 3 | 0 | 0 | 4·72 | 4·22 | 3·97 | ||
| ″Retropharyngeal L | 0 | 0 | 0 | 4·16 | 0 | 0 | 0 | 0 | 0 | 4·05 | ||
| Total no. of tissues affected | 0 | 0 | 2 | 3 | 3 | 0 | 2 | 3 | 4 | 6 | ||
Viable count (log10 cfu/g tissue). The limit of detection using the isolation technique is 5 colonies. Six lymph nodes examined for each animal.
Effect of prior exposure to M. avium on development of skin test responses
Reactions to avian and/or bovine PPD in the skin test were observed in 14/15 animals exposed to either M. bovis or M. avium(Table 3). Four of 4 animals in the M. bovis alone group with lesions at post mortem would be identified as M. bovis reactor animals, compared with only one of four animals in the M. avium—M. bovis group that had lesions of TB. The PPD-B minus PPD-A response was significantly higher in the M. bovis alone group compared to the M. avium—M. bovis group (P = 0·02).
Table 3. Skin test responses 16 weeks post challenge with M. bovis.
| Increase (mm)‡ | ||||
|---|---|---|---|---|
| Group Animal/lesion score | PPD-A | PPD-B | B-A | Skin test interpretation§ |
| M. avium-M. bovis | ||||
| ″270† (0) | 17 | 2 | −15 | Negative |
| ″291 (1) | 13 | 11 | −2 | Negative |
| ″356 (4) | 14 | 18 | 4 | I |
| ″349 (6) | 14 | 21 | 7 | R |
| ″282 (9) | 3 | 3 | 0 | Negative |
| ″Median | 13 | 11 | 0* | |
| M. bovis alone | ||||
| ″336 (0) | 1 | 0 | −1 | Negative |
| ″286 (5) | 7 | 23 | 16 | R |
| ″277 (9) | 7 | 25 | 18 | R |
| ″353 (12) | 9 | 21 | 12 | R |
| ″343 (15) | 9 | 37 | 28 | R |
| ″Median | 7 | 23 | 16* | |
| M. avium alone | ||||
| ″279 | 13 | 4 | −9 | |
| ″283 | 7 | 1 | −6 | |
| ″304 | 11 | 5 | −6 | |
| ″339 | 4 | 0 | −4 | |
| ″329 | 8 | 3 | −5 | |
| ″Median | 8 | 3 | −5·5 | |
| Control | ||||
| ″273 | 0 | 0 | 0 | |
| ″289 | 0 | 1 | 1 | |
| ″333 | −1 | −1 | 0 | |
| ″368 | 0 | −1 | 1 | |
| ″Median | 0 | 0 | 0·5 | |
Animal number (lesion score)
Increase in skin thickness from day 0 to day 3
Interpretation of tuberculin skin test (standard interpretation according to EC Regulation 1226/2002). R, reactor; I, inconclusive reactor.
Statistically significant difference between these groups P = 0·03.
Skin test reactions were strongly PPD-A biased in the M. avium alone group and skin test reactivity in the mock control group was insignificant.
Cytokine secretion in whole blood following M. avium exposure
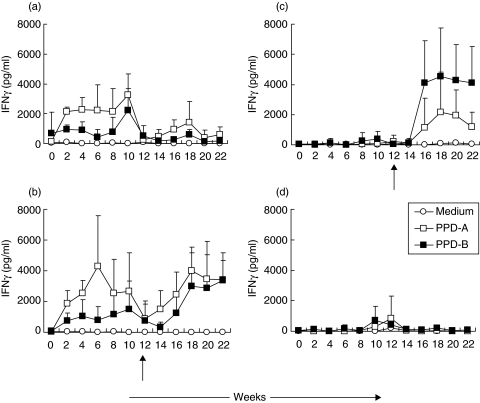
Immune responses were monitored for 12 weeks in calves inoculated with M. avium(Fig. 1a,b) and in mock-inoculated animals (Fig. 1c,d). Exposure to M. avium induced increases in IFNγ secretion in response to PPD-A which were evident at 2 weeks, peaked at 6 weeks but which were reduced significantly by week 12. Inoculation of M. avium also induced IFNγ secretion in response to PPD-B but these responses were significantly lower. No significant secretion of IFNγ was observed in the control mock-inoculated calves.
Fig. 1.
IFNγ secretion post M. avium exposure and post-M. bovis challenge. Calves were inoculated with M. avium (a, b) or mock inoculated (c, d). Twelve weeks later calves were challenged with M. bovis (b, c) or were untreated (a, d). The time of challenge is indicated by an arrow. Responses were assessed bi-weekly throughout the experiment. Whole blood was stimulated with PPD-A (□), PPD-B (▪) or medium control (○) and IFNγ secretion in supernatants was assessed by ELISA. Means ± SD for groups of 5 calves are shown.
Cytokine responses post M. bovis challenge
At 12 weeks two groups of calves were challenged with M. bovis (Fig. 1b,c). In the M. avium-M. bovis group (Fig. 1b), increased IFNγ secretion to PPD-A was observed by 2 weeks. In the M. bovis alone group (Fig. 1c) strong PPD-B biased IFNγ secretion was observed at 4 weeks post challenge. A lower level of IFNγ was secreted in response to PPD-A in these calves. Overall the ratio of PPD-B: A induced IFNγ was higher in the M. bovis group compared to the M. avium—M. bovis calves.
Differences between calves in reactivity to ESAT-6 and CFP-10 were noted (Fig. 2). Overall, responses were lower in the M. avium—M. bovis group compared to the M. bovis alone calves. Two calves that failed to respond to either antigen (no. 270 and no. 336) did not appear to have been productively infected with M. bovis. Two of 4 animals in the M. avium—M. bovis group with disease (no. 291 and no. 356) did not have PPD-B specific IFNγ but showed intermittent responses to ESAT-6 and CFP-10. Animal no. 349 did not display ESAT-6 or CFP-10 reactivity despite having significant disease. Calf no. 282 responded to CFP-10 on only 2 occasions and did not have PPD-B biased skin test reactivity, but did have significant disease. The calves in the M. bovis alone group also showed variable reactivity to ESAT-6 and CFP-10. With the exception of no. 336 all calves showed consistent IFNγ responses.
Fig. 2.
IFNγ secretion in response to M. bovis specific antigens ESAT-6 and CFP-10. Calves were inoculated with M. avium (a–e) or sham inoculated (f–j). Twelve weeks later all calves were challenged with M. bovis. Responses were assessed bi-weekly post challenge (week 0). Whole blood was stimulated with ESAT-6 (□), CFP-10 (▴) or medium control (▪) and IFNγ secretion in supernatants was assessed by ELISA. Individual animal data is shown.
IFNγ secretion by lymphocyte subsets
Four weeks following inoculation of M. avium, significant (P < 0·05) stimulation of CD4+ cells by M. avium infected DC was observed (Fig. 3a). This response was transiently boosted in the M. avium—M. bovis group 4 weeks post challenge (Fig. 3a). In those animals which were inoculated only with M. bovis, no M. avium reactive CD4+ cells were observed (Fig. 3a). High non specific responses of CD8+ T cells to M. avium-infected DC were observed at time 0 in the majority of animals (Fig. 3b) and these did not appear to be altered by exposure of the animals to M. avium.
Fig. 3.
Responses of CD4+ and CD8+ T lymphocytes to M. avium or M. bovis infected DC. Calves were inoculated with M. avium alone (▪), M. avium followed by M. bovis (□), M. bovis alone (•) or were sham inoculated (○). At the indicated time points PBMC were isolated and cultured for 5 days with monocyte derived DC that were infected with either M. avium (a, b) or M. bovis (c, d). Expression of intracellular IFNγ by CD4+ or CD8+ T cells was assessed by flow cytometry. Mean values ± SE for groups of 3 calves are shown.
No consistent responses to M. bovis infected DC were seen as a consequence of exposure to M. avium. Post challenge increased IFNγ secretion by CD4+ T lymphocytes in response to M. bovis infected DC was observed (Fig. 3c; P < 0·05) that was greatest in M. avium primed animals but reduced significantly in these (P < 0·05) by week 8 post challenge (Fig. 3c). In the M. bovis alone group a response of the CD8+ T cells to M. bovis DC was evident and still increasing at week 8 (Fig. 3d).
Discussion
The objective of this study was to assess the effect of exposure to M. avium, taken as a model of infection with an environmental mycobacterium, on the response of calves to virulent M. bovis. We showed that prior exposure to M. avium can induce a level of protection against M. bovis but that it also interferes with the diagnosis of M. bovis infection.
Calves inoculated with M. avium had fewer lesions at necropsy and significantly less bacteria present in the lymph nodes following intranasal inoculation of M. bovis, compared to animals that did not have prior experience of M. avium. Associated with this level of immunity was significantly less extensive skin test responses to PPD-B and more rapid, but lower, specific IFNγ and T cell responses in the animals exposed to M. avium. This suggests that the memory T cell response resulting from M. avium infection leads to a secondary response on challenge that results in reduced bacterial replication and as a consequence reduced pathology and immunological responses.
Other studies reported that exposure to environmental bacteria can provide a degree of protection against challenge with virulent mycobacteria. Humans with a higher sensitivity to M. avium antigens had a lower risk of developing tuberculosis [3]. Studies by Palmer and Long [7] in a guinea pig model reported that environmental mycobacteria induced a level of protection against aerosol challenge with M. tuberculosis. Some studies in mice showed that aerosol inoculation with M. avium partially protected against M. tuberculosis[9,10], whereas others where M. avium was inoculated subcutaneously [5] showed no protection. Only limited data are available for cattle. Amadori et al. [13] reported that cattle which were naturally responding to M. avium before M. bovis experimental challenge were not protected against disease. Their study also indicated that cattle with evidence for sensitization by M. avium showed masked responses to M. bovis in terms of both IFNγ and skin test reactivity.
We showed previously that exposure to M. avium prior to BCG vaccination induced an anamnestic response that was biased towards antigens present in PPD-A [19]. This implied that M. avium infection would prime the immune system and imprint a memory of the exposure onto the T cell repertoire that might also affect exposure to virulent M. bovis. Differences in the responses of T lymphocytes to DC infected with M. avium or M. bovis were observed. Thus, in calves exposed to M. avium, challenge with M. bovis boosted IFNγ expression by M. avium specific CD4+ T cells suggesting that prior exposure had primed the immune response. In addition, the M. bovis specific T lymphocyte response in the M. avium—M. bovis calves appeared shorter in duration than in calves exposed to M. bovis alone. Possibly, animals pre-exposed to M. avium were mounting a more rapid response that was aiding control of the infection while the animals exposed to M. bovis alone were developing a more intense immune response as the infection was progressing.
Memory of a prior exposure to M. avium also results in a boosting of the response to antigens common to M. avium and M. bovis which affects diagnostic tests for M. bovis infection. Thus, 4 of 4 calves exhibiting lesions following exposure to M. bovis would have been diagnosed as infected by the standard skin test and by the IFNγ test on 15 of 20 sampling points. In contrast only 1 of 4 animals that were exposed to both M. avium and M. bovis and which had lesions evident at necropsy would have been diagnosed as infected by the standard interpretation of the skin test. Furthermore, on only 3 of 20 occasions would assessing the IFNγ response have diagnosed infection in these 4 animals.
The use of M. bovis or M. tuberculosis-specific antigens has the potential to improve specificity and effective diagnosis and allows the distinction of BCG vaccinates from humans or animals with tuberculosis [25–29]. In this study, although standard diagnostic tests were affected in the calves exposed to M. avium before challenge with M. bovis, the use of ESAT-6 and CFP-10 increased the likelihood of detection of some animals as M. bovis positive. Thus, in a group of 5 calves exposed to M. avium prior to M. bovis only one (no. 349) would have been detected by the standard interpretation of the skin test, and by the IFNγ test, as having tuberculosis. Interestingly, this animal showed no response to ESAT-6 or CFP-10. Three further animals which were skin test negative and showed no or low intermittent IFNγ responses to PPD-B responded to either ESAT-6 or CFP-10 (or both) on at least some sampling points. Overall, compared to the use of PPD-A and PPD-B as test antigens, application of the specific antigens ESAT-6 and CFP-10 lead to improved test sensitivity in the animals pre-exposed to M. avium (lesioned animals detected by standard interpretation of the skin test: 1/4, PPD-B IFN-γ: 2/4, ESAT-6/CFP-10 IFN-γ: 3/4). Nevertheless, some animals would escape detection even when specific antigens are used particularly as the overall level of response to these antigens was much lower than that seen in calves exposed to M. bovis alone. The ESAT-6 and CFP-10 responses were correlated with both lesion score and bacterial burden, and lower responses to these antigens in the M. avium—M. bovis group likely reflects reduced bacterial replication within these animals. This study emphasizes the need to identify more specific antigens to complement ESAT-6 and CFP-10 thereby allowing the consistent identification of cattle with bovine tuberculosis. Such antigens have been identified recently [30] and efforts to identify further antigens are on-going.
In summary, we have demonstrated that exposure to the model environmental organism M. avium imparts a degree of immunity to M. bovis. However, it can interfere with the response to M. bovis such that diagnostic tests are compromised, although the use of specific antigens could improve test sensitivity. Though the dose and route of M. avium exposure used here may not necessarily reflect the field situation, this study clearly indicates the potential effects of exposure to environmental mycobacteria on the outcome of M. bovis infection. Although immunity to tuberculosis as a consequence of exposure to environmental mycobacteria may be advantageous in a natural environment it clearly has the potential to reduce the effectiveness of diagnosis and the removal of infected animals.
Acknowledgments
This work was funded by the Department for the Environment, Food and Rural Affairs (DEFRA), UK. We gratefully acknowledge the staff in the animal care facilities at the Institute for Animal Health, Marc Martin for bacteriology and Sue Stephens for histopathology and post mortem examinations.
References
- 1.Cosivi O, Grange JM, Daborn CJ, et al. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg Infect Dis. 1998;4:59–70. doi: 10.3201/eid0401.980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosismeta-analyses of the published literature. Pediatrics. 1995;96:29–35. [PubMed] [Google Scholar]
- 3.Fine PE. Variation in protection by BCG. implications of and for heterologous immunity. Lancet. 1995;346:1339–45. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 4.Buddle BM, Wards BJ, Aldwell FE, Collins DM, de Lisle GW. Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine. 2002;20:1126–33. doi: 10.1016/s0264-410x(01)00436-4. [DOI] [PubMed] [Google Scholar]
- 5.Brandt L, Feino Cunha J, Weinreich Olsen A, Chilima B, Hirsch P, Appelberg R, Andersen P. Failure of the Mycobacterium bovis BCG vaccine. some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun. 2002;70:672–8. doi: 10.1128/iai.70.2.672-678.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanford JL, Shield MJ, Rook GA. How environmental mycobacteria may predetermine the protective efficacy of BCG. Tubercle. 1981;62:55–62. doi: 10.1016/0041-3879(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 7.Palmer CE, Long MW. Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am Rev Respir Dis. 1966;94:553–68. doi: 10.1164/arrd.1966.94.4.553. [DOI] [PubMed] [Google Scholar]
- 8.Edwards ML, Goodrich JM, Muller D, Pollack A, Ziegler JE, Smith DW. Infection with Mycobacterium avium-intracellulare and the protective effects of Bacille Calmette-Guerin. J Infect Dis. 1982;145:733–41. doi: 10.1093/infdis/145.2.733. [DOI] [PubMed] [Google Scholar]
- 9.Orme IM, Roberts AR, Collins FM. Lack of evidence for a reduction in the efficacy of subcutaneous BCG vaccination in mice infected with nontuberculous mycobacteria. Tubercle. 1986;67:41–6. doi: 10.1016/0041-3879(86)90030-9. [DOI] [PubMed] [Google Scholar]
- 10.Orme IM, Collins FM. Efficacy of Mycobacterium bovis BCG vaccination in mice undergoing prior pulmonary infection with atypical mycobacteria. Infect Immun. 1984;44:28–32. doi: 10.1128/iai.44.1.28-32.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hewinson RG, Vordermeier HM, Buddle BM. Use of the bovine model of tuberculosis for the development of improved vaccines and diagnostics. Tuberculosis (Edinb) 2003;83:119–30. doi: 10.1016/s1472-9792(02)00062-8. [DOI] [PubMed] [Google Scholar]
- 12.Buddle BM, Wedlock DN, Parlane NA, Corner LA, De Lisle GW, Skinner MA. Revaccination of neonatal calves with Mycobacterium bovis BCG reduces the level of protection against bovine tuberculosis induced by a single vaccination. Infect Immun. 2003;71:6411–9. doi: 10.1128/IAI.71.11.6411-6419.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amadori M, Tagliabue S, Lauzi S, Finazzi G, Lombardi G, Telo P, Pacciarini L, Bonizzi L. Diagnosis of Mycobacterium bovis infection in calves sensitized by mycobacteria of the avium/intracellulare group. J Vet Med B Infect Dis Vet Public Health. 2002;49:89–96. doi: 10.1046/j.1439-0450.2002.00513.x. [DOI] [PubMed] [Google Scholar]
- 14.Buddle BM, Keen D, Thomson A, et al. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res Vet Sci. 1995;59:10–6. doi: 10.1016/0034-5288(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 15.Buddle BM, de Lisle GW, Pfeffer A, Aldwell FE. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine. 1995;13:1123–30. doi: 10.1016/0264-410x(94)00055-r. [DOI] [PubMed] [Google Scholar]
- 16.Marchant A, Goetghebuer T, Ota MO, et al. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J Immunol. 1999;163:2249–55. [PubMed] [Google Scholar]
- 17.Vekemans J, Amedei A, Ota MO, et al. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol. 2001;31:1531–5. doi: 10.1002/1521-4141(200105)31:5<1531::AID-IMMU1531>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Hope JC, Thom ML, Villarreal-Ramos B, Vordermeier HM, Hewinson RG, Howard CJ. Vaccination of neonatal calves with Mycobacterium bovis BCG induces protection against intranasal challenge with virulent M. Bovis. Clin Exp Immunol. 2005;139:48–56. doi: 10.1111/j.1365-2249.2005.02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard CJ, Kwong LS, Villarreal-Ramos B, Sopp P, Hope JC. Exposure to Mycobacterium avium primes the immune system of calves for vaccination with Mycobacterium bovis BCG. Clin Exp Immunol. 2002;130:190–5. doi: 10.1046/j.1365-2249.2002.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallagher J, Horwill DM. A selective oleic acid albumin agar medium for the cultivation of Mycobacterium bovis. J Hyg (Lond) 1977;79:155–60. doi: 10.1017/s0022172400052943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vordermeier HM, Chambers MA, Cockle PJ, Whelan AO, Simmons J, Hewinson RG. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect Immun. 2002;70:3026–32. doi: 10.1128/IAI.70.6.3026-3032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison WI, Bourne FJ, Cox DR, Donnelly CA, Gettinby G, McInerney JP, Woodroffe R. Pathogenesis and diagnosis of infections with Mycobacterium bovis in cattle. Independent Scientific Group on Cattle TB. Vet Rec. 2000;146:236–42. [PubMed] [Google Scholar]
- 23.Kwong LS, Hope JC, Thom ML, Sopp P, Duggan S, Bembridge GP, Howard CJ. Development of an ELISA for Bovine IL-10. Vet Immunol Immunopathol. 2002;85:213–23. doi: 10.1016/s0165-2427(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 24.Hope JC, Kwong LS, Sopp P, Collins RA, Howard CJ. Dendritic cells induce CD4+ and CD8+ T-cell responses to Mycobacterium bovis and M. avium antigens in Bacille Calmette Guerin vaccinated and nonvaccinated cattle. Scand J Immunol. 2000;52:285–91. doi: 10.1046/j.1365-3083.2000.00780.x. [DOI] [PubMed] [Google Scholar]
- 25.McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, Fletcher HA, Hill AV. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240–4. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 26.van Pinxteren LA, Ravn P, Agger EM, Pollock J, Andersen P. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin Diagn Laboratory Immunol. 2000;7:155–60. doi: 10.1128/cdli.7.2.155-160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock JM, Andersen P. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J Infect Dis. 1997;175:1251–4. doi: 10.1086/593686. [DOI] [PubMed] [Google Scholar]
- 28.Vordermeier HM, Cockle PJ, Whelan AO, Rhodes S, Hewinson RG. Toward the development of diagnostic assays to discriminate between Mycobacterium bovis infection and bacille Calmette-Guerin vaccination in cattle. Clin Infect Dis. 2000;30:S291–8. doi: 10.1086/313877. [DOI] [PubMed] [Google Scholar]
- 29.Vordermeier HM, Whelan A, Cockle PJ, Farrant L, Palmer N, Hewinson RG. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin Diagn Laboratory Immunol. 2001;8:571–8. doi: 10.1128/CDLI.8.3.571-578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cockle PJ, Gordon SV, Lalvani A, Buddle BM, Hewinson RG, Vordermeier HM. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect Immun. 2002;70:6996–7003. doi: 10.1128/IAI.70.12.6996-7003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]