Abstract
Kawasaki disease (KD) is an acute illness of early childhood characterized by prolonged fever, diffuse mucosal inflammation, indurative oedema of the hands and feet, a polymorphous skin rash and nonsuppurative lymphadenopathy. The histopathological findings in KD comprise panvasculitis with endothelial necrosis, and the infiltration of mononuclear cells into small and medium-sized blood vessels. The levels of many proinflammatory cytokines, chemokines and adhesion molecules can be elevated in sera from children with KD at the acute stage. Although many immunological studies on KD involving peripheral blood have been reported, the data obtained remain controversial. This review focuses on the immune response of peripheral blood lymphocytes and monocytes/macrophages during acute KD.
Keywords: Kawasaki disease, peripheral blood, T lymphocytes, monocytes/macrophages, intravenous immnoglobulin
Introduction
Kawasaki disease (KD) is an acute illness of early childhood characterized by prolonged fever, diffuse mucosal inflammation, indurative oedema of the hands and feet, a polymorphous skin rash and nonsuppurative lymphadenopathy [1]. The histopathological findings in KD comprise panvasculitis with endothelial necrosis, and the infiltration of mononuclear cells into small and medium-sized blood vessels [2]. Coronary artery involvement is the most important complication of KD and may cause significant coronary stenosis resulting in ischemic heart disease [3]. Less than 1% of patients with KD may actually die from an aneurysm and/or thrombosis caused by coronary arteritis. Serological testing of patients with this disease reveals nonspecific severe inflammation. The levels of many proinflammatory cytokines, chemokines, and soluble adhesion molecules can be elevated in sera from children with KD at the acute stage [4–9]. In spite of the long history of aetiological investigation in Japan, the cause(s) of KD remains unclear. Recently, two review articles on KD have been reported in 2004 [10,11]. These reviews include the diagnosis, epidemiology, aetiology, pathology, immunopathogenesis and therapy of KD. Although many immunological studies on KD involving peripheral blood have been reported, the data obtained remain controversial. This review focuses on the immune response of peripheral blood lymphocytes and monocytes/macrophages during acute KD, including the effect of intravenous immunoglobulin (IVIG) on peripheral blood monocytes/macrophages.
Numerical changes of peripheral blood lymphocytes and monocytes/macrophages
Table 1 shows the absolute counts of white blood cells, mononuclear cells, monocytes/macrophages and lymphocytes during the acute stage before treatment and during the convalescent stage of KD together with age-matched control subjects [12]. The absolute counts of CD14+ monocytes/macrophages and CD19+ B cells in KD were increased during the acute stage. On the contrary, the absolute counts of CD4+ and CD8+ T cells in KD decreased during the acute stage. These numerical changes of peripheral blood immunocompetent cells are an important finding for investigating the immune response of peripheral blood lymphocytes and monocytes/macrophages during acute KD.
Table 1. Absolute counts of white blood cells, mononuclear cells, monocytes/macrophages and lymphocytes during the acute stage before treatment and during the convalescent stage of KD and of control subjects.
| Acute stage (n = 106) | Convalescent stage (n = 68) | Control subjects (n = 22) | |
|---|---|---|---|
| Mean (SD) day sample taken after onset of fever | 5·8 (1·6) | 33·9 (19·9) | |
| White blood cells | 15·59 (0·47)† | 8·56 (0·29) | 8·41 (0·39) |
| Mononuclear cells | 4·58 (0·21) | 5·27 (0·20) | 4·86 (0·25) |
| CD14 + monocytes/macrophages | 0·52 (0·04)† | 0·25 (0·02) | 0.18 (0·02) |
| Lymphocytes | 4·06 (0·20) | 4·89 (0·19) | 4·68 (0·25) |
| CD4+ T cell | 1·81 (0.11)* | 2·27 (0·11) | 2·33 (0·19) |
| CD8+ T cell | 0·78 (0·04)* | 1·14 (0·06) | 0·94 (0·06) |
| CDl9 + B cell | 1·23 (0·09)* | 1·00 (0·06) | 0·94 (0·10) |
All results for cell counts are expressed as ×109/l and mean (SEM).
Significant at P < 0·05 versus control subjects.
Significant at P < 0·01 versus control subjects.
Activation of peripheral blood lymphocytes
The aetiology of KD, particularly the role of staphylococcal and streptococcal superantigens, which have the unique ability to activate a large number of lymphocytes, remains controversial. Following superantigen activation, T cells with particular T cell receptor β-chain (TCR Vβ) rapidly proliferate [13]. This is followed by T cell Vβ-restricted deletion mediated by Fas-Fas ligand from the peripheral blood, which is one of the processes of apoptosis [14,15]. Thus, the characteristic immunologocal features mediated by superantigens are the response of T cell Vβ expansion and deletion in the peripheral blood of patients exposed to these toxins. Recently, Brogan et al. [16] have reported that Class II MHC-positive endothelial cells operate as competent superantigen-presenting cells for CD4 and CD8 lymphocytes, suggesting that activated T cells are temporarily withdrawn from peripheral circulation during acute KD. In addition, the up-regulatinon of MHC Class II expression on lesion endothelial cells has been reported in a patient with fatal KD [17]. This might be related with slight increase of serum interferon γ (IFN-γ) levels in acute KD patients with coronary artery lesions (CAL) reported by us, since IFN-γ increases MHC Class II exression on endothelial cells [18]. At present, conflicting data have been reported regarding expanded T cell populations with particular TCR Vβ gene segments, suggesting either a superantigen- or a conventional antigen-mediated immune response in KD. Although some studies have demonstrated a significant increase or decrease in the percentage of peripheral blood T cells with any particular TCR Vβ family [19–23], the findings have not been confirmed by other investigators [24–27]. We have reported the lack of increases in the serum levels of soluble Fas and Fas ligand during acute KD [28].
The infiltration of activated T cells expressing HLA-DR antigen in biopsy skin lesions and coronary vascular lesions at autopsy has been reported [17,29]. However, it remains uncertain whether peripheral blood T cells are largely activated in acute KD, as some reports have provided evidence of peripheral blood T cell activation [30,31], whereas other reports suggested that there is a low level of activation of peripheral blood T cells during acute KD [32–34]. Recently, we demonstrated a decrease in the number of IFN-γ-producing, but not IL-4-producing, CD3+ T cells in the peripheral blood obtained from KD patients without CAL, suggesting an imbalance of the peripheral blood T cell function at the acute stage [35]. In addition, it has been reported that plasma levels of IL-4 were significantly higher in the acute KD than control children [36]. Our results suggest that some population of peripheral blood T cells, such as IL-4 producing T cells may be activated, while IFN-γ producing T cells (Th1 and Tc1-type CD3+ T cells) develop hypofunction during acute KD. These results further suggest that great caution should be taken in studies on peripheral blood T cell-mediated responses during acute KD.
Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4, CD152) is a receptor present on T cells that plays a critical role in the down-regulation of antigen-activated immune responses. The expression of CTLA-4 on the T cells depends on cell activation induced by CD28–B7 interaction, which is essential for T cell activation. CTLA-4 is a surface molecule on activated T cells exhibiting sequence homology to CD28 [37]. The essential inhibitory function of CTLA-4 is to maintain the homeostasis of the immune system [38]. We demonstrated that the intracellular T cell expression of CTLA-4 is up-regulated in peripheral blood CD3+, CD4+ and CD8+ T cells in the early part of the acute stage in KD [39]. However, there was a mild increase in intracellular T cells expressing CTLA-4 in KD compared with in Epstein-Barr virus infectious mononucleosis and influenza virus-associated encephalopathy [39,40]. It is an important finding that there is down-regulation of antigen-activated peripheral blood T cell during acute KD, in spite of a mild increase in intracellular CTLA-4 in T cells.
A few studies have been reported on the activation of peripheral blood B cells in KD. The findings included polyclonal B cell activation, an increase in the absolute number of B cells and increased expression of CD23 on B cells [12,41–43]. Recently, it was reported that circulating IgA B cells are reduced in acute KD, while IgA plasma cells infiltrate vascular tissue, including coronary arterial walls, in fatal KD [44,45].
Few studies have been reported concerning natural killer (NK) cells in KD. A significant reduction in the absolute number of circulating CD16+ NK cells was observed during the acute phase of KD [46]. It remains unclear whether the alteration in the number of peripheral blood NK cells is primary or secondary to the pathologic condition of acute KD.
Activation of peripheral blood monocytes/macrophages
The immunological features of monocytes/macrophages observed in patients with KD can be summarized as follows:
infiltration by the cells is notable in affected tissues in autopsy cases and in skin biopsy specimens from KD patients [17,29];
the numbers of peripheral blood CD14+ monocytes/macrophages and activated CD14+CD23+ monocytes/macrophages increase during the acute stage of KD [12];
there are elevated levels of a variety of serum cytokines, such as tumour necrosis factor α (TNF-α, IL-1 and IL-6, which are considered to be produced by monocytes/macrophages during acute KD [4–6,47];
peripheral blood mononuclear cells from patients with acute KD spontaneously secrete high levels of TNF-α and IL-1 [48,49];
increases in the number of peripheral blood CD14+ monocytes/macrophages, serum TNF-α level, IL-6 activity in serum and secretion of IL-1 from mononuclear cells are more evident in KD patients with than in ones without CAL [4,6,12,47,49];
KD patients with a high level of soluble TNF receptor in their serum seem to be susceptible to CAL [50];
predominant vascular endothelial growth factor expression and enhanced nitric oxide synthase expression in monocytes have been demonstrated in patients with KD [51,52];
immunocytochemical and immunoelectron microscopic studies have shown that monocytes partly differentiate into macrophages in the peripheral circulation during the acute stage of KD [53,54].
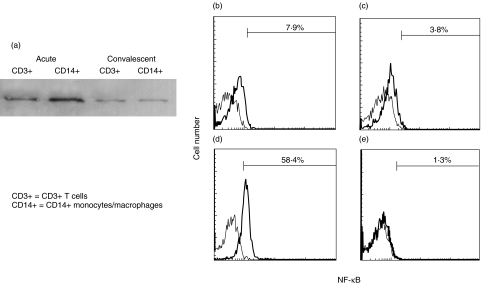
It has been reported that the CD14+CD16+(FcγRIII) monocyte/macrophage subpopulation plays a more important role in inflammation [55]. We observed an increase in the number of peripheral blood CD14+CD16+(FcγRIII) monocytes/macrophages in acute KD, which showed positive correlation with the disease severity [56]. Furthermore, we investigated the activation of nuclear factor kappa B (NF-κB) in peripheral blood CD14+ monocytes/macrophages and CD3+ T cells by means of Western blotting and flow cytometric analyses. NF-κB is a pivotal transcription factor for genes that encode the proinflammatory cytokines, chemokines and adhesion molecules that mediate inflammation [57–59]. As shown in Fig. 1, NF-κB activation was more increased in peripheral blood CD14+ monocytes/macrophages than in CD3+ T cells in KD patients during the acute stage [60]. These findings suggest that the activation of peripheral blood monocytes/macrophages plays an important role during acute KD.
Fig. 1.
NF-κB activation in peripheral blood CD3+ T cells and CD14 + monocytes/macrophages of a 2-month-old boy with KD. (a) Nuclear extracts were harvested from CD14+ monocytes/macrophages or CD3+ T cells. The nuclear extracts were used as the sample for Western blotting because activated NF-κB existed in the nucleus. Rabbit polyclonal antibodies against NF-κB-p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as the primary antibodies.Western blot analysis demonstrated that intranuclear amount of NF-κB was increased in CD14 + monocytes/macrophages and CD3+ T cells at the acute stage compared with that at the convalescent stage. (b–e) Whole blood was labelled with phycoerythrin-conjugated with anti-CD14 moclonal antibodies and peridinin chlorophyll protein-conjugated anti-CD3 monoclonal antibodies and then permeabilized in 4% paraformaldehyde in phosphate-buffered saline, pH 7·2, containing 0·1% saponin and 10 mm HEPES. The cells were then labelled with a mouse anti-NF-κB (nuclear-localized signal) antibody (IgG3; Boehringer Mannheim, Mannheim, Germany). The mouse anti-NF-κB (nuclear-localized signal) antibody recognizes an epitope overlapping the nuclear location signal of NF-κB-p65 and therefore selectively recognizes the activated form of NF-κB. The cells were then labelled with a FITC-conjugated rat antimouse IgG3 monoclonal antibody (Pharmingen, San Diego, CA, USA). Immunofluorescence was analysed with a FACScan flow cytometer equipped with CellQuest software (Becton-Dickinson Biosciences, San Jose, CA, USA). The percentages of cells with intranuclear NF-κB in CD14+ monocytes/macrophages and CD3+ T cells by flow cytometric analysis are indicated. (b) CD3+ T cells at the acute stage; (c) CD3+ T cells at the convalescent stage; (d) CD14 + monocytes/macrophages at the acute stage; (e) CD14 + monocytes/macrophages at the convalescent stage.
Effect of intravenous immunoglobulin on peripheral blood monocytes/macrophages
IVIG therapy has been reported to be effective in reducing the incidence of CAL in patients with KD [61–63]. There have been a few reports on the effect of IVIG on peripheral blood lymphocytes, neutrophils and cytokines in acute KD, including lymphocyte activation and apoptosis, neutrophil apoptosis and cytokine modulation [42,64–66]. The mechanism of IVIG in immune thrombocytopenic purpura (ITP) has been elucidated. In a murine model of ITP, IVIG increases the expression of inhibitory Fc receptor FcγRIIB on splenic macrophages [67]. However, the mode of action of IVIG in monocytes/macrophages during acute KD is not clearly understood.
IVIG therapy for acute KD seems to decrease the absolute number of circulating CD14+ monocytes/macrophages [68]. We revealed that NF-κB activation in peripheral blood CD14+ monocytes/macrophages is significantly decreased after IVIG therapy during acute KD [60]. Recently, we demonstrated that IVIG inhibits NF-κB activation induced by TNF-α, while it remains unclear whether IVIG acts extracellularly and/or intracellularly in U-937 cells, human monocytic leukaemia cell line. Western blotting of cytoplasmic extracts of U-937 cells revealed that IVIG inhibited the degradation of the IκBα protein, which suppresses NF-κB activation [69]. Further examination is necessary to determine whether or not the data in vitro reflects those in vivo.
We previously observed an increase in the number of CD14+CD16+(FcγRIII) monocytes/macrophages in acute KD and a decrease after IVIG therapy, as shown in Table 2[56]. In vitro study of activation of Fc receptor FcγRIII by flow cytometry demonstrated that IVIG decreased the expression of FcγRIII in U-937 cells and peripheral blood CD14+ monocytes/macrophages, and that this phenomenon is transient [69]. On the other hand, IVIG did not affect FcγRIIB expression on the membranes of U-937 cells or peripheral blood CD14+ monocytes/macrophages. More recently, we observed that CD14+CD32B+(FcγRIIB) monocytes/macrophages were not increased during subacute KD after IVIG therapy [70]. Regarding FcγR expression in peripheral blood monocytes/macrophages during acute KD, the main effect of IVIG therapy may be based on a decrease in CD14+CD16+ (FcγRIII) monocytes/macrophages, and not an increase in CD14+CD32B+ (FcγRIIB) monocytes/macrophages.
Table 2. CD14+ CD16+ (FcγRIII) monocytes/macrophages in the patients with KD during the acute stage and the convalescent stage, and in control subjects.
| KD (n = 28) | ||||
|---|---|---|---|---|
| Acute | Control subjects | |||
| Before IVGG | After IVGG | Convalescent | (n = 20) | |
| Mononuclear cells (cells/µl) | 5271 ± 2705 | 5779 ± 2354 | 5374 ± 2274 | 5585 ± 1783 |
| CD14+CD16+monocytes/macrophages (%) | 3·6 ± 3·5* | 0·6 ± 0·6 | 0·5 ± 0·3 | 0·7 ± 0·3 |
| CD14+CD16+monocytes/macrophages (cells/µl) | 155 ± 132* | 35 ± 32 | 25 ± 18 | ″35 ± 18 |
| Percentage of CD14+CD16+ monocytes/macrophages among CD14+ monocytes/macrophages (%) | 21·6 ± 12·5* | 6·6 ± 6·8 | 6·7 ± 3·7 | 10·1 ± 4·3 |
Values are expressed as mean ± s.d.
Significant at P < 0·01 versus convalescent stage and control subjects.
Concluding remarks
Many conflicting data regarding peripheral blood T cell activation during acute KD have been reported. We speculate that these conflicting data might be due to the bipolarity of the peripheral blood T cell function observed in patients with acute KD. There is now ample evidence of a central role of peripheral blood monocytes/macrophages during acute KD, including the observation of an anti-inflammatory action of IVIG on monocytes/macrophages.
Informed consent for participation was obtained from the subjects’ parents in our studies. Our studies were approved by the Institutional Review Board of Yamaguchi University Hospital.
References
- 1.Kawasaki T, Kosaki F, Osawa S, Shigemitsu I, Yanagawa SA. new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–6. [PubMed] [Google Scholar]
- 2.Fujiwara H, Hamashima Y. Pathology of the heart in Kawasaki's disease. Pediatrics. 1978;61:100–7. [PubMed] [Google Scholar]
- 3.Kato H, Sugimura T, Akagi T, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–85. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa S, Matsubara T, Jujoh K, et al. Peripheral blood monocyte/macrophages and serum tumor necrosis factor in Kawasaki disease. Clin Immunol Immunopathol. 1988;48:247–51. doi: 10.1016/0090-1229(88)90088-8. [DOI] [PubMed] [Google Scholar]
- 5.Maury CPJ, Salo E, Pelkonen P. Circulating interleukin-1β in patients with Kawasaki disease. N Engl J Med. 1988;319:1670–1. doi: 10.1056/NEJM198812223192515. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa S, Matsubara T, Yone K, Hirano Y, Okumura K, Yabuta K. Kawasaki disease differs from anaphylactoid purpura and measles with regard to tumour necrosis factor-α and interleukin 6 serum. Eur J Pediatr. 1992;151:44–7. doi: 10.1007/BF02073890. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa S, Imai K, Matsubara T, et al. Increased levels of circulating intercellular adhesion molecule 1 in Kawasaki disease. Arthritis Rheum. 1992;35:672–7. doi: 10.1002/art.1780350611. [DOI] [PubMed] [Google Scholar]
- 8.Lin CY, Lin CC, Hwang B, Chiang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor α among patients with Kawasaki disease. J Pediatr. 1992;121:924–6. doi: 10.1016/s0022-3476(05)80343-9. [DOI] [PubMed] [Google Scholar]
- 9.Schiller B, Elinder G. Inflammatory parameters and soluble cell adhesion molecules in Swedish children with Kawasaki disease. relationship to cardiac lesions and intravenous immunoglobulin treatment. Acta Paediatr. 1999;88:844–8. doi: 10.1080/08035259950168766. [DOI] [PubMed] [Google Scholar]
- 10.Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364:533–44. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 11.Shulman ST, Rowley AH. Advances in Kawasaki disease. Eur J Pediatr. 2004;163:285–91. doi: 10.1007/s00431-004-1431-z. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa S, Matsubara T, Yabuta K. Mononuclear cell subsets and coronary artery lesions in Kawasaki disease. Arch Dis Child. 1992;67:706–8. doi: 10.1136/adc.67.6.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoukry NH, Lavoie PM, Thibodeau J, D'Souza S, Sekaly RP. MHC class II-dependent peptide antigen versus superantigen presentation to T cells. Hum Immunol. 1997;54:194–201. doi: 10.1016/s0198-8859(97)00074-8. [DOI] [PubMed] [Google Scholar]
- 14.Webb SR, Hutchinson J, Hayden K, Sprent J. Expansion/deletion of mature T cells exposed to endogenous superantigens in vivo. J Immunol. 1994;152:586–97. [PubMed] [Google Scholar]
- 15.Mingari MC, Cambiaggi A, Vitale C, Schiavetti F, Bellomo R, Poggi A. Effect of superantigens on human thymocytes. selective proliferation of V beta 2+ cells in response to toxic shock syndrome toxin-1 and their deletion upon secondary stimulation. Int Immunol. 1996;8:203–9. doi: 10.1093/intimm/8.2.203. [DOI] [PubMed] [Google Scholar]
- 16.Brogan PA, Shah V, Klein N, Dillon MJ. V-restricted T cell adherence to endothelial cells: a mechanism for superantigen-dependent vascular injury. Arthritis Rheum. 2004;50:589–97. doi: 10.1002/art.20021. [DOI] [PubMed] [Google Scholar]
- 17.Terai M, Kohno Y, Namba M, et al. Class II major histocompatibility antigen expression on coronary arterial endothelium in a patient with Kawasaki disease. Hum Pathol. 1990;21:231–4. doi: 10.1016/0046-8177(90)90135-r. [DOI] [PubMed] [Google Scholar]
- 18.Matsubara T. Serum gamma interferon levels in relation to tumor necrosis factor and interleukin 2 receptor in patients with Kawasaki disease involving coronary-artery lesions. Arerugi. 1990;39:118–23. [PubMed] [Google Scholar]
- 19.Abe J, Kotzin BL, Jujo K, et al. Selective expansion of T cells expressing T cell receptor variable lesions Vβ2 and Vβ8 in Kawasaki disease. Proc Natl Acad Sci USA. 1992;89:4066–70. doi: 10.1073/pnas.89.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung DYM, Meissner HC, Fulton DR, Murry DL, Kotzin BL, Schlievert PM. Toxic shock syndrome toxin-secreting Staphylococcus aureus in Kawasaki syndrome. Lancet. 1994;342:1385–8. doi: 10.1016/0140-6736(93)92752-f. [DOI] [PubMed] [Google Scholar]
- 21.Nomura Y, Masuda K, Shinkoda Y, et al. Twenty-five types of T-cell receptor Vbeta family repertoire in patients with Kawasaki syndrome. Eur J Pediatr. 1998;157:981–6. doi: 10.1007/s004310050982. [DOI] [PubMed] [Google Scholar]
- 22.Yoshioka T, Matsutani T, Iwagami S, et al. Polyclonal expansion of TCRBV2-and TCRBV6-bearing T cells in patients with Kawasaki disease. Immunology. 1999;96:465–72. doi: 10.1046/j.1365-2567.1999.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brogan PA, Shah V, Bagga A, Klein N, Dillon MJ. T cell Vβ repertoires in childhood vasculitides. Clin Exp Immunol. 2003;131:517–27. doi: 10.1046/j.1365-2249.2003.02081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietra BA, De Inocencio J, Giannini EH, Hirsch R. TCR Vβ family repertoire and T cell activation markers in Kawasaki disease. J Immunol. 1994;153:1881–8. [PubMed] [Google Scholar]
- 25.Marchette NJ, Cao X, Kihara S, Martun J, Melish ME. Staphylococcal toxic shock syndrome toxin-1, one possible cause of Kawasaki syndrome? In: Kato H, editor. Kawasaki Disease. The Netherlands: Elsevier Science BV; 1995. pp. 149–55. [Google Scholar]
- 26.Choi IH, Chwae YJ, Shim WS, et al. Clonal expansion of CD8+ T cells in Kawasaki disease. J Immunol. 1997;159:481–6. [PubMed] [Google Scholar]
- 27.Mancia L, Wahlstrom J, Schiller B, et al. Characterization of the T-cell receptor V-beta repertoire in Kawasaki disease. Scand J Immunol. 1998;48:443–9. doi: 10.1046/j.1365-3083.1998.00415.x. [DOI] [PubMed] [Google Scholar]
- 28.Koga M, Hasegawa S, Furukawa S. No increase in soluble Fas and Fas ligand in Kawasaki disease. Arthritis Rheum. 1998;41:568–70. doi: 10.1002/1529-0131(199803)41:3<568::AID-ART32>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 29.Sugawara T, Hattori S, Hirose S, Furukawa S, Yabuta K, Shirai T. Immunopathology of the skin lesions of Kawasaki disease. In: Schulman ST, editor. Kawasaki Disease. New York: Alan R. Liss; 1987. pp. 185–92. [PubMed] [Google Scholar]
- 30.Leung DYM, Chu ET, Wood N, Grady S, Meade R. Geha RS. Immunoregulatory T cell abnormalities in mucocutaneous lymph node syndrome. J Immunol. 1983;130:2002–4. [PubMed] [Google Scholar]
- 31.Barron K, DeCunto C, Montalvo J, Orson F, Lewis D. Abnormalities of immunoregulation in Kawasaki syndrome. J Rheumatol. 1988;15:1243–9. [PubMed] [Google Scholar]
- 32.Terai M, Kohno Y, Niwa K, Toba T, Sakurai N, Nakajima H. Imbalance among T-cell subsets in patients with coronary arterial aneurysms in Kawasaki disease. Am J Cardiol. 1987;60:555–9. doi: 10.1016/0002-9149(87)90304-3. [DOI] [PubMed] [Google Scholar]
- 33.Furukawa S, Matsubara T, Tsuji K, Motohashi T, Okumura K, Yabuta K. Serum soluble CD4 and CD8 levels in Kawasaki disease. Clin Exp Immunol. 1991;86:134–9. doi: 10.1111/j.1365-2249.1991.tb05785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furukawa S, Matsubara T, Tsuji K, Okumura K, Yabuta K. Transient depletion of T cells with bright CD11a/CD18 expression from peripheral circulation during acute Kawasaki disease. Scand J Immunol. 1993;37:377–80. doi: 10.1111/j.1365-3083.1993.tb02567.x. [DOI] [PubMed] [Google Scholar]
- 35.Matsubara T, Katayama K, Matsuoka T, Fujiwara M, Koga M, Furukawa S. Decreased interferon-gamma (IFN-γ)-producing T cells in patients with acute Kawasaki disease. Clin Exp Immunol. 1999;116:554–7. doi: 10.1046/j.1365-2249.1999.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirao J, Hibi S, Andoh T, Ichimura T. High levels of circulating interleukin-4 and interleukin-10 in Kawasaki disease. Int Arch Allergy Immunol. 1997;112:152–6. doi: 10.1159/000237447. [DOI] [PubMed] [Google Scholar]
- 37.Slavik JM, Hutchcroft JE, Bierer BE. CD28/CTLA-4 and CD80/CD86 families. Immunol Res. 1999;19:1–24. doi: 10.1007/BF02786473. [DOI] [PubMed] [Google Scholar]
- 38.Brunner MC, Chambers CA, Chan FK-M, Hanke J, Winoto A, Allison JP. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–20. [PubMed] [Google Scholar]
- 39.Matsubara T, Anwar R, Fujiwara M, Ichiyama T, Furukawa S. CTLA-4 (CD152) expression in peripheral blood T cells in Kawasaki disease. Clin Exp Immunol. 2003;132:169–73. doi: 10.1046/j.1365-2249.2003.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayukawa H, Matsubara T, Kaneko M, Hasegawa M, Ichiyama T, Furukawa S. Expression of CTLA-4 (CD152) in peripheral blood T cells of children with influenza virus infection including encephalopathy in comparison with respiratory syncytial virus infection. Clin Exp Immunol. 2004;137:151–5. doi: 10.1111/j.1365-2249.2004.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung DY, Siegel R, Grady S, et al. Immunoregulatory abnormalities in mucocutaneous lymph node syndrome. Clin Immunol Immunopathol. 1982;23:100–12. doi: 10.1016/0090-1229(82)90075-7. [DOI] [PubMed] [Google Scholar]
- 42.Leung DY, Burns J, Newburger J, Geha R. Reversal of lymphocyte activation in vivo in Kawasaki syndrome by intravenous gammmaglobulin. J Clin Invest. 1987;79:468–72. doi: 10.1172/JCI112835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furukawa S, Matsubara T, Motohashi T, et al. Increased expression of Fc∈R2/CD23 on peripheral blood B lymphocytes and serum IgE levels in Kawasaki disease. Int Arch Allergy Appl Immunol. 1991;95:7–12. doi: 10.1159/000235446. [DOI] [PubMed] [Google Scholar]
- 44.Shingadia D, O'Gorman M, Rowley AH, Shulman ST. Surface and cytoplasmic immunoglobulin expression in circulating B-lymphocytes in acute Kawasaki disease. Pediatr Res. 2001;50:538–43. doi: 10.1203/00006450-200110000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Rowley AH, Eckerley CA, Jack HM, Shulman ST, Baker SC. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J Immunol. 1997;159:5946–55. [PubMed] [Google Scholar]
- 46.Furukawa S, Matsubara T, Tsuji K, et al. Comparison of Kawasaki disease and infectious mononucleosis in terms of natural killer cell and CD8+ T cell subsets. J Infect Dis. 1991;163:416–7. doi: 10.1093/infdis/163.2.416. [DOI] [PubMed] [Google Scholar]
- 47.Maury CPJ, Salo E, Pelkonen P. Elevated circulating tumor necrosis factor-α in patients with Kawasaki disease. J Laboratory Clin Med. 1989;113:651–4. [PubMed] [Google Scholar]
- 48.Lang GA, Silverman ED, Laxer RM, Lau AS. Spontaneous tumor necrosis factor production in Kawasaki disease. J Pediatr. 115:939–43. doi: 10.1016/s0022-3476(89)80746-2. [DOI] [PubMed] [Google Scholar]
- 49.Leung DYM, Cotran RS, Kurt-Johnes E, Burns JC, Newburger JW, Pober JS. Endothelial cell activation and high interleukin−1 secretion in the pathogenesis of acute Kawasaki disease. Lancet. 1989;2:1298–302. doi: 10.1016/s0140-6736(89)91910-7. [DOI] [PubMed] [Google Scholar]
- 50.Furukawa S, Matsubara T, Umezawa Y, Okumura K, Yabuta K. Serum levels of p60 soluble tumor necrosis factor receptor during acute Kawasaki disease. J Pediatr. 1994;124:721–5. doi: 10.1016/s0022-3476(05)81361-7. 1989. [DOI] [PubMed] [Google Scholar]
- 51.Hamamichi Y, Ichida F, Yu X, et al. Neutrophils and mononuclear cells express vascular endothelial growth factor in acute Kawasaki disease: its possible role in progression of coronary artery lesions. Pediatr Res. 2001;49:74–80. doi: 10.1203/00006450-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 52.Yu X, Hirono K, Ichida F, et al. Enhanced iNOS expression in leukocytes and circulating endothelial cells is associated with the progression of coronary artery lesions in acute Kawasaki disease. Pediatr Res. 2004;55:688–94. doi: 10.1203/01.PDR.0000113464.93042.A4. [DOI] [PubMed] [Google Scholar]
- 53.Koga M, Ishihara T, Takahashi M, Umezawa Y, Furukawa S. Activation of peripheral blood monocytes and macrophages in Kawasaki disease. ultrastructural and immunocytochemical investigation. Pathol Int. 1998;48:512–7. doi: 10.1111/j.1440-1827.1998.tb03942.x. [DOI] [PubMed] [Google Scholar]
- 54.Ariga S, Koga M, Takahashi M, Ishihara T, Matsubara T, Furukawa S. Maturation of macrophages from peripheral blood monocytes in Kawasaki disease: immunocytochemical and immunoelectron microscopic study. Pathol Int. 2001;51:257–63. doi: 10.1046/j.1440-1827.2001.01202.x. [DOI] [PubMed] [Google Scholar]
- 55.Ziegler-Heitbrock HWL. Heterogeneity of human blood monocytes: the CD14+ CD16+ subpopulation. Immunol Today. 1996;17:424–8. doi: 10.1016/0167-5699(96)10029-3. [DOI] [PubMed] [Google Scholar]
- 56.Katayama K, Matsubara T, Fujiwara M, Koga M, Furukawa S. CD14+ CD16+ monocyte subpopulation in Kawasaki disease. Clin Exp Immunol. 2000;121:566–70. doi: 10.1046/j.1365-2249.2000.01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages. involvement of four κB-like motifs and of constitutive and inducible forms of NF-κB. Mol Cell Biol. 1990;10:1498–506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kunsch C, Lang RK, Rosen CA, Shannon MF. Synergistic transcriptional activation of the IL-8 gene by NF-κB p65 (RelA) and NF-IL-6. J Immunol. 1994;153:153–64. [PubMed] [Google Scholar]
- 59.Chen CC, Rosenbloom CL, Anderson DC, Manning AM. Selective inhibition of E-selectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 expression by inhibitors of IκB-α phosphorylation. J Immunol. 1995;155:3538–45. [PubMed] [Google Scholar]
- 60.Ichiyama T, Yoshitomi T, Nishikawa M, et al. NF-κB activation in peripheral blood monocytes/macrophages and T cells during acute Kawasaki disease. Clin Immunol. 2001;99:373–7. doi: 10.1006/clim.2001.5026. [DOI] [PubMed] [Google Scholar]
- 61.Furusho K, Kamiya T, Nakano H, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984;2:1055–8. doi: 10.1016/s0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 62.Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–7. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 63.Newburger JW, Takahashi M, Beiser AS, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–9. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 64.Yi QJ, Li CR, Yang XQ. Effect of intravenous immunoglobulin on inhibiting peripheral blood lymphocyte apoptosis in acute Kawasaki disease. Acta Paediatr. 2001;90:623–7. [PubMed] [Google Scholar]
- 65.Tsujimoto H, Takeshita S, Nakatani K, Kawamura Y, Tokutomi T, Sekine I. Intravenous immunoglobulin therapy induces neutrophil apoptosis in Kawasaki disease. Clin Immunol. 2002;103:161–8. doi: 10.1006/clim.2002.5209. [DOI] [PubMed] [Google Scholar]
- 66.Gupta M, Noel GJ, Schaefer M, Friedman D, Bussel J, Johann-Liang R. Cytokine modulation with immune γ-globulin in peripheral blood of normal children and its implications in Kawasaki disease treatment. J Clin Immunol. 2001;21:193–9. doi: 10.1023/a:1011039216251. [DOI] [PubMed] [Google Scholar]
- 67.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–6. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 68.Furukawa S, Matsubara T, Jujoh K, et al. Reduction of peripheral blood macrophages/monocytes in Kawasaki disease by intravenous gammaglobulin. Eur J Pediatr. 1990;150:43–7. doi: 10.1007/BF01959479. [DOI] [PubMed] [Google Scholar]
- 69.Ichiyama T, Ueno Y, Isumi H, Niimi A, Matsubara T, Furukawa S. Intravenous immunoglobulin inhibits NF-κB activation and affects Fcγ receptor expression in monocytes/macrophages. Naunyn-Schmiedeberg's Arch Pharmacol. 2004;369:428–33. doi: 10.1007/s00210-004-0877-x. [DOI] [PubMed] [Google Scholar]
- 70.Ichiyama T, Ueno Y, Hasegawa M, Ishikawa Y, Matsubatra T, Furukawa S. Intravenous immunoglobulin does not increase FcγRIIB expression on monocytes/macrophages during acute Kawasaki disease. Rheumatology. 2005;44:314–7. doi: 10.1093/rheumatology/keh488. [DOI] [PubMed] [Google Scholar]