Abstract
CD4+ T-cell levels are an important criterion for categorizing HIV-related clinical conditions according to the CDC classification system and are therefore important in the management of HIV by initiating antiretroviral therapy and prophylaxis for opportunistic infections due to HIV among HIV-infected individuals. However, it has been observed that the CD4 counts are affected by the geographical location, race, ethnic origin, age, gender and changes in total and differential leucocyte counts. In the light of this knowledge, we classified 600 HIV seropositive antiretroviral treatment (ART)-naïve Indian individuals belonging to different CDC groups A, B and C on the basis of CDC criteria of both CD4% and CD4 counts and receiver operating characteristic (ROC) curves were generated. Importantly, CDC staging on the basis of CD4% indicated significant clinical implications, requiring an early implementation of effective antiretroviral treatment regimen in HIV-infected individuals deprived of treatment when classified on the basis of CD4 counts.
Keywords: HIV, CDC staging, CD4 count, CD4%, India
Introduction
Acquired immunodeficiency syndrome (AIDS) is a progressive deterioration of the immune status of the individual. It is characterized by the progressive depletion of the CD4 T lymphocyte population, which represents a major target of viral infection by the causative human immunodeficiency virus (HIV). Low absolute CD4 counts and the perturbed cytokine network manifest havoc at clinical level. The clinical consequences of HIV infection encompass a spectrum ranging from an acute syndrome associated with primary infection to prolonged asymptomatic state to advanced disease [1–8]. Consequently, with the staggering worldwide growth of HIV pandemic, the US Public Health Service (PHS) recommended that CD4+ T-cell levels be monitored every 3–6 months in all HIV-infected persons to decrease the clinical complications by initiating prophylaxis for various opportunistic infections due to HIV and for initiating and monitoring the efficacy of antiretroviral therapy [9–11].
Hence, the Center for Disease Control and Prevention (CDC) defined a set of guidelines and recommendations for HIV-infected adolescents and adults on the basis of clinical conditions associated with the HIV infection and CD4+ T-lymphocyte counts [12–15]. The system is based on three ranges of CD4+ T-lymphocyte counts or CD4% and three clinical categories and is represented by a matrix of nine mutually exclusive categories. This complex yet comprehensive case definition of AIDS enables the clinician to view HIV disease as a spectrum ranging from primary acute phase to advanced clinical disease and thus plays an important role in AIDS surveillance [13]. These CDC guidelines have been based on studies done mostly in developed countries. A few studies have been carried out in developing countries on the basis of the present staging and monitoring system [16].
The influence of geographical location, racial and ethnic background, age, sex and conditions of living, on the distribution of human peripheral blood T-lymphocyte subpopulations have already been documented in various studies [17–26]. We have previously determined the lymphocyte subset reference range in HIV-seronegative North Indian adults [27]. In an extension of this previous study, we tried to classify HIV-seropositive antiretroviral treatment (ART)-naïve Indian individuals on CDC criteria of clinical symptoms and CD4% and CD4 counts. The optimum cut-off values of CD4 counts and CD4% obtained were compared with the CDC recommended values. The present study also aimed to investigate the CDC staging of HIV-1 patients, on the basis of CD4 counts and CD4%, and the clinical implications in terms of HIV treatment and prophylaxis of these two staging criteria in an Indian population.
Materials and methods
Study population
The study was conducted from January 2003 to July 2004. We included 600 consecutive ART-naïve HIV-seropositive patients at various stages of disease progression: approximately 200 each in CDC group A, B and C. The groups A, B and C are based on clinical symptoms and further divided into subgroups on the basis of CD4 counts or CD4% (Table 1). All these patients were attending the AIDS clinic at the Department of Microbiology, AIIMS, Ansari Nagar, New Delhi.
Table 1. CDC classification system for HIV infection.
| Clinical categories | |||
|---|---|---|---|
| A | B | C | |
| CD4+ T-cell count (cells/µl.) (CD4%) | Asymptomatic, acute (primary) HIV or PGL* | Symptomatic, not A or C conditions† | AIDS-indicator conditions‡ |
| > 500 (28%) | A1 | B1 | C1 |
| 200–499 (15–28%) | A2 | B2 | C2 |
| < 200 (14%) | A3 | B3 | C3 |
Category A: asymptomatic HIV infection, persistent generalized lymphadenopathy (PGL).
Category B: oropharyngeal and vulvovaginal candidiasis, constitutional symptoms such as fever (38·5°C) or diarrhea lasting >1 month, herpes zoster (shingles).
Category C: Mycobacterium tuberculosis (pulmonary and disseminated), Pneumocystis carinii pneumonia, candidiasis of bronchi; trachea or lungs, extrapulmonary cryptococcosis, CMV, HIV-related encephalopathy, Kaposi's sarcoma, wasting syndrome due to HIV.
Sample collection
Five ml of whole blood sample was collected from each of the HIV-infected individuals by venipuncture in K3EDTA vacutainer tubes after pre-test counselling and informed consent. To exclude the influence of circadian variation on lymphocyte subpopulations, samples were collected between 0800 and 1200 h. An aliquot of the sample was kept for haematological analysis. All the samples were held at room temperature and were processed within 2 h of collection.
Flow cytometry
Dual-colour immunophenotyping was performed using standard whole blood methodology, four tube panel: CD45/CD14; CD3/CD4; CD3/CD8 and isotype controls IgG1/IgG1 [14,15]. A total number of 10 000 events were acquired for each tube on a FACSCalibur (Becton Dickinson, San Jose, CA, USA) flow cytometer immediately after processing. The flow cytometer was calibrated with CaliBrite beads (BD) using FACSComp software. The compensation settings were verified using CD8+ bright population for both FITC and PE fluorescent markers, respectively. Background staining (< 5%) was assessed using appropriate isotype controls. Analysis was done by the Cell Quest software (Becton Dickinson) using a set of criteria for quality control [15,18].
Haematology
Absolute counts of cells were calculated by multiplication of the percentage of respective lymphocyte subset by the differential lymphocyte percentage and total leucocyte counts obtained using an automated cell counter (MS9 cell counter analyser, Melet Schloesing Laboratories, Pontoise, France).
Statistical analysis
The mean values and 95% confidence intervals of different lymphocyte subsets among the HIV-infected individuals belonging to CDC groups A, B and C were calculated for the middle 95% values, excluding the outlier 2·5% observations on each side. The patients were classified according to CDC criteria of CD4 counts and CD4% into different subgroups in each of the symptom groups A, B and C. Patients belonging to the same subgroup in each CDC group A, B and C were pooled (A1 + B1 + C1, A2 + B2 + C2, A3 + B3 + C3) and collectively assigned as group 1, 2 and 3, respectively. These groups were compared using receiver operating characteristics (ROC) curve analysis, to determine the optimum cut off values. Classification based on recommended absolute counts was used to determine the optimum cut-off values for CD4% and classification based on recommended CD4% was used to determine optimum absolute CD4 count cut-offs. All patients were also classified into a CDC matrix on the basis of clinical symptoms and CD4 counts and also on clinical symptoms and CD4%, as recommended by the CDC. Both these classifications were compared. STATA 7·0 software was used for all statistical analysis.
Results
A total of 217 HIV-infected individuals belonging to CDC group A comprised 137 males and 80 females with a mean (SD) age of 30·6 (9·8) years. A total of 180 HIV-infected individuals (135 men, 45 women) belonged to CDC group B with a mean (SD) age of 32·2 (9·7) years. A total of 202 patients (168 men, 34 women) were in CDC group C with a mean (SD) age of 34·3 (9·8) years. Flow cytometric analysis (Cell Quest software) generated values for CD4% and CD3%. Absolute CD4+ T cell counts were derived using the differential lymphocyte percentage and the total leucocyte count (TLC) values obtained from the auto-analyser [19]. The mean values of different lymphocyte subsets (excluding the outliers) among the HIV-infected individuals belonging to CDC groups A, B and C are represented in Table 2. All the measurements were done using the same sample preparation technique, abiding by the same set of quality control criterion, using the same flow cytometer and auto-analyser for the entire duration of study.
Table 2. Distribution of lymphocyte subsets among HIV-infected CDC groups A, B and C.
| CDC group A (n = 212) | CDC group B (n = 172) | CDC group C (n = 194) | ||||
|---|---|---|---|---|---|---|
| Lymphocyte subset | Mean | 95%CI | Mean | 95%CI | Mean | 95%CI |
| CD4 | ||||||
| ″Count/µl. | 379·2 | 348·5–410 | 218·9 | 195·4–242·3 | 163·4 | 147·0–179·8 |
| ″CD4% | 15·9 | 15·0–16·7 | ″11·6 | 10·5–12·8 | ″9·7 | ″8·9–10·6 |
| CD8 | ||||||
| ″Count/µl. | 1351·2 | 1250–1452·3 | 1164·3 | 1061·5–1261·0 | 1046·5 | 936·7–1156·3 |
| ″CD8% | 54·9 | 53·3–56·5 | 58·7 | 56·8–60·7 | ″58·5 | 56·6–60·3 |
| CD4/CD8 ratio | ″0·32 | 0·30–0·35 | ″0·20 | 0·19–0·25 | ″0·18 | 0·16–0·20 |
| WBC count/µl. | ″7·1 | 6·6–7·5 | ″6·3 | 5·8–6·8 | ″6·0 | ″5·5–6·5 |
| Lymphocyte% | 35·3 | 34·2–36·4 | 32·6 | 31·0–34·2 | 30·6 | 29·1–32·0 |
n, number of individuals (excludes outliers).
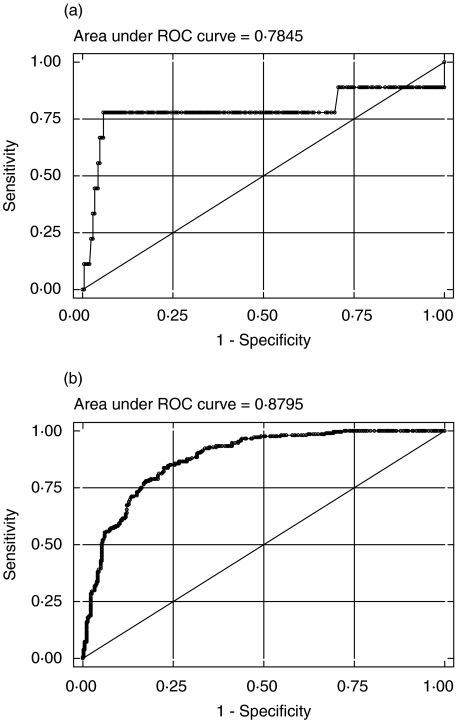
The patients were classified according to CDC criteria of CD4% into different groups. The CDC subgroups A1, B1 and C1 were pooled as group 1 and compared with the pooled group 2 comprising of A2, B2 and C2. Similarly, the pooled group 3 of A3, B3 and C3 was compared with the pooled group 2. The optimum cut-off values of CD4 counts were established by generating ROC curves between the above-mentioned CDC groups 1 and 2 and between CDC groups 2 and 3 (Fig. 1a,b). Cut-off values of 475 and 250 cells/µl for CD4 counts were deduced from these ROC curves. The sensitivity (Sn) and specificity (Sp) at these cut-offs were compared with the CDC classification values (Table 3). Our observed Sn and Sp values for CD4 counts ≥475 to categorize as subgroup 1 in CDC groups A, B and C were 77·78% and 77·88%, respectively, which corresponded well with the values for the recommended CDC cut-off. Similar findings were also observed for the obtained optimum cutoff of >250 between subgroup 2 and subgroup 3.
Fig. 1.
(a) ROC curve for determining the optimum cut-off of CD4 count between CDC groups A1 + B1 + C1 and A2 + B2 + C2, based on CD4 percentage. (b) ROC curve for determining the optimum cut-off of CD4 count between CDC groups A2 + B2 + C2 and A3 + B3 + C3, based on CD4 percentage.
Table 3. Comparison of CDC and observed Indian cut off values for CD4 count with patient distribution on CD4 percentage.
| CDC cut-off | Observed Indian cut-off | ||||
|---|---|---|---|---|---|
| CD4 count | Sensitivity (Sn) | Specificity (Sp) | CD4 count | Sensitivity (Sn) | Specificity (Sp) |
| ≥ 500 | 77·78 | 81·73 | > 475 | 77·78 | 77·88 |
| 201–500 | 86·54 | 72·02 | 251–475 | 79·81 | 79·22 |
| ≤ 200 | – | – | ≤ 250 | – | – |
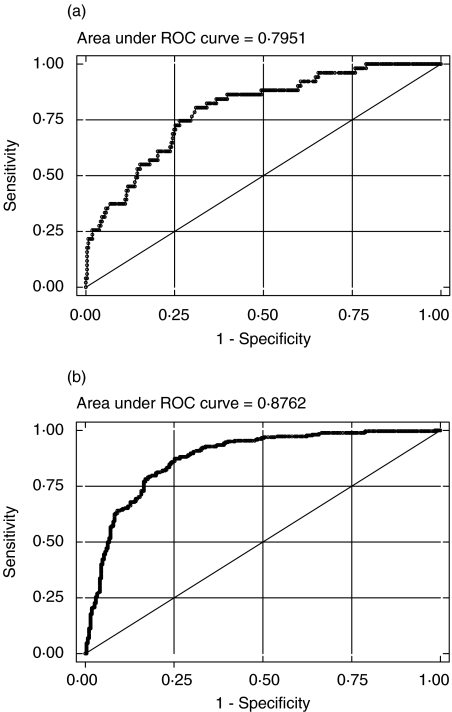
The analysis was repeated after classifying the patients according to CDC criteria of CD4 counts into different subgroups with ROC analysis to determine the optimum cut-off values of CD4%. The threshold values of >18% and ≤10% were obtained for CD4% (Fig. 2a,b). The sensitivity and specificity values for our observed cut-offs of >18% and ≤10% for CD4% indicate a substantial deviation from those obtained with the recommended CDC cut-off values of >28% and ≤14% (Table 4). For example, the sensitivity with the CDC recommended cut-off of 28% was only 13·7% as compared to 73·6% with the obtained cut-off of 18%.
Fig. 2.
(a) ROC curve for determining the optimum cut-off of CD4 percentage between CDC groups A1 + B1 + C1 and A2 + B2 + C2, based on CD4 counts. (b) ROC curve for determining the optimum cut-off of CD4 percentage between CDC groups A2 + B2 + C2 and A3 + B3 + C3 based on CD4 counts.
Table 4. . Comparison of CDC and observed Indian cut off values for CD4 percentage with patient distribution on CD4 counts.
| CDC cut-off | Observed Indian cut-off | ||||
|---|---|---|---|---|---|
| CD4% | Sensitivity (Sn) | Specificity (Sp) | CD4% | Sensitivity (Sn) | Specificity (Sp) |
| > 28 | 13·73 | 99·62 | > 18 | 74·51 | 73·56 |
| 15–28 | 49·43 | 93·61 | 11–18 | 84·67 | 76·32 |
| ≤ 14 | – | – | ≤ 10 | – | – |
The patients were also classified into a CDC matrix on the basis of clinical symptoms, and CDC recommended CD4 counts and CD4% values. The cross-classification of different subgroups within each of CDC A, B and C groups by these two criteria are shown in Table 5. As can be seen, there are differences in the distribution of patients with respect to the subcategorization based on CD4 counts and CD4% values. Among the 41 patients classified in category CDC A1 according to CD4 counts, more than three-quarters (85·4%) were classified as CDC A2 or CDC A3 by the CD4% criteria. Similarly, of the 130 patients classified as CDC A2 by CD4 criteria, 38·5% were categorized as CDC A3 by the CD4% criteria. The classifications among CDC B and CDC C groups also show similar trends.
Table 5. Classification of patients in CDC matrix on basis of CD4 counts and CD4 percentage for CDC groups A, B and C.
| CD4 counts | ||||
|---|---|---|---|---|
| CD4% | A/B/C1* | A/B/C2* | A/B/C3* | Total |
| Group A | A1 | A2 | A3 | |
| A1 | 6 (14·6%) | 0 (0·0%) | 0 (0·0%) | 6 (2·8%) |
| A2 | 30 (73·2%) | 80 (61·5%) | 7 (17·1%) | 117 (55·2%) |
| A3 | 5 (12·2%) | 50 (38·5%) | 34 (82·9%) | 89 (42·0%) |
| Total | 41 (100·0%) | 130 (100·0%) | 41 (100·0%) | 212 (100·0%) |
| Group B | B1 | B2 | B3 | |
| B1 | 1 (10·0%) | 1 (1·5%) | 1 (1·1%) | 3 (1·7%) |
| B2 | 8 (80·0%) | 33 (49·3%) | 6 (6·3%) | 47 (27·3%) |
| B3 | 1 (10·0%) | 33 (49·3%) | 88 (92·6%) | 122 (70·9%) |
| Total | 10 (100·0%) | 67 (100·0%) | 95 (100·0%) | 172 (100·0%) |
| Group C | C1 | C2 | C3 | |
| C1 | 0 | 0 (0·0%) | 0 (0·0%) | 0 (0·0%) |
| C2 | 0 | 37 (57·8%) | 7 (5·4%) | 44 (22·7%) |
| C3 | 0 | 27 (42·2%) | 123 (94·6%) | 150 (77·3%) |
| Total | 0 | 64 (100·0%) | 130 (100·0%) | 194 (100·0%) |
Groups A1–3, B1–3, C1–3 as defined in Table 1
Discussion
The obtained optimum cut-off values of CD4 counts with the patient distribution on CD4% corresponded well with the values for the recommended CDC cut-off for CD4 counts. However, similar findings were not observed for threshold CD4% values when the patients were classified according to the recommended CDC cut-off for CD4 counts.
The matrix also indicated some differences in the distribution of patients with respect to CD4 counts and CD4% into various CDC categories. The CD4% criterion tends to divide more patients into subgroups 2 and 3 as compared to the absolute CD4 count criteria. As can be seen from Table 5, of the total 212 patients of CDC A, 41 (19·3%) were found to be of A1 by CD4 values as against only 6 (2·8%) by the CD4% values. The same observations are reflected in CDC groups B and C (Table 5). This also emphasizes that CD4 expressed as a percentage is a better prognostic marker than the absolute CD4 counts because CD4 expressed as a percentage is not affected by changes in total and differential leucocyte counts as compared to the absolute counts in dual-platform technology [27].
Hence, the results from the present study indicate that HIV-infected Indian patients, who require effective initiation of antiretroviral treatment on the basis of their clinical category and CD4%, may be deprived of it in lieu of adopting a staging criterion for HIV infection based on their clinical category and absolute CD4 counts. Therefore, we propose a new classification system based on clinical category and CD4% for an HIV-infected Indian population (Table 6).
Table 6. Proposed classification system for an HIV-infected Indian population.
| Clinical categories | |||
|---|---|---|---|
| A | B | C | |
| CD4+ T-cell count (cells/µl.) (CD4%) | Asymptomatic, acute (primary) HIV or PGL* | Symptomatic, not A or C conditions† | AIDS-indicator conditions‡ |
| > 475 (18%) | A1 | B1 | C1 |
| 251–475 (11–18%) | A2 | B2 | C2 |
| ≤ 250 (10%) | A3 | B3 | C3 |
Category A: asymptomatic HIV infection, persistent generalized lymphadenopathy (PGL).
Category B: oropharyngeal and vulvovaginal candidiasis, constitutional symptoms such as fever (38·5°C) or diarrhea lasting >1 month, herpes zoster (shingles).
Category C: Mycobacterium tuberculosis (pulmonary and disseminated), Pneumocystis carinii pneumonia, candidiasis of bronchi; trachea or lungs, extrapulmonary cryptococcosis, CMV, HIV-related encephalopathy, Kaposi's sarcoma, wasting syndrome due to HIV.
Conclusions
The present study in an Indian population raises a few questions regarding the relevance of the current CDC staging system based on absolute CD4 counts in the HIV-infected population. It also emphasizes the importance of CD4% values in the staging of HIV patients so that effective antiretroviral treatment can be initiated at an appropriate time during the surveillance of the HIV-infected population. However, similar investigations need to be done in different settings to arrive at a conclusion about the utility of CDC staging based on absolute CD4 count vis-à-vis CD4% based staging. Therefore, HIV-1 risk management remains a tight-rope walk that has to be balanced between the initiation of antiretroviral treatment and the potential benefit of this prophylaxis in delaying the onset of clinical events, thus decreasing the physical and psychological morbidities.
References
- 1.DeWolf F, Roos M, Lange JM, et al. Decline in CD4+ cell numbers reflects increase in HIV-1 replication. AIDS Res Hum Retroviruses. 1988;4:433–40. doi: 10.1089/aid.1988.4.433. [DOI] [PubMed] [Google Scholar]
- 2.Giorgi JV, Nishanian PG, Schmid I, Hultin LE, Cheng H, Detels R. Selective alterations in immunoregulatory lymphocyte subsets in early HIV (human T-lymphotropic virus type III/lymphadenopathy-associated virus) infection. J Clin Immunol. 1987;7:140–50. doi: 10.1007/BF00916008. [DOI] [PubMed] [Google Scholar]
- 3.Lang W, Perkins H, Anderson RE, Royce R, Jewell N, Winkelstein W., Jr Patterns of T-lymphocyte changes with human immunodeficiency virus infection: from seroconversion to the development of AIDS. J Acquir Immune Defic Syndr. 1989;2:63–9. [PubMed] [Google Scholar]
- 4.Masur H, Ognibene FP, Yarchoan R, et al. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1989;111:223–31. doi: 10.7326/0003-4819-111-3-223. [DOI] [PubMed] [Google Scholar]
- 5.Smith RD. The pathobiology of HIV infection: a review. Arch Pathol Laboratory Med. 1990;114:235–9. [PubMed] [Google Scholar]
- 6.Hanson DL, Chu SY, Farizo KM, Ward JW. Distribution of CD4+ T lymphocytes at diagnosis of acquired immunodeficiency syndrome-defining and other human immunodeficiency virus-related illnesses. The Adult and Adolescent Spectrum of HIV Disease Project Group. Arch Intern Med. 1995;155:1537–42. [PubMed] [Google Scholar]
- 7.Stein DS, Korvick JA, Vermund SH. CD4+ lymphocyte cell enumeration for prediction of clinical course of human immunodeficiency virus disease: a review. J Infect Dis. 1992;165:352–63. doi: 10.1093/infdis/165.2.352. [DOI] [PubMed] [Google Scholar]
- 8.Fahey JL, Taylor JM, Detels R, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–72. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 9.Friedland GH. Early treatment for HIV. the time has come. N Engl J Med. 1990;322:1000–2. doi: 10.1056/NEJM199004053221409. [DOI] [PubMed] [Google Scholar]
- 10.De Gruttola V, Gelman R, Lagakos S. Uses of CD4-lymphocyte count in AIDS treatment decisions. Infect Agents Dis. 1994;2:304–13. [PubMed] [Google Scholar]
- 11.CDC. USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus: a summary. MMWR. 1995;44 (no. RR–8):1–34. [PubMed] [Google Scholar]
- 12.CDC. Perspectives in disease prevention and health promotion update. universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other blood borne pathogens in health-care settings. MMWR. 1988;37:377–88. [PubMed] [Google Scholar]
- 13.CDC. 1992 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1993;41 (no. RR–17):961–2. [PubMed] [Google Scholar]
- 14.CDC. 1997 Revised Guidelines for Performing CD4+ T-Cell Determinations in Persons Infected with Human Immunodeficiency Virus (HIV) MMWR. 1997;46 (no. RR–2):1–29. [PubMed] [Google Scholar]
- 15.Mandy F, Nicholson J, McDougal J. Revised guidelines for performing single-platform absolute CD4+ T-Cell determinations with CD45 gating for persons infected with human immunodeficiency virus. MMWR. 2003;52 (no. RR–02):1–13. [PubMed] [Google Scholar]
- 16.Kam KM, Wong KH, et al. Proposed CD4+ T-cell criteria for staging HIV-infected Chinese adults. Clin Immunol Immunopathol. 1998;89:11–22. doi: 10.1006/clin.1998.4570. [DOI] [PubMed] [Google Scholar]
- 17.McNerlan SE, Alexander HD, Rea IM. Age related Reference intervals for lymphocyte subsets in whole blood of healthy individuals. Scan J Clin Laboratory Invest. 1999;59:89–92. doi: 10.1080/00365519950185805. [DOI] [PubMed] [Google Scholar]
- 18.Vithayasai V, Sirisanthana T, Sakonwasun C, et al. Flow Cytometric analysis of T lymphocyte subsets in adult Thais. Asian Pacific J Allergy Immunology. 1997;15:141–6. [PubMed] [Google Scholar]
- 19.Webster HK, Pattanapanyasat K, Phanupak P, et al. Lymphocyte Immunophenotype reference ranges in Healthy Thai Adults: Implications for management of HIV/AIDS in Thailand. Southeast Asain J Trop Med Public Health. 1996;27:418–29. [PubMed] [Google Scholar]
- 20.Pagleironi TG, Holland PV. Circannual variation in lymphocyte subsets, revisited. Transfusion. 1994;34:512–6. doi: 10.1046/j.1537-2995.1994.34694295067.x. [DOI] [PubMed] [Google Scholar]
- 21.Hulstaert F, Hannet I, et al. Age-related changes in Human blood lymphocyte subpopulations. Clin Immunol Immunopathol. 1994;70:152–8. doi: 10.1006/clin.1994.1023. [DOI] [PubMed] [Google Scholar]
- 22.Senju M, Makiyama K, Hara K, et al. Two-color immunofluorescence and Flow cytometric analysis of peripheral blood lymphocyte subsets in Caucasian and Japanese Healthy subjects. Jpn J Med. 1991;30:509–15. doi: 10.2169/internalmedicine1962.30.509. [DOI] [PubMed] [Google Scholar]
- 23.Zakeng L, Sadjo A, Meli J, et al. T lymphocyte subset values Among Healthy Cameroonians. J Acquir Immuno Defic Syndr. 1997;14:82–3. doi: 10.1097/00042560-199701010-00016. [DOI] [PubMed] [Google Scholar]
- 24.Worku S, Christensson B, Bjorkman A, et al. Higher Proportion of CD8+ T cells in the blood in healthy adults from Ethiopia and Bangladesh compared with Sweden. Transactions Royal Soc Trop Med Hygiene. 1997;91:618–22. doi: 10.1016/s0035-9203(97)90051-1. [DOI] [PubMed] [Google Scholar]
- 25.Reichert T, De Brujere M, Deneys V, et al. Lymphocyte subset reference ranges in Adult Caucasians. Clin Immunol Immunopathol. 1991;60:190–208. doi: 10.1016/0090-1229(91)90063-g. [DOI] [PubMed] [Google Scholar]
- 26.Messele T, Abdulkadir M, Fontanet AL, et al. Reduced naive and increased activated CD4 and CD8 cells in healthy adult Ethiopians compared with their Dutch counterparts. Clin Exp Immunol. 1999;115:443–50. doi: 10.1046/j.1365-2249.1999.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amatya R, Vajpayee M, Kaushik S, et al. Lymphocyte immunophenotype reference ranges in healthy Indian adults: implications for management of HIV/AIDS in India. Clin Immunol. 2004;112:290–5. doi: 10.1016/j.clim.2004.04.008. [DOI] [PubMed] [Google Scholar]