Abstract
Atherosclerosis is a complex disease involved in major fatal events such as myocardial infarction and stroke. It is the result of interactions between metabolic, dietetic and environmental risk factors acting on a genetic background that could result in endothelial susceptibility. Our aim was to determine the patterns of expression of adhesion molecules and whether phosphatidylserine is translocated to the cell surface of human umbilical vein endothelial cells (HUVECs) isolated from healthy newborns born to parents with a strong family history of myocardial infarction under TNF-α or oxLDL stimulated conditions. Compared to control HUVECs, experimental cords showed: (a) a four-fold increase in VCAM-1 expression under basal conditions, which showed no change after stimulation with the pro-atherogenic factors; (b) a two-fold increase in basal P-selectin expression that reached a 10-fold increase with any of the pro-atherogenic factors; (c) a basal ICAM-1 expression similar to P-selectin that was not modified by the pro-atherogenic molecules; (d) a similar PECAM-1 expression. Unexpectedly, phospathidylserine expression in experimental cord HUVECs was significantly increased (211 817 versus 3354 TFU) but was not associated to apoptotic death as the percentage of dead cells induced by TNF-α treatment was very low (0·55 versus 9·87% in control HUVECs). The latter result was corroborated by TUNEL staining. T cell adherence to HUVECs was highly up-regulated in the genetically predisposed samples. The analysis of nonpooled HUVECs, from newborns to family predisposed myocardial-infarction individuals, might represent a useful strategy to identify phenotypical and functional alterations, and hopefully, to take early preventive actions.
Keywords: HUVECs, infarction, inflammation, adhesins, atherosclerosis
Introduction
Myocardial infarction (MI) is a severe disabling and very often fatal event associated with the atherosclerotic process [1,2]. It is well recognized that members of families with a history of heart infarction are prone to develop MI; however, the way in which this background influences future atherosclerotic development is unclear. The progression of atherosclerosis is characterized by endothelial dysfunction, subendothelial mononuclear cells infiltration, arterial wall remodelation, lipid deposition and fibrosis [3,4]. Initial lesions might occur at a very early stage, but the full manifestation of atherosclerotic plaque usually occurs after the fourth decade of life [5].
Apparently, full plaque development needs cumulative aggression [6]. However a positive family history of coronary artery disease (which has been associated with an impaired endothelium [7]) has effects that add up to those exerted by other risk factors, such as hypercholesterolaemia, increased age, hypertension, smoking, etc. [8,9]. Nevertheless, the specific weight of each individual risk factor varies between individuals depending on an unclear genetic background that includes differences in endothelial susceptibility [10,11].
During the last two decades, studies have shown that the pathogenesis of atherosclerosis is immune-related and seems to be initiated by the early tethering and rolling of leucocytes along the endothelial cell lining, using adhesins expressed on the surface of endothelial cells. The expression of these molecules, which define the type of cell to be recruited [12], is induced and regulated by cytokines liberated in the milieu [13]. Thus, the endothelial cell plays a major role in determining and maintaining the inflammatory reactions that occur in response to various inflammatory stimuli [14]. Why endothelial cells respond in such an heterogeneous way is not known [15]. However, in animal models the overexpression of VCAM-1 and ICAM-1 has been observed in areas prone to developing atherosclerotic lesions [16].
This pro-inflammatory status could also be maintained by human umbilical vein endothelial cells undergoing apoptotic cell death. These HUVECs exhibit a more rapid exposure of membrane phosphatidylserine [17] which activates the classical pathway of complement [18], thus contributing to the inflammatory response.
Experiments performed with HUVECs frequently use cells pooled from several umbilical cords. Interestingly, comparisons of individually derived cultures from newborns with distinct genetic background are scarce. If endothelial susceptibility is a predisposing factor, some individual abnormalities should be discernible in newborns and we could expect differences in the response of endothelial cells to different pro-atherogenic factors, only if nonpooled HUVEC cultures are analysed.
In this paper we describe the different cell adhesion capabilities of nonpooled HUVECs isolated form newborns with and without strong heart infarction background. We assessed surface expression of: P-selectin, ICAM-1, VCAM-1, PECAM-1, T lymphocyte adherence as well as surface exposure of phosphatidylserine, of nonstimulated and TNF-α or ox-LDL-stimulated endothelial cells.
Materials and methods
Umbilical cords
We questioned 372 pregnant women concerning familial and personal history of heart infarction, hypertension, diabetes, abortions, fetal death, cigarette smoking, obesity and the appearance of complications during pregnancy. Eight of the women had strong family history of ischaemic heart disease defined as three or more first degree relatives (grandparents, parents and brothers/sisters) with a background of myocardial infarction. All the participants gave their informed consent. Two of the mothers (patients 4 and 6) were diabetic. Patient 4 had a heart infarct at 30 years of age, that is three years before giving birth to the newborn we evaluated; patients 2 and 6 had maternal grandmothers with elevated serum cholesterol levels. None of the mothers had hypercholesterolaemia and their serum lipids were within normal values (Table 1).
Table 1. Serum lipid and glucose concentrations in mothers from which pathological HUVECs were obtained.
| HDL (>70 mg/dl) | LDL (<160 mg/dl) | Triglyceride (<300 mg/dl) | Cholesterol (<380 mg/dl) | Glucose (80–120 mg/dl) | |
|---|---|---|---|---|---|
| 1 | 72 | 174 | 321 | 298 | 96 |
| 2 | 76 | 139 | 233 | 253 | 63 |
| 3 | 58 | 97 | 332 | 209 | 74 |
| 4 | 50 | 101 | 243 | 209 | 157 |
| 5 | 58 | 174 | 258 | 57 | 112 |
| 6 | 31 | 135 | 320 | 218 | 63 |
| 7 | 50 | 101 | 243 | 190 | 157 |
| 8 | 63 | 146 | 281 | 277 | 94 |
Blood samples were obtained in the delivery room. HDL, High density lipoproteins; LDL, low density lipoproteins. Normal values at the end of pregnancy are shown in brackets.
Blood samples and an EKG were obtained from each patient before entering the delivery room where umbilical cords were obtained. The cords were immediately kept in M-199 medium (Gibco/BRL, Grand Island, NY, USA) containing 10% heat-inactivated fetal calf serum (Hyclone Logan, Utah, USA) and supplemented with 10 mM HEPES, 100 µg/ml of penicillin, 100 µg/ml of streptomycin, 2 mM l-glutamine, and 5 IU/ml porcine heparin (all from Sigma, St. Louis, Missouri, USA), and HUVECs extracted immediately upon arrival. The period between the time when the umbilical cord was obtained and the time it was processed was never longer than 6 h.
Human umbilical vein endothelial cells
HUVECs were extracted with 0·2% type II collagenase (Roche, Hertfordshire, UK) by conventional techniques and were cultured until confluence at 37°C in a 7% CO2 humidified atmosphere. HUVECs were recovered with saline solution containing 0·5% trypsin/5 mM EDTA. Before being used, the cells were washed three times with saline solution supplemented with 2% fetal calf serum, 1 M Hepes, 2·2 g/l glucose, 4 mM CaCl2 (all from Sigma). The cells were used within three passages and were identified as endothelial by their characteristic morphology and presence of endoglin (CD105) surface antigen (BD PharMingen San Diego, CA, USA).
Flow cytometry
Two × 106 endothelial cells fixed in 2 ml of a solution containing 25% ethanol with 15 mM magnesium, were exhaustively washed with PBS and were resuspended in 1 ml of PBS containing 1% bovine albumin (Sigma). 100 µl aliquots of the cell suspension were incubated 1 h at 37°C with anti-human ICAM-1, VCAM-1, PECAM, and P-selectin monoclonal antibodies (R & D Systems, Minneapolis, Minnesota, USA). FITC-labelled annexin V was from (Roche). Stimulation experiments were done using human recombinant TNF-α (R & D Systems) or oxidized LDL (kindly donated by Dr Guillermo Cardoso-Saldaña) [19] using different concentrations previously determined as optimal and nontoxic. At the end, the cells incubated with nonlabelled antibodies were incubated with FITC-labelled rabbit anti-mouse IgG (Roche), for 1 h at 37°C. Fluorescence was measured in a Becton Dickinson FACScan (San Jose, CA, USA). For all the experiments 10 thousand events were recorded for each molecule. In order to determine phosphatidylserine translocation to the cell membrane; the endothelial cells were stained for 15 min with FITC-labelled annexin V before measuring fluorescence in the FACS. Propidium iodide (final concentration of 150 ng/µl) was used to detect dead cells. Negative controls and an immunoglobulin isotype controls were included in each experiment and fluorescence values were corrected when appropriate. Results, standarized to the 10 000 events already mentioned, showing the differences between groups are expressed as total fluorescence units (TFU which is the result of multiplying the geometrical mean fluorescence by the total number of cells that responded to the stimulus; these units reflect the total number of molecules expressed on the cell membrane of the cell population being studied, regardless of whether there were a greater number of molecules per cell or a greater number of cells expressing similar number of molecules each).
TUNEL reaction
In order to evaluate endothelial cell death in HUVECs isolated from newborns with a strong familial history of myocardial infarction (experimental group) and those without strong family history of myocardial infarction (control group) a TUNEL reaction was performed in nonstimulated and TNF-α-stimulated (10 ng/ml) endothelial cells. The assay was performed with the In Situ Cell Death Detection Kit, Fluorescein (Roche) according to the manufacturer's instructions. Briefly, the cells were cultured to confluence in glass slides as described in the section Human umbilical vein endothelial cells. Cultured cells were rinsed twice in freshly prepared Phosphate buffered saline (PBS), pH 7·4, before fixing them for 20 min at room temperature with a gentle shake. Afterwards the slides were extensively washed in PBS before incubating them in the permeabilization solution (0·1% Triton X-100, 0·1% sodium citrate) for 2 min on ice. The slides were then incubated with 50 µl of TUNEL reaction mixture in a humidified atmosphere for 1 h at 37 °C in the dark, washed in PBS and counter-stained with propidium iodide. The cells were analysed by fluorescent microscopy in a LEICA DMLS microscope equipped with a LEICA DF 300× digital camera and a LEICA IM-1000 software. Propidium iodide stains red at a wavelength of 600 nm or higher and the fluorescein labels incorporated in nucleotide polymers stain green at 515–565 nm. Merging both colours gives a yellow stain in apoptotic cells.
Crystal violet staining
Cell proliferation was evaluated by crystal violet staining [20,21]. Experimental and control HUVECs were plated in 96-multiwell plates and cultured for 24 and 48 h at 37°C in a 7% CO2 humid atmosphere. At the end of the incubation, cells were fixed with 100 µl of ice cold glutaraldehyde (1·1% in PBS) for 15 min at 4°C. Plates were washed three times by submersion in de-ionized water, air-dried and stained for 20 min with 100 µl of a 0·1% crystal violet solution (in 200 mM phosphoric acid buffer at pH 6). After careful aspiration of the crystal violet solution the plates were extensively washed with de-ionized water, and air-dried prior to the solubilization of the bound dye with 100 µl of a 10% acetic acid solution incubated during 30 min. The O.D. of the plates was measured at 595 nm in a multi-well plate spectrophotometer.
Adhesion assay
Jurkat cells (CD3 + lymphoblasts) or U-937 cells (C3R + monocytes) were grown in RPMI-1640 medium with 10% fetal calf serum, 10 mM Hepes and 2 mM l-glutamine (all from Sigma), at 37 °C in a 7% CO2 humid atmosphere. The cells were grown in 20–40 ml sterile tissue culture flasks (Nunclon, Nalgene Nunc Intl., Rochester, NY, USA) and split every 3–5 days to maintain a cell concentration of 1·0–2·0 × 106 cells/ml. The cells to be used in the adhesion assays were centrifuged and resuspended at 1·5 × 106 cells/ml in M-199 medium containing 15 mM HEPES to maintain pH. The cells were labelled with BCECF-AM according to the manufacturer's instructions (Molecular Probes, Eugene, OR, USA). The adhesion assay was carried out in 24 well tissue culture plates (Nunclon) seeded with 1 × 105 endothelial cells cultured during 24 h to allow the formation of monolayers, before adding 3 × 105 labelled Jurkat or U-937 cells; the cells were allowed to interact with the HUVECs for 1 h; nonadherent cells were then removed by gentle washing with M-199 medium before adding 1·5 ml of lysis buffer (10% Triton X-100 in 10 mM Tris, pH 9·0) (all from Sigma) per well. The percentage of cell adherence was calculated according to the fluorescence quantified at 490 nm in a fluorescence spectrophotometer LS 50B (Perkin-Elmer, Norwalk, CT, USA).
Statistical analysis
The results were analysed by means of a Student's T-test using the SPSS software, 11·0.0 release; a P-value of <0·05 was regarded as significant.
Results
Endothelial cells obtained from each individual umbilical cord were grouped between those with a strong familial history of myocardial infarction (experimental group) and those without strong family history of myocardial infarction (control group).
Adhesion molecule expression in basal and TNF-α stimulated conditions
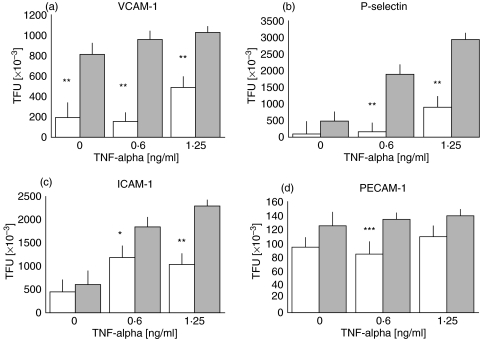
Interestingly, even under nonstimulated conditions, an important difference could be seen in the membrane expression of VCAM-1 between HUVECs derived from experimental and control cords (1·91 × 105versus 8·12 × 105 TFU; P < 0·001). Control cord cells stimulated with recombinant TNF-α increased the expression of VCAM-1 whereas experimental cords cells did not show a significant increase in VCAM-1 expression after stimulation with this cytokine (Fig. 1).
Fig. 1.
(a) VCAM-1 expression showed similar total fluorescence units (TFU) in the experimental cords HUVECs (░) under basal nonstimulated conditions as well as under TNF-α stimulated conditions; control HUVECs (□) showed significatively lower basal values (*P < 0·005). (b) P-selectin expression showed similar values under nonstimulated conditions in both groups, nevertheless, the experimental cords expressed significatively higher amounts of this adhesin (**P < 0·001) than control cords HUVECs under TNF-α stimulation; (c) ICAM-1 expression behaved almost similarly to P-selectin although the number of molecules induced by the maximal TNF-α dose was somehow smaller 2·2 × 106versus 2·9 × 106 for P-selectin. (d) PECAM although not over-expressed in either group under basal conditions showed a significant (***P < 0·05) but transient overexpression when the cells were stimulated with 0·6 ng/ml of recombinant TNF-α. All the results are expressed as the mean ± standard deviation of the mean for the eight experimental umbilical vein endothelial cells.
P-selectin expression was higher under nonstimulated conditions in experimental cords, however, this difference did not reach statistical significance when compared to control cells. Unlike the expression of VCAM-1, there was a progressive increase in the expression of P-selectin in the experimental cord cells stimulated with recombinant TNF-α, which reached a six-fold increase (4·76 × 105versus 2·94 × 106 TFU; P < 0·001) when 1·25 ng/ml of TNF-α was used to stimulate the cells (Fig. 1).
The expression of ICAM-1 was similar to that of P-selectin, however, the increase observed in the unstimulated controls and samples stimulated with the highest TNF-α concentration, while statistically significant (6·5 × 105versus 1·85 and 2·2 × 106; P < 0·001), was moderate (Fig. 1). There were no significant differences in the expression of PECAM-1 under basal nonstimulated conditions, nevertheless, when a 0·6 ng/ml of recombinant TNF-α was used to stimulate the cells, there was a transient but significant (P< 0·05) increase in PECAM-1 expression in the experimental HUVECs which did not augment when the cells were stimulated with a higher dose of TNF-α (Fig. 1).
Phosphatidylserine surface/membrane exposure
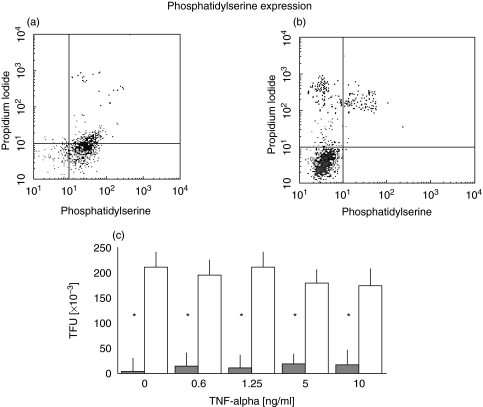
There was an important difference in the basal expression of phosphatidylserine (PS) as determined by annexin V staining, between experimental and control cord cells (Fig. 2a,b). This difference was confirmed by measuring total fluorescence units of both HUVECs groups (211 817 versus 3354 TFU; P < 0·001) (Fig. 2c). TNF-α stimulation did not increase the translocation of PS in either group at concentrations as high as 10 ng/ml. It was worth noting that the majority of the experimental cord cells expressed phosphatidylserine (89·3 ± 12%versus 3·10 ± 2%) and that this expression was not modified by TNF-α. Interestingly, 10 ng/ml of TNF-α induced a 9·87% cell death in the control cord cells, but when the experimental cord cells were treated with the same concentration of TNF-α, the percentage of dead cells was negligible (0·55 ± 0·46%), as determined through the use of propidium iodide (data not shown). Cell death without TNF-α stimulation was less than 1%. TUNEL assays, done to corroborate the cell death, showed a very low amount of endothelial cells containing apoptotic bodies even in the presence of 10 ng/ml of TNF-α (Fig. 3b,d,f) as opposed to control cords which under TNF-α-stimulated conditions showed a significant number of endothelial cells containing apoptotic bodies (Fig. 3a,c,e).
Fig. 2.
Flow cytometry results of (a) experimental and (b) control HUVECs stained with annexin V to detect phosphatidylserine translocation to the cell membrane, and propidium iodide to determine cell viability. (c) PS expression in HUVECs stimulated with different doses of TNF-α, showing a statistically significant increased expression in the experimental cords HUVECs (□) versus control cords ( ), independently of TNF-α concentration (*P < 0·001). Results are expressed as mean ± standard deviation of the mean.
), independently of TNF-α concentration (*P < 0·001). Results are expressed as mean ± standard deviation of the mean.
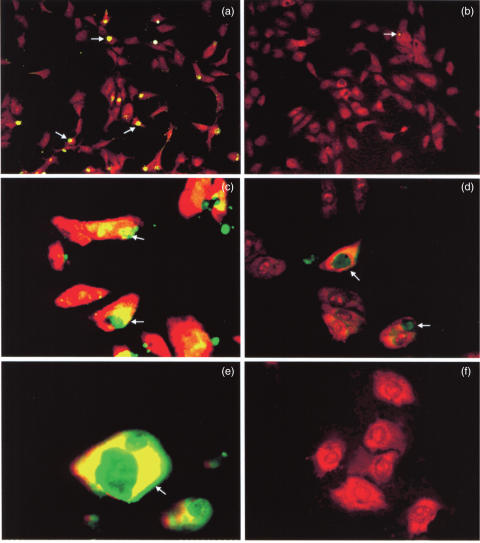
Fig. 3.
Microphotographs (a,b, ×20; c,d, ×63; e,f, ×100 original magnification) of the TUNEL assays performed in control and experimental HUVECs stimulated with 10 ng/ml of TNF-α. The arrows show the presence of apoptotic bodies (white arrows) in control cells (a,c,e) The scarcity of apoptotic bodies in experimental cords HUVECs (b,d,f) is evident.
Adhesion molecule expression in oxLDL stimulated conditions
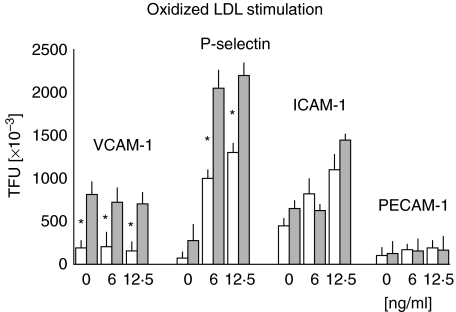
The stimulation of endothelial cells with an optimal dose of oxidized LDL (12·5 ng/ml) induced a small decrease in the expression of VCAM-1, which did not reach statistical significance (Fig. 4). This result further emphasizes the observation we made when TNF-α was used for stimulation purposes. P-selectin expression reached 2·2 × 106 TFU, a cipher similar to the one obtained when TNF-α was used to stimulate those cells. It was interesting to note that the expression of P-selectin was higher in control cells stimulated with ox-LDL (1·3 × 106 TFU) than with TNF-α (9 × 105 TFU). Expression of ICAM-1 was enhanced by ox-LDL but its maximum value (1·45 × 106 TFU) was below that obtained with TNF-α (2·2 × 106 TFU). PECAM-1 expression showed no differences when compared to the control cells.
Fig. 4.
The expression of different adhesins induced by oxidized LDL was higher in the experimental cords HUVECs ( ) than in the control cords (□). TFUs values never reached the same values as those obtained under TNF-α stimulation. *P < 0·001. Results are expressed as mean ± standard deviation of the mean.
) than in the control cords (□). TFUs values never reached the same values as those obtained under TNF-α stimulation. *P < 0·001. Results are expressed as mean ± standard deviation of the mean.
Jurkat and U-937 adhesion assays
Based on the differences observed in the expression of adhesion molecules, we evaluated the competence of endothelial cells to adhere to T cell and pro-monocytes (JURKAT and U-937 cell lines, respectively) under nonstimulated and TNF-α (2·5 ng/ml) stimulated conditions. HUVECs from the experimental group showed an enhanced binding to lymphocytes or pro-monocytes, which was not the consequence of an increase in the rate of growth of experimental HUVECs as the crystal violet staining results showed (Table 2). The result of the fluorescence spectroscopy with the BCECF-labelled JURKAT or U-937 cells, corroborate the intrinsic augmented binding capacity of experimental versus control HUVECs. Both HUVEC types were stimulated with TNF-α, a major pro-atherogenic stimulus and a well established factor influencing endothelial adhesive properties. We found that there was a statistical significant increase in the adhesion of both mononuclear cell types to stimulated HUVECs in the experimental group, 4·6 fold for Jurkat (P< 0·0002) and 2·7 fold for U-937 (P< 0·002) cells (Table 3 and Fig. 5).
Table 2. Experimental and control HUVECs proliferation as determined by crystal violet staining.
| HUVECs | 24 h | 48 h |
|---|---|---|
| Experimental | 0·319 ± 0·081 | 0·367 ± 0·050 |
| Control | 0·339 ± 0·068 | 0·390 ± 0·055 |
The results represent the O.D. at 595 nm of triplicate cell cultures maintained for 24 and 48 h before being stained with crystal violet as described in material and methods. There was no statistical significant difference between groups and culture times.
Table 3. Adherence of Jurkat (T-cell like) and U-937 (monocyte-like) cells to experimental and control HUVECs.
| Non-stimulated | TNF-α stimulated | |||
|---|---|---|---|---|
| Control HUVECs | Experimental HUVECs | Control HUVECs | Experimental HUVECs | |
| Jurkat cells | 4 ± 1 | 6 ± 2 (a) | 15 ± 6 (b) | 28 ± 12 (c) |
| U-937 cells | 5 ± 2 | 7 ± 2 (d) | 6 ± 2 (e) | 19 ± 9 (f) |
Percentage of BCECF-AM labelled Jurkat or U-937 cell adhered to either control or experimental endothelial cells. The adhesion of Jurkat or U-937 cells to control or to experimental HUVECs did not show differences under basal conditions. The adhesion of Jurkat (CD3+) cells under TNF-α stimulated (2·5 ng/ml) conditions was significantly higher ((a) versus (c) P < 0·0002) in the experimental versus the control ((b) versus (c) P < 0·01) HUVECs. There was a significant increase in the adhesion of U-937 cells to TNF-α-stimulated experimental HUVECs ((d) versus (f) P < 0·002) versus the control ((e) versus (f) P < 0·001). Results representing the percentage of fluorescence binding are expressed as mean ± standard deviation of the mean.
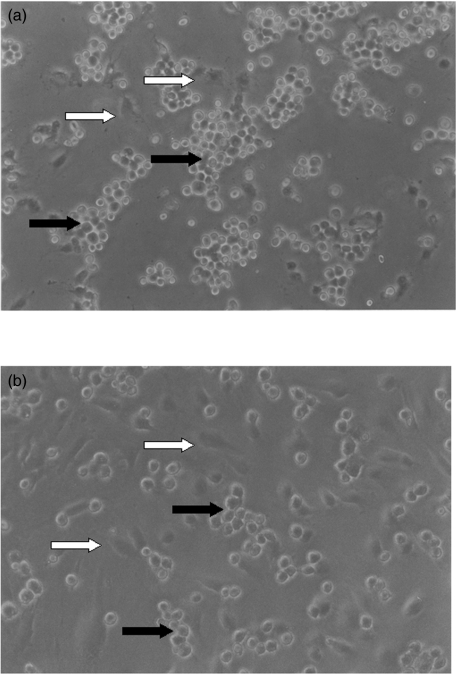
Fig. 5.
Microphotograph (original magnification ×60) showing the enhanced adhesion of Jurkat cells (dark arrows) to endothelial cells (white arrows) obtained from (a) experimental cords versus (b) control cords. The existence of clusters of HUVECs is evident in those areas where adhesion is more pronounced.
Discussion
Atherosclerosis is the result of a complex interaction between several risk factors, one of them being a familial history of coronary artery disease, which has been linked to a strong possibility of developing the disease [22]. The formation of characteristic lesions is secondary to a tightly regulated migration of blood monocytes and lymphocytes into the arterial intima, which suggests that endothelial cells play a significant role in the development of atherosclerosis and that local events might contribute to the recruitment of monocytes and lymphocytes [3, 4, 13, 23]. One such event could be a distinct expression pattern of local adhesion molecules in the endothelial cells of individuals with inherited susceptibility, as recent experiments done with mice and rabbits suggest [16].
The expression of VCAM-1, P-selectin and ICAM-1 adhesion molecules was higher in the experimental cords under basal, nonstimulated conditions. It was surprising to find that the expression of VCAM-1 did not increase when the cells were stimulated with TNF-α, an observation suggesting that the latter cells are already in a stimulated status. Cell adhesion molecules expressed in endothelial cell are controlled by cytokines, specially IL-1, IL-4 and TNF-α[24], but the surface expression of VCAM-1 depends on the activation of NF-kb [25,26] and on a functioning actin cytoskeleton [27].
Our observations in HUVECs, a venous endothelium, may not be entirely representative of the responses of other endothelia. It has been shown that human heart-derived endothelial cells have different expression levels of adhesion molecules under basal and cytokine-stimulated conditions [28]. Whether these differences are shared among other endothelia or are particular to a specific subpopulation [29], remains to be determined. Our preliminary results in experimental cord HUVECs have shown the existence of a large granular endothelial cell subpopulation more reactive to pro-atherogenic factors.
Experimental cords under non TNF-α-stimulated conditions over-expressed adhesion molecules. This expression was not enhanced upon stimulation with TNF-α, suggesting a loss of the down-regulation mechanism which is probably genetically predetermined [30]. Under normal conditions, cytokines modulate the expression of adhesion molecules on HUVECs either in a synergistic or antagonistic manner [31–33]. It has recently been demonstrated that basal expression of adhesion molecules does not change with age whereas the response to cytokine stimulation increases [34]. Enhanced T and pro-monocyte cell adhesion by experimental cord HUVECs confirmed the over-expression of adhesion molecules; nevertheless, it is possible that adhesion to monocytes is actually greater than that which we observed with the U-937 cells, since the latter require phorbol ester 12-O-tetradecanoylphorbol-13-acetate stimulation to reach terminal monocytic differentiation [35]. Similarly, it was interesting to observe that not all HUVECs bound JURKAT or U-937 cells, reinforcing the clonal composition of the endothelium [36]. All these results strengthen the likelihood of an atherosclerosis-prone status in experimental cords’ HUVECs.
Oxidized low density lipoprotein as opposed to TNF-α, had a different effect upon cell adhesion molecule expression in experimental cord HUVECs. A recent report by Vielma et al. [37] confirms that once endothelial cells display adhesion molecules, oxLDL has a selective and limited enhancing effect. This differential regulation had already been observed for matrix metalloproteinase-1 (MMP-1) expression in human vascular endothelial cells [38]. The inducing effect of oxLDL is highly dependent on the source of endothelial cell [39].
Atherosclerosis development requires several concurrent factors [5, 6, 22], namely
Inflammatory or injury risk factors such as TNF-a, hypertension, diabetes mellitus, viral or bacterial infection, etc;
deposition factors such as familial hypercholesterolaemia, elevated plasma Lpa(a) isoforms, homocysteine and/or LDL and oxidized LDL, Lpa (a) isoforms, etc;
genetic risk factors.
Our results showed that the effect of two different pro-atherogenic factors in the experimental cords’ HUVECs was not identical, suggesting the need for a more profound analysis of their effect upon the endothelium in risk conditions.
A major observation of this study was the increased membrane exposure of phosphatidylserine in the outer membrane of experimental cord HUVECs, which was not modified by TNF-α or oxLDL pretreatment. It was interesting to observe that TNF-α-induced cell death, as determined by propidium iodide, was less in comparison to the control cords. The experimental cells stained positively with annexin V and were negative to propidium iodide. This observation was corroborated by the TUNEL assay, which showed the absence of apoptotic bodies. Propidium iodide stain nucleic acids; in integral cells it only stains the nuclei but in paraformaldehyde fixed cells which do not have an intact membrane it can stain the DNA o RNA that has abandoned the nuclei, the latter being the cause of the cytoplasmic red labelling. It was possible that in vitro culture conditions might have induced apoptosis, but this event is only possible in serum deprivation conditions [17], which was considered in our experiments. It is possible that enhanced VCAM-1 expression and very low apoptotic cell death rate in the experimental HUVECs might depend on signalling intermediates such as TRAF-2/5 or RIP-1, generating NF-kB and AP-1-dependent gene transcription of VCAM-1 [25] and cellular inhibitors which block the function of caspases [40].
Translocation of phosphatidylserine to the outer leaflet of the cellular membrane seems to be a key signal for apoptotic cells to be engulfed by phagocytes. The absence of apoptotic bodies despite PS over-expression in the experimental HUVECs is consistent with reports that suggest that the maintenance of outer membrane exposure of PS is not always associated with inevitable apoptosis [41,42]. It has also been reported that HUVECs undergoing cell death exhibited typically a more rapid exposure of membrane phosphatidylserine (PS) than DNA fragmentation [17]
In this scenario, increased PS surface exposure could make the endothelium prone to become a target for macrophage attachment via the scavenger receptors CD36 and CD68, and the LOX-1 receptor [43,44]. We can not conclude that the over-expression of PS on endothelial cells is a marker for inherited cardiovascular risk, but it could be a potent atherogenic stimulus inducing enhanced monocyte- and lymphocyte-endothelial adhesion and possibly thrombosis as well as activating complement [18] and initiating coagulation [45–47].
The importance of determining the degree of responsiveness of pro-inflammatory and pro-atherogenic stimuli in endothelial cells from newborns with a strong family history of ischaemic heart disease, could lead to the identification of potential links between genetic predisposition and endothelial dysfunction in ischaemic heart disease.
Acknowledgments
The authors wish to acknowledge the assistance of Dr Alberto Valero, Head of the Reproductive Medicine Unit, Hospital Angeles del Pedregral, for umbilical cords and critical evaluation of the patients, and Jorge Galicia, M.Sc. for statistical analysis. Oxidized LDL was a kind gift of Dr Guillermo Cardoso-Saldaña, Depto. Endocrinología, Instituto Nacional de Cardiología ‘Ignacio Chávez’, México. This work was supported by FOSISS-CONACyT grant Salud-2002-C01-7630, CONACyT-MO334-FUNSALUD and DGAPA-UNAM (PAPIIT-IN224598) grants.
References
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Eng J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Chilton RJ. Recent discoveries in assessment of coronary heart disease impact of vascular mechanisms on development of atherosclerosis. J Am Osteopath Assoc. 2001;101:S1–5. [PubMed] [Google Scholar]
- 3.Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nature Med. 2002;8:1211–7. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- 4.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nature Med. 2002;8:1249–56. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 5.Kelishadi R, Zadegan S, Naderi G, Asgary S, Bashardoust N. Atherosclerosis risk factors in children and adolescents with or without family history of premature coronary artery disease. Med Sci Monit. 2002;8:425–9. [PubMed] [Google Scholar]
- 6.Zieske AW, Malcom GT, Strong JP. Natural history and risk factors of atherosclerosis in children and youth: the PDAY study. Pediatr Pathol Mol Med. 2002;21:213–37. doi: 10.1080/15227950252852104. [DOI] [PubMed] [Google Scholar]
- 7.Lind L, Sarabi M, Millgard J, Kahan T. Endothelium-dependent vasodilation is impaired in apparently healthy subjects with a family history of myocardial infarction. J Cardiovasc Risk. 2002;9:53–7. doi: 10.1177/174182670200900108. [DOI] [PubMed] [Google Scholar]
- 8.de Jongh S, Lilien MR, Bakker HD, Hutten BA, Kastelein JJ, Stroes ES. Family history of cardiovascular events and endothelial dysfunction in children with familial hypercholesterolemia. Atherosclerosis. 2002;163:193–7. doi: 10.1016/s0021-9150(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 9.Schachinger V, Britten MB, Elsner M, Walter DH, Scharrer I, Zeiher AM. A positive family history of premature coronary artery disease is associated with impaired endothelium-dependent coronary blood flow regulation. Circulation. 1999;100:1502–8. doi: 10.1161/01.cir.100.14.1502. [DOI] [PubMed] [Google Scholar]
- 10.Paigen B, Holmes PA, Mitchell D, Albee D. Ath-1, a gene determining atherosclerosis susceptibility and high density lipoprotein levels in mice. Proc Natl Acad Sci USA. 1987;84:3763–7. doi: 10.1073/pnas.84.11.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi W, Haberland ME, Jien ML, Shih DM, Lusis AJ. Endothelial responses to oxidized lipoproteins determine genetic susceptibility to atherosclerosis in mice. Circulation. 2000;101:75–81. doi: 10.1161/01.cir.102.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 13.Mehta JL, Saldeen TG, Rand K. Interactive role of infection, inflammation, and traditional risk factors in atherosclerosis and coronary artery disease. J Am Coll Cardiol. 1998;31:1217–25. doi: 10.1016/s0735-1097(98)00093-x. [DOI] [PubMed] [Google Scholar]
- 14.Mehta JL, Li DY. Inflammation in ischemic heart disease: response to tissue injury or a pathogenetic villain? Cardiovascular Res. 1999;43:291–9. doi: 10.1016/s0008-6363(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 15.Ribatti D, Nico B, Vacca A, Roncali L, Dammacco F. Endothelial cell heterogeneity and organ specificity. J Hematother Stem Cell Res. 2002;11:81–90. doi: 10.1089/152581602753448559. [DOI] [PubMed] [Google Scholar]
- 16.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 17.Bombeli T, Karsan A, Tait JF, Harlan JM. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89:2429–42. [PubMed] [Google Scholar]
- 18.Mold C, Morris CA. Complement activation by apoptotic endothelial cells following hypoxia/reoxygenation. Immunology. 2001;102:359–64. doi: 10.1046/j.1365-2567.2001.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posadas-Romero C, Torres-Tamayo M, Zamora-Gonzalez J, et al. High insulin levels and increased low-density lipoprotein oxidizability in pediatric patients with systemic lupus erythematosus. Arthritis Rheum. 2004;50:160–5. doi: 10.1002/art.11472. [DOI] [PubMed] [Google Scholar]
- 20.Kueng W, Silber E, Eppenberg V. Quantification of cells cultured on 96-well plates. Anal Biochem. 1989;186:16–9. doi: 10.1016/0003-2697(89)90710-0. [DOI] [PubMed] [Google Scholar]
- 21.Zapata E, Ventura JL, De la Cruz K, Rodriguez E, Damian P, Masso F, Montaño LF, Lopez-Marure R. Dehydroepiandrosterone inhibits the proliferation of human umbilical vein endothelial cells by enhancing the expression of p53 and p21, restricting the phosphorylation of retinoblastoma protein, and is androgenand estrogen-receptor independent. FEBS J. 2005;272:1343–53. doi: 10.1111/j.1742-4658.2005.04563.x. [DOI] [PubMed] [Google Scholar]
- 22.Libby P. Atherosclerosis. In: Fauci AS, Braunwald E, Isselbacher KJ, et al., editors. Harrison's Principles of Internal Medicine. 14th edn. New York: Mc Graw Hill; 1998. pp. 1345–52. [Google Scholar]
- 23.Flores-Romo L, Estoppey D, Bacon KB. Anti-CD40 antibody stimulates the VLA-4-dependent adhesión of normal and LFA-1-deficient B cells to endothelium. Immunology. 1993;79:445–51. [PMC free article] [PubMed] [Google Scholar]
- 24.Haraldsen G, Kvale D, Lien B, Farstad IN, Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J Immunol. 1996;156:2558–65. [PubMed] [Google Scholar]
- 25.Jiang MZ, Tsukahara H, Ohshima Y, Todoroki Y, Hiraoka M, Maeda M, Mayumi M. Effects of antioxidants and nitric oxide on TNF-alpha-induced adhesion molecule expression and NF-kappaB activation in human dermal microvascular endothelial cells. Life Sci. 2004;75:1159–70. doi: 10.1016/j.lfs.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 26.Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–63. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 27.Vandenberg E, Reid MD, Edwards JD, Davis HW. The role of the cytoskeleton in cellular adhesion molecule expression in tumor necrosis factor-stimulated endothelial cells. J Cell Biochem. 2004;91:926–37. doi: 10.1002/jcb.20011. [DOI] [PubMed] [Google Scholar]
- 28.McDouall RM, Farrar MW, Khan S, Yacoub MH, Allen SP. Unique sensitivities to cytokine regulated expression of adhesion molecules in human heart-derived endothelial cells. Endothelium. 2001;8:25–40. doi: 10.3109/10623320109063155. [DOI] [PubMed] [Google Scholar]
- 29.Wu H, Yao Q, Lumsden A, Chen C. Characterization of two populations of human coronary artery endothelial cells (I) J Surg Res. 2004;118:38–44. doi: 10.1016/j.jss.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Shi Q, Wang J, Wang XL, VandeBerg JL. Comparative Analysis of Vascular Endothelial Cell Activation by TNF-alpha and LPS in Humans and Baboons. Cell Biochem Biophys. 2004;40:289–304. doi: 10.1385/CBB:40:3:289. [DOI] [PubMed] [Google Scholar]
- 31.Raab M, Daxecker H, Markovic S, Karimi A, Griesmacher A, Mueller MM. Variation of adhesion molecule expression on human umbilical vein endothelial cells upon multiple cytokine application. Clin Chim Acta. 2002;321:11–6. doi: 10.1016/s0009-8981(02)00048-7. [DOI] [PubMed] [Google Scholar]
- 32.Murakami S, Morioka T, Nakagawa Y, Suzuki Y, Arakawa M, Oite T. Expression of adhesion molecules by cultured human glomerular endothelial cells in response to cytokines: comparison to human umbilical vein and dermal microvascular endothelial cells. Microvasc Res. 2001;62:383–91. doi: 10.1006/mvre.2001.2356. [DOI] [PubMed] [Google Scholar]
- 33.Voisard R, Osswald M, Baur R, et al. Expression of intercellular adhesion molecule-1 in human coronary endothelial and smooth muscle cells after stimulation with tumor necrosis factor-alpha. Coron Artery Dis. 1998;9:737–45. doi: 10.1097/00019501-199809110-00006. [DOI] [PubMed] [Google Scholar]
- 34.Shi Q, Aida K, Vandeberg JL, Wang XL. Passage-dependent changes in baboon endothelial cells — relevance to in vitro aging. DNA Cell Biol. 2004;23:502–9. doi: 10.1089/1044549041562294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hass R, Meinhardt G, Hadam M, Bartels H. Characterization of human TUR leukemia cells: continued cell cycle progression in the presence of phorbol ester is associated with resistance to apoptosis. Eur J Cell Biol. 1994;65:408–16. [PubMed] [Google Scholar]
- 36.Schwartz SM, Murry CE. Proliferation and the monoclonal origins of atherosclerotic lesions. Annu Rev Med. 1998;49:437–60. doi: 10.1146/annurev.med.49.1.437. [DOI] [PubMed] [Google Scholar]
- 37.Vielma SA, Mironova M, Ku JR, Lopes-Virella MF. Oxidized LDL further enhances expression of adhesion molecules in Chlamydophila pneumoniae-infected endothelial cells. J Lipid Res. 2004;45:873–80. doi: 10.1194/jlr.M300456-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y, Song L, Wu S, Fan F, Lopes-Virella MF. Oxidized LDL differentially regulates MMP-1 and TIMP-1 expression in vascular endothelial cells. Atherosclerosis. 2001;156:119–25. doi: 10.1016/s0021-9150(00)00638-9. [DOI] [PubMed] [Google Scholar]
- 39.Amberger A, Maczek C, Jurgens G, Michaelis D, Schett G, Trieb K, Eberl T, Jindal S, Xu Q, Wick G. Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial and venous endothelial cells in response to cytokines and oxidized low-density lipoproteins. Cell Stress Chaperones. 1997;2:94–103. doi: 10.1379/1466-1268(1997)002<0094:ceoive>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karin M, Lin A. NF-κb at the crossroads of life and death. Nature Immunol. 2002;3:221–7. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 41.Maderna P, Godson C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim Biophys Acta. 2003;1639:141–51. doi: 10.1016/j.bbadis.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Span LF, Pennings AH, Vierwinden G, Boezeman JB, Raymakers RA, de Witte T. The dynamic process of apoptosis analyzed by flow cytometry using Annexin-V/propidium iodide and a modified in situ end labeling technique. Cytometry. 2002;47:24–31. [PubMed] [Google Scholar]
- 43.Minami M, Kume N, Shimaoka T, Kataoka H, Hayashida K, Yonehara S, Kita T. Expression of scavenger receptor for phosphatidylserine and oxidized lipoprotein (SR-PSOX) in human atheroma. Ann NY Acad Sci. 2001;947:373–6. doi: 10.1111/j.1749-6632.2001.tb03966.x. [DOI] [PubMed] [Google Scholar]
- 44.Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–87. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 45.Chiu GN, Bally MB, Mayer LD. Targeting of antibody conjugated, phosphatidylserine-containing liposomes to vascular cell adhesion molecule 1 for controlled thrombogenesis. Biochim Biophys Acta. 2003;1613:115–21. doi: 10.1016/s0005-2736(03)00142-1. [DOI] [PubMed] [Google Scholar]
- 46.Zwaal RFA, Comfurius P, Bevers EM. Lipid—protein interactions in blood coagulation. Biochim Biophys Acta. 1998;1376:433–53. doi: 10.1016/s0304-4157(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 47.Tedgui A, Mallat Z. Apoptosis as a determinant of atherothrombosis. Thromb Haemost. 2001;86:420–6. [PubMed] [Google Scholar]