Abstract
BALB/c mice immunized with recombinant Trypanosoma cruzi ribosomal P2β protein (TcP2β) develop a strong and specific antibody response against its 13 residue-long C-terminal epitope (peptide R13: EEEDDDMGFGLFD) that has a concomitant β1-adrenergic stimulating activity. However, other animals that undergo similar immunizations seem tolerant to this epitope. To evaluate further the antibody response against the ribosomal P proteins, 25 BALB/c and 25 Swiss mice were immunized with TcP2β. From the 50 animals, 31 developed a positive anti-R13 response, whereas 19 were non-responsive. From the 31 anti-R13 positive mice, 25 had anti-R13 antibodies that recognized the discontinuous motif ExDDxGF, and their presence correlated with the recording of supraventricular tachycardia. The other six had anti-R13 antibodies but with a normal electrocardiographic recording. These anti-R13 antibodies recognized the motif DDxGF shared by mammals and T. cruzi and proved to be a true anti-P autoantibody because they were similar to those elicited in Swiss, but not in BALB/c mice, by immunization with the C-terminal portion of the mouse ribosomal P protein. Our results show that the recognition of the glutamic acid in position 3 of peptide R13 defines the ability of anti-R13 antibodies to react with the motif AESDE of the second extracellular loop of the β1-adrenergic receptor, setting the molecular basis for their pathogenic β1 adrenoceptor stimulating activity.
Keywords: β1-adrenergic receptor, pathogenic antibodies, ribosomal P proteins, Trypanosoma cruzi
Introduction
Circulating antibodies with agonist-like properties on cardiac membrane receptors have been reported to exist in chronic Chagas’ heart disease (cChHD), the most frequent and severe consequence of the chronic infection by the haemoflagellate parasite Trypanosoma cruzi[1–4]. Our studies on the nature of these antibodies enabled us to propose that these autoreactive specificities were, in fact, antibodies directed against intracellular parasite antigens such as the T. cruzi ribosomal P proteins, with the ability to cross-react and stimulate cardiac receptors [5–7]. This assumption was proved in mice immunized with T. cruzi recombinant ribosomal P2β protein (TcP2β) that developed a strong and specific antibody response against its 13 residue-long C-terminal epitope (peptide R13: EEEDDDMGFGLFD, R13+ mice) [8,9]. The elicited anti-R13 antibodies had a concomitant β1-adrenergic stimulating activity, whose appearance correlated strictly with the recording of supraventricular tachycardia and premature death. Fine epitope mapping using alanine mutation scanning allowed the identification within peptide R13 of a discontinuous motif ExDDxGF targeted by the pathogenic anti-P antibodies. This motif mimics the ESDE acidic amino acid sequence present in the second extracellular loop of the β1-adrenergic receptor, and sets the molecular basis for the anti-β1 receptor activity of the antibodies reactive to R13 [8].
In the same experiment, half the mice that displayed antibodies against the immunizing antigen TcP2β, but were negative for R13, lived to the end of the experiment without developing any cardiac symptoms. A probable explanation for the lack of R13 reactivity is its similarity with its mammalian counterpart, peptide H13 (EESDDDMGFGLFD) [8].
In order to evaluate the antibody response against the C-terminal end of TcP2β protein, we monitored the results of immunizing a large cohort of mice with either TcP2β or a mammalian ribosomal P protein. Surprisingly, in addition to the R13+ and R13– mice, we detected immunized animals that had antibodies reactive to R13, albeit with no functional activity. The analysis of this particular reactive pattern showed that the mentioned anti-R13 antibodies were, in fact, true anti-P autoantibodies directed against self ribosomal P proteins. Comparison of the P auto-epitope with the epitope recognized by anti-R13 antibodies with adrenoceptor stimulating properties confirmed the importance of the third E residue of peptide R13 in the generation of the cardioreactive anti-R13 response.
Materials and methods
Cloning, expression and purification of recombinant proteins
A cDNA encoding the 28 amino acids long C-terminal end of Mus musculus ribosomal P protein (MmP0) was isolated by screening a λgt11 mouse cDNA library with sera from a P positive SLE patient. This cDNA was amplified by polymerase chain reaction (PCR) using oligonucleotide S1 (GAGCACGTCAGGATCCGCGGAAT) and S2 (GCGAC CGAAGCTTAGCTGGAATTC) and cloned into pMal-c2 (New England Biolabs, Cambridge, MA, USA) and pGex-1lT (Pharmacia Biotech, Uppsala, Sweden) vectors in the BamHI-HindIII sites. The TcP2β gene was cloned into pMal-c2 and pGex-1λT vectors in the EcoRI site. Production and purification of the maltose binding protein (MBP) and gluthatione-S-transferase (GST) fusion proteins, MBP-MmP0, GST-MmP0, MBP-TcP2β and GST-TcP2β were performed as indicated by the manufacturers.
Synthetic peptides
Peptides were prepared by solid-phase method of Merrifield as described by Müller et al. [10] with a semi-automatic multi-synthesizer NPS 4000 (Neosystem, Strasbourg, France). Peptide R13 (EEEDDDMGFGLFD) was derived from the 13 carboxyl-terminal amino acids of TcP2β, while C10 (DDDMGFGLFD) was derived from a consensus sequence in the ribosomal P protein family [11]. Peptide H13 (EESDDDMGFGLFD) was derived from the mammalian ribosomal P proteins [12] and H26R (HWWRAESDEARRCYNDPKCCDFVTNR) corresponds to amino acids 197–222 of the human β1-adrenergic receptor [13]. Peptide TMVP (AEAALUKMALMKV), from tobacco mosaic virus coat protein, was used as a negative control in enzyme-linked immunosorbent assay (ELISA). Peptides were coupled at a molar ratio of 1 : 30 to bovine serum albumin (BSA) (Sigma, St Louis, MO, USA) with 0·05% glutaraldehyde as described [10].
Immunization schedule
Twenty-five Swiss and BALB/c (H-2d) mice, aged 6–8 weeks, were immunized intraperitoneally with five doses (days 1, 14, 28, 42 and 56) of purified MBP-TcP2β or MBP-MmP0 (50 µg/mouse) plus incomplete Freund's adjuvant (IFA) (Sigma). A similar protocol was used for sex- and age-matched control groups receiving MBP plus IFA or IFA alone. Mice were bled on days 0 (bleed 1), 26 (bleed 2), 52 (bleed 3) and 66 (bleed 4).
Antibodies
The IgG fraction was prepared by diluting the sera 1 : 5 in phosphate-buffered saline (PBS), pH 7·4, and further precipitation with 40% (NH4)2SO4. The precipitate was redissolved in PBS at 1 : 1.
ELISA determinations
ELISA assays were performed as described by Mesri et al. [14]. Briefly, polystyrene immunoplates were coated with 2 µm BSA-conjugated peptides or with 2 µg/ml of GST recombinant proteins in 0·05 M bicarbonate–carbonate buffer (pH 9·6). BSA or GST were used as controls. Titration of the sera was performed as described previously [8]. In inhibition experiments, diluted sera were first incubated for 2 h at 37°C with increasing amounts of peptides and the decrease in reactivity was expressed as percentage of inhibition. Cut-off values corresponded to the mean plus 2 s.d. of the reactivity measured in mice immunized with MBP. The ratio R13/H13 was calculated as the rate of the ELISA measurements obtained for peptides R13 and H13.
Electrocardiographic records (ECG)
Electrocardiograms were performed as reported previously [8]. Records were taken on 12 days after the last antigen inoculation. ECGs were obtained with the six standard leads (I, II, III, AVR, AVL, AVF) at 50 mm/s of paper speed and a 20 mm/mV amplitude using a Fukuda-Denshi Fx-2111 electrocardiograph (Tokyo, Japan). Electrocardiographic analysis included measurements of heart rate, P wave duration and amplitude, ventricular depolarization (QRS) complex duration and amplitude, P–R interval duration and a search for disturbances of rhythm, conduction and repolarization.
Functional assay on neonatal rat cardiomyocytes
Spontaneously beating cultured neonatal rat cardiomyocytes were used to assess the functional effects of IgG fractions from immunized mice. Single cells were dissociated from the minced heart of Wistar rats with a trypsin–collagenase solution. Myocytes were cultured for 4 days at 37°C in a 5% CO2 atmosphere as monolayers in Dulbecco's minimum essential medium (DMEM)/F-12 (Life Technologies, Gaithersburg, MD, USA) containing 5% calf serum. The baseline-beating rate, measured in 10 different fields at 37°C on the heated stage of an inverted microscope, was 120 ± 19 beats per minute (b.p.m.). Measurements were repeated 1 h after exposure to IgG fractions at a 1 : 50 dilution and after subsequent addition of 1 µM of atropine, bisoprolol or propranolol, or 60 µM of R13 or H26R peptides.
Epitope mapping and alanine mutation scanning
The fine epitope mapping of the C-terminal region of TcP2β (R13) was performed with the SPOTs® kit (Sigma-Genosys, St. Louis, MO, USA). Two sets of 14 peptides representing the R13 sequence and its 13 alanine-replacement analogues were synthesized by the supplier. The interactive residues were defined by incubation with diluted sera for 2 h at room temperature followed by 1 h incubation with peroxidase-conjugated anti-mouse IgG (Sigma) and revealed by the ECL technique (Amersham Bioscience, Piscataway, NJ, USA).
Statistical analysis
Statistical analyses were performed by paired Student's t-test or Wilcoxon's rank sum test (Fig. 2). The level for statistical significance for all tests was set at P < 0·05.
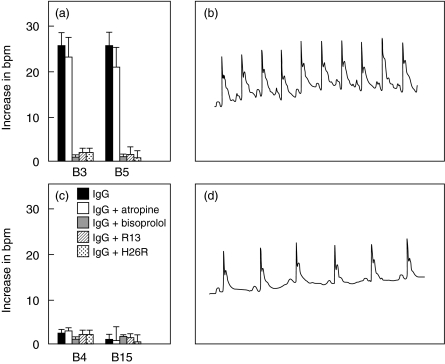
Fig. 2.
Functional effect of anti-P antibodies from BALB/c mice immunized with TcP2β. Chronotropic effect on neonatal rat cardiomyocytes of IgGs from mice displaying R13+/C10– (a) or R13+/C10+ (c) profile. The effect of the antibodies was also assessed in the presence of the muscarinic acethylcholine antagonist atropine, β-adrenergic antagonist bisoprolol or after preincubation with H26R or R13 peptides. Mean and s.e. from 10 observations are given. Results show the increase in beats per minute with respect to the baseline beating rate from two representative serum samples from each group. Representative electrocardiograms from mice displaying R13+/C10– (b) or R13+/C10+ profile (d).
Results
Antibody response induced by immunization with recombinant TcP2β protein
Previous results indicated that immunization with TcP2β induced, in all mice, antibodies against TcP2β but only half of the mice developed an antibody response against the C-terminal end of the protein [8]. To evaluate the antibody response to the C-terminal R13 epitope, we immunized 25 BALB/c and 25 Swiss mice with the MBP-TcP2β recombinant protein, as described in Materials and methods.
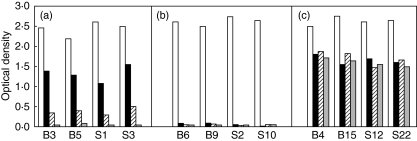
To outline a reactive profile of each animals, antibody levels against recombinant TcP2β and synthetic peptide R13 (representing the TcP2β C-terminal region), H13 (representing the C-terminal region of the mammalian P proteins) and C10 (representing the sequence shared by TcP2β and mammalian P protein) were measured. Immunizations generated three reactive phenotypes, all positive for TcP2β but differing in their reactivity to R13, H13 and C10. A first group, group A in Fig. 1, included mice positive for peptides R13, H13 and negative for C10 (Chagas’-like phenotype, also depicted as R13+/C10–); a second group, named B in Fig. 1, negative for all peptides (non-responsive phenotype, or R13–/C10–) and a third group, C in Fig. 1, with a similar positive response to all three peptides (autoimmune phenotype, also named R13+/C10+). Both Swiss and BALB/c mice elicited a similar response against the parasite ribosomal protein reaching median titres of 12 800 as measured using GST-TcP2β as reactive reagent (Table 1). Mice immunized with MBP alone or IFA failed to react with GST-TcP2β (data not shown). Fourteen of 25 Swiss (56%), and 11 of 25 BALB/c mice (44%) developed a R13+/C10– profile characterized by high anti-R13 antibody levels, with antibody titres ranging between 800 and 6400, low anti-H13 antibody titres and no detectable binding to peptide C10, a reactive pattern that revealed the induction in these mice of the anti-P antibody characteristic of cChHD (Table 1 and Fig. 1a). Peptides C10 and H13 failed to inhibit the anti-R13 reactivity, confirming direct ELISA measurements (data not shown).
Fig. 1.
Reactivity to TcP2β and C-terminal peptides in sera from mice immunized with MBP-TcP2β. Sera (1 : 200 dilution) from BALB/c (B sera) and Swiss (S sera) mice immunized with MBP-TcP2β were assayed against the recombinant protein GST-TcP2β (white bars) and against peptides R13 (black bars), H13 (hatched bars) and C10 (grey bars). The optical density reading at 405 nm is shown. (a) Mice positive for TcP2β, R13 and H13 and negative for C10 (Chagas’-like phenotype); (b) mice positive for TcP2β and negative for R13, H13 and C10 (non-responsive phenotype) and (c) mice positive for TcP2β, R13, H13 and C10 (autoimmune phenotype).
Table 1. Median antibody titre against TcP2β and P protein C-terminal peptides.
| n (%) | TcP2β | R13 EEEDDDMGFGLFD | H13 EESDDDMGFGLFD | C10 DDDMGFGLFD | |
|---|---|---|---|---|---|
| BALB/c | |||||
| Chagasa’-likeb | 11 (44%) | 12 800 | 1600 | 400 | 0 |
| Non-responsiveb | 11 (44%) | 12 800 | 0 | 0 | 0 |
| Autoimmunec | 3 (12%) | 12 800 | 6400 | 6400 | 6400 |
| Swiss | |||||
| Chagas’-like | 14 (56%) | 12 800 | 1600 | 400 | 0 |
| Non-responsive | 8 (32%) | 12 800 | 0 | 0 | 0 |
| Autoimmune | 3 (12%) | 12 800 | 6400 | 6400 | 6400 |
Mice with an R13+/C10− profile.
Mice with an R13–/C10– profile.
Mice with an R13+/C10+ profile.
On the other hand, 32% of the Swiss mice and 44% of the BALB/c were non-responsive to R13, giving an R13–/C10– reactive profile (Table 1 and Fig. 1b). To our surprise, 12% of both Swiss and BALB/c mice were R13+/C10+ (Table 1 and Fig. 1c), a profile reminiscent of the autoimmune anti-P antibody specificity that is generated in the course of human systemic lupus erythematosus (SLE), or developed spontaneously in autoimmune lpr mice [15]. This finding was confirmed by the fact that the anti-R13 reactivity of these sera was abolished by C10, H13 and R13 to a similar extent, signifying that in this case, the minimal epitope was encompassed within the peptide that represents the sequence common to both parasite and mammalian proteins (data not shown).
Functional properties of anti-TcP2β antibodies
Because the immunization with TcP2β induced supraventricular tachycardia in mice that developed a response against R13, we decided to screen all R13 positive mice for alterations of the cardiac rhythm. The characteristics of the normal ECG for BALB/c and Swiss mice did not differ significantly and were similar to those described previously (Fig. 4d) [8]. From all 31 BALB/c and Swiss mice presenting anti-R13 antibodies, only 25 presented a significant increase in heart rate. These mice belonged to the R13+/C10– phenotype (Fig. 2b). On the contrary, mice that had anti-R13 antibodies but normal ECGs, presented a R13+/C10+ phenotype (Fig. 2d). To discriminate further between both groups, we compared the functional activity of the corresponding IgG fractions on spontaneously beating neonatal rat cardiomyocytes. The chronotropic effect of the IgGs is shown in Fig. 2(a and c). IgGs from mice with the R13+/C10– phenotype had a marked positive chronotropic effect (Fig. 2a), whereas IgGs from R13+/C10+ mice failed to modify the baseline beating rate (Fig. 2c). Clearly, the recording of normal ECGs in R13+ mice was linked to the recognition of peptide C10, the reactive feature of anti-R13 specificities devoid of an adrenergic-stimulating activity. Moreover, it is important to note that mice with phenotype R13–/C10– presented a normal ECG (data not shown).
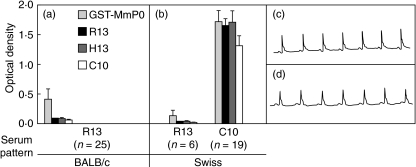
Fig. 4.
Reactivity to the MmP0 and C-terminal peptides in sera from mice immunized with MBP-MmP0. Sera (1 : 200 dilution) from BALB/c (a) and Swiss (b) mice immunized with MBP-MmP0 were assayed against the recombinant protein GST-MmP0 and against peptides R13, H13 and C10. The optical density reading at 405 nm is shown. Results from Swiss mice were grouped on profiles C10+ and R13–. (c) Representative electrocardiogram from mice immunized with MmP0 displaying C10+ profile. (d) Electrocardiogram from normal BALB/c mouse.
The onset of the anti-R13 antibody response
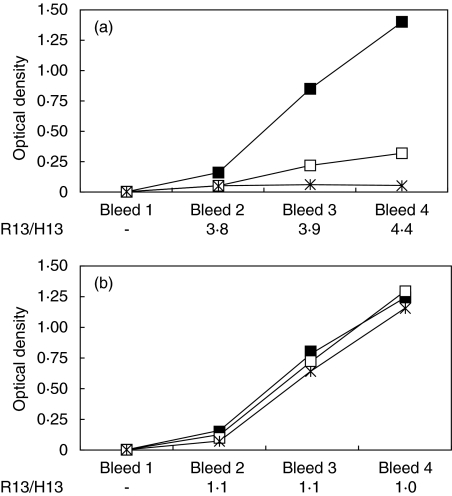
Shifts in anti-P antibody specificity during the time-course of experimental T. cruzi infections have been proposed as an explanation to the changing profile of anti-cardiac receptor activities of total IgG fractions from chronically infected mice [5,16]. In consequence, it seemed relevant to determine the time-course of the induction of each of the anti-R13+ reactive patterns during immunization. In Fig. 3 we plotted the antibody levels against peptides C10, H13 and R13 measured in blood samples obtained after each boost. The ratio R13/H13 was calculated as a useful parameter to detect changes of the reacting profile. Anti-TcP2β antibodies were detected early on the second bleeding, whereas antibodies against the C-terminal peptides that allowed discrimination of different reactive patterns were detected from the third bleed onwards (data not shown). The ratio R13/H13 was above 3 for all mice displaying the R13+/C10– phenotype (4·22 ± 0·84). In contrast, the R13/H13 ratio for R13+/C10+ mice in all cases was very close to 1 (mean: 1·05 ± 0·11). Remarkably, the intrinsic R13/H13 ratio of each mouse remained constant for each time-point, as shown in Fig. 3.
Fig. 3.
Evolution of the reactive profile along the immunization protocol. Serial serum samples (1 : 200 dilution) from BALB/c mice immunized with TcP2β were assayed by enzyme-linked immunosorbent assay (ELISA) for reactivity against peptides R13 (▪), H13 (□) and C10 (*). Results from a representative mouse from both the R13+/C10– (a) and R13+/C10+ (b) profiles are plotted. The ratio R13/H13 at each bleed is also shown.
Eliciting an autoimmune response
To gain insight into the nature of the R13+/C10+ reactive pattern, we tried to induce a fully autoimmune response against the C-terminal epitope of the mouse ribosomal P protein, although previous reports indicated that immunization with P autoantigens was not an appropriate stimulus to generate autoanti-P responses [15]. To this end a cDNA encoding the peptide EFPAAPAKAEAKEESEESDEDMGFGLFD representing the C-terminal end of the ribosomal P0 protein of Mus musculus (MmP0) was cloned into the expression vectors pMal and pGex.
Thereafter, 25 BALB/c and 25 Swiss mice were immunized with MBP-MmP0 and sera were collected to assess the induction of specific antibodies by ELISA. Twenty of 25 (80%) Swiss mice immunized with MmP0 reacted strongly with GST-MmP0 with median antibody titre of 51 200, whereas only six of 25 (24%) BALB/c mice reacted with GST-MmP0, albeit weakly (Fig. 4a). Sera from BALB/c mice immunized with MmP0 failed to develop antibodies against the C-terminal peptides (Fig. 4), confirming results by Hines et al. [13]. However, the 19 sera from Swiss mice immunized with MmP0 that developed anti-MmP0 antibodies reacted with the self C-terminal ribosomal P protein epitope represented by peptide H13, with median titre of 3200. These sera also reacted strongly against peptides R13 and C10 and presented a R13/H13 ratio of 0·98 ± 0·09 (Fig. 4b, C10+ pattern). Competitive ELISA confirmed these results as peptides C10, H13 and R13 abolished the anti-H13 reactivity to a similar extent (data not shown). This reactive profile resembles the R13+/C10+ pattern induced by immunization with TcP2β (Fig. 1c). As in mice immunized with TcP2β with the R13+/C10+ phenotype, MmP0 R13+/C10+ mice showed a normal ECG (Fig. 4c) and the corresponding IgG fractions did not exert any functional effect on cardiomyocytes (data not shown). It is worth noting that the response elicited in Swiss mice is comparable to the autoanti-P response described for MRL/lpr mice [15] and anti-P positive SLE patients [17].
IgG isotypes of the anti-P response
The isotype involved in the antibody response against GST-TcP2β and GST-MmP0 in both BALB/c and Swiss mice immunized with MBP-TcP2β and MBP-MmP0 was mainly of the IgG1 isotype, and to a lesser extent IgG2a and IgG2b (data not shown). The response against R13 in mice immunized with TcP2β displaying an R13+/C10− phenotype was also IgG1, IgG2a and IgG2b. Remarkably, BALB/c and Swiss mice with a C10+ phenotype following immunization either with MmP0 or TcP2β displayed antibodies to R13, H13 and C10 exclusively of the IgG1 isotype (data not shown).
Fine epitope mapping of anti-TcP2β and anti-MmP0 antibodies
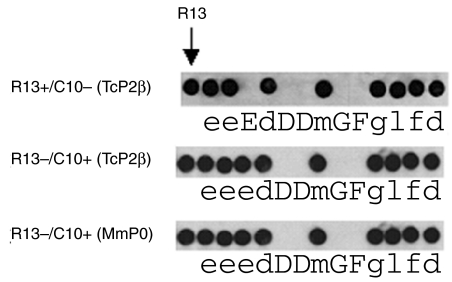
To identify the amino acids that define the epitopes recognized by R13+/C10+ and R13+/C10– antibodies, we performed alanine scanning mutagenesis (ASM) of peptide R13 using IgGs from immunized mice. In agreement with ELISA measurements, similar ASM patterns were obtained for C10+ antibodies regardless of the antigen used in the immunization protocol (MmP0 or TcP2β, Fig. 5). Amino acids essential for recognition by C10+ antibodies were the two Asp residues at positions 5 and 6, the Gly at position 8 and the contiguous Phe, resulting in the antigenic motif DDxGF (Fig. 5). The epitope recognized by sera from mice immunized with TcP2β that developed an R13+/C10– profile, and carry IgG that exert a functional effect on heart cells, is ExDDxGF (Fig. 5).
Fig. 5.
Alanine scanning mutagenesis patterns of anti-P antibodies.The indicated amino acids of R13 were replaced by Ala and the reactivity of the mutated peptides was assayed using polyclonal C10+ or R13+ sera from mice immunized with TcP2β or MmP0. Amino acids involved in antibody binding are in capital letters.
Discussion
In human and experimental chronic infections with T. cruzi, the antibodies against the ribosomal P2β protein are characterized by their marked preference for the C-terminal parasite peptide R13, and their weak reactivity towards the C-terminal end of the mammalian ribosomal P protein, H13. This is the hallmark of Chagas’ disease, and recent estimates indicate that more than 80% of infected individuals contain different levels of these antibodies, differentially characterized in serum samples by R13/H13 ratios above 3 [18,19]. The prevalence of this antibody response in immunizations with recombinant TcP2β is clearly not the same as in infections [18,19].
In expanding the number of animals immunized with recombinant TcP2β protein, we observed that although all mice developed a strong response to this protein, three different patterns of reactivity against its C-terminal end were evident. Two of them have been previously reported. First, the non-responsive profile (R13–/C10–) characterized by a strong reactivity to the TcP2β protein, no reactivity to its C-terminal end, and IgG that caused no increase in beating frequency of neonatal rat cardiomyocytes [16]. Second, the recognition of peptide R13 linked to the recording of supraventricular tachicardia, represented by mice with the R13+/C10– pattern, or Chagas’ disease profile with R13/H13 ratio above 3 (Fig. 1). Most interestingly, a novel reactive profile was characterized, R13+/C10+, that did not associate with the recording of electrocardiographic abnormalities, and was characterized by a R13/H13 ratio around 1 (Figs 1, 2 and 3). In accordance, purified IgG from R13+/C10+ mice did not have functional activity on neonatal rat cardiomiocytes. Alanine scanning showed that the antigenic motif of the R13 peptide recognized by these IgGs was DDxGF (Fig. 5). Comparison with the motif recognized by the R13+/C10– mice, ExDDxGF, shows that functional activity of antibodies and abnormal ECG recordings associate with the recognition of the third E residue of R13. This generates an antibody specificity that is able to recognize the AESDE sequence of the second extracellular loop of the β1 adrenergic receptor, motif ExDE, exerting a functional effect on cardiomiocytes that is reflected in the ECG recordings (Fig. 2).
It has been suggested that maturation of the immune response during Chagas’ disease could bring about changes in the affinity of antibodies [5,16]. However, it is evident that no mouse presented a mixed pattern of R13 recognition, indicating that there was no shift in antibody specificity during the immunization period (Fig. 3). In this regard, the R13/H13 ratio was a useful parameter during the follow-up of the immunization (Fig. 3, bottom line). These results suggest that the two different anti-R13 antibody responses evolved independently from each other, and were fixed very early during the immunization protocol. Although we do not know how the immune system of each animal selected their type of anti-R13 response, it is seems clear that once either R13+/C10– or R13+/C10– was opted, the other anti-R13 response was excluded.
Remarkably, immunization with MmP0 that contains a sequence identical to the one present in the second extracellular loop of the β1 adrenergic receptor, namely EESEESDED (seven residues apart from the C-terminal D residue), did not induce functionally cardioactive antibodies. On the contrary, the anti-MmP0 response produced an autoimmune R13+/C10+ reactive profile (Fig. 4) confirming, on the other hand, that the R13+/C10+ profile induced in 12% of animals immunized with TcP2β is clearly due to autoimmunity. The fact that MmP0 did not induce a response in BALB/c mice was as expected, because induction of anti-P autoantibodies by immunization with autologous ribosomes or purified protein has been unsuccessful [14]. Notably, in BALB/c, immunization with only TcP2β protein was able to induce anti-P autoantibodies, and in a similar proportion to that in Swiss mice. None the less, only the outbred Swiss strain broke tolerance by immunization with the self-protein. Comparison of the reactivity and functional properties of these antibodies with those elicited in autoimmune diseases such as SLE revealed striking similarities. Indeed, the epitope of SLE auto anti-P antibodies is located within the 10 C-terminal residues common to the three human P proteins [20,21] region in which the DDxGF motif recognized by IgG of MmP0 and TcP2β -R13+/C10+ animals was found.
It remains to be explained why Swiss mice, when immunized with an autologous P protein, produced true anti-P autoantibodies while BALB/c mice did not. This may be related to the ability of each strain of mice to generate Th1- or Th2- responses [22–24]. For instance, BALB/c mice have the H-2d haplotype and are considered typical Th2 responders, whereas Swiss mice are random-bred and different Th responses are possible [22–24].
In conclusion, we have shown that the immune response against the T. cruzi ribosomal P protein in the absence of parasites is variable, including the possibility to generate true anti-P autoantibodies, hence preventing the production of pathogenic anti-R13 antibodies. Remarkably, in natural and experimental infections only the pathogenic response characterized by R13/H13 ratios above 3 is measured in serum samples [16,18,19].
Acknowledgments
This research has been supported financially by grants from the World Health Organization/Special Program for Research and Training in Tropical Diseases, Universidad de Buenos Aires, Fundación Bunge y Born, Ministerio de Salud y Acción Social-Beca Ramón Carrillo-Arturo Oñativia and the National Agency of Scientific and Technological Promotion (FONCYT BID 1201/OC-AR 01–14389). The work of M. J. L. is supported partially by an International Research Grant from the Howard Hughes Medical Institute (Chevy Chase, MD, USA).
References
- 1.Elizari MV, Chiale PA. Pharmacologic treatment of arrhythmias related to chronic Chagas' heart disease. In: Tentori MC, Segura E, Hayes DL, editors. Arrhytmia management in Chagas' disease. Armonk, New York: Futura Publishing Co.; 2000. pp. 95–116. [Google Scholar]
- 2.Chiale PA, Ferrari I, Mahler E, et al. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation. 2001;103:1765–71. doi: 10.1161/01.cir.103.13.1765. [DOI] [PubMed] [Google Scholar]
- 3.Borda E, Sterin-Borda L. Antiadrenergic and muscarinic receptor antibodies in Chagas' cardiomyopathy. Int J Cardiol. 1996;54:149–56. doi: 10.1016/0167-5273(96)02592-2. [DOI] [PubMed] [Google Scholar]
- 4.Kierszenbaum F. Chagas' disease and the autoimmunity hypothesis. Clin Microbiol Rev. 1999;12:210–23. doi: 10.1128/cmr.12.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan D, Ferrari I, Lopez Bergami P, et al. Antibodies to ribosomal P proteins of Trypanosoma cruzi in Chagas' disease possess functional autoreactivity with heart tissue and differ from anti-P autoantibodies in lupus. Proc Natl Acad Sci USA. 1997;94:10301–6. doi: 10.1073/pnas.94.19.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elies R, Ferrari I, Wallukat G, et al. Structural and functional analysis of the B cell epitopes recognized by anti-receptor autoantibodies in patients with Chagas' disease. J Immunol. 1996;157:4203–11. [PubMed] [Google Scholar]
- 7.Masuda M, Levin MJ, Farias de Oliveira S, et al. Functionally active cardiac antibodies in chronic Chagas' disease are specifically blocked by Trypanosoma cruzi antigens. FASEB J. 1998;12:1551–8. doi: 10.1096/fasebj.12.14.1551. [DOI] [PubMed] [Google Scholar]
- 8.Lopez Bergami P, Scaglione J, Levin MJ. Antibodies against the carboxyl-terminal end of the Trypanosoma cruzi ribosomal P proteins are pathogenic. FASEB J. 2001;15:2602–12. doi: 10.1096/fj.01-0132com. [DOI] [PubMed] [Google Scholar]
- 9.Lopez Bergami P, Cabeza Meckert P, Kaplan D, et al. Immunization with recombinant Trypanosoma cruzi ribosomal P2β protein induces changes in the electrocardiogram of immunized mice. FEMS Immunol Med Microbiol. 1997;18:75–85. doi: 10.1111/j.1574-695X.1997.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 10.Muller S, Couppez M, Briand JP, Gordon J, Sautiere P, van Regenmortel MH. Antigenic structure of histone H2B. Biochim Biophys Acta. 1985;827:235–46. doi: 10.1016/0167-4838(85)90208-0. [DOI] [PubMed] [Google Scholar]
- 11.Levin MJ, Vazquez M, Kaplan D, Schijman AG. The Trypanosoma cruzi ribosomal P protein family: classification and antigenicity. Parasitol Today. 1993;9:381–4. doi: 10.1016/0169-4758(93)90088-w. [DOI] [PubMed] [Google Scholar]
- 12.Elkon K, Bonfa E, Llovet R, Danho W, Weissbach H, Brot N. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proc Natl Acad Sci USA. 1988;85:5186–9. doi: 10.1073/pnas.85.14.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnusson Y, Hoyer S, Lengagne R, et al. Antigenic analysis of the second extra-cellular loop of the human beta-adrenergic receptors. Clin Exp Immunol. 1989;78:42–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Mesri E, Levitus G, Hontebeyrie-Joskowicz M, et al. Major Trypanosoma cruzi determinant in Chagas' heart disease shares homology with the systemic lupus erythematosus ribosomal P protein epitope. J Clin Microbiol. 1990;28:1219–24. doi: 10.1128/jcm.28.6.1219-1224.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hines JJ, Weissbach H, Brot N, Elkon K. Anti-P autoantibody production requires P1/P2 as immunogen but is not driven by exogenous self-antigen in MLR mice. J Immunol. 1991;146:3386–95. [PubMed] [Google Scholar]
- 16.Sepulveda P, Liegeard P, Wallukat G, Levin MJ, Hontebeyrie M. Modulation of cardiocyte functional activity by antibodies against Trypanosoma cruzi ribosomal P2 protein C-terminus. Infect Immunol. 2000;68:5114–9. doi: 10.1128/iai.68.9.5114-5119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkon K, Bonfa E, Skeky S, et al. Ribosomal protein autoantibodies in systemic lupus erythematosus. Bioessays. 1987;7:258–61. doi: 10.1002/bies.950070607. [DOI] [PubMed] [Google Scholar]
- 18.Mahler E, Hoebeke J, Levin MJ. Structural and functional complexity of the humoral response against the Trypanosoma cruzi ribosomal P2 β protein in patients with chronic Chagas' heart disease. Clin Exp Immunol. 2004;136:527–34. doi: 10.1111/j.1365-2249.2004.02480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahler E. PhD thesis. University of Buenos Aires; 2002. Rol de las proteínas P de Trypanosoma cruzi en la generación de anticuerpos con autorreactividad funcional en la cardiomiopatía crónica chagásica. [Google Scholar]
- 20.Mahler M, Kessenbrock K, Raats J, Williams R, Fritzler MJ, Bluthner M. Characterization of the human autoimmune response to the major C-terminal epitope of the ribosomal P proteins. Mol Med. 2003;81:194–204. doi: 10.1007/s00109-003-0423-1. March. [DOI] [PubMed] [Google Scholar]
- 21.Zampieri S, Mahler M, Bluthner M, et al. Recombinant anti-P protein autoantibodies isolated from a human autoimmune library: reactivity, specificity and epitope recognition. Cell Mol Life Sci. 2003;60:588–98. doi: 10.1007/s000180300050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun B, Rizzo LV, Sun S-H, et al. Genetic susceptibility to experimental autoimmune uveitis involves more than a predisposition to generate a T helper-1-like or a T helper-2-like response. J Immunol. 1997;159:1004–11. [PubMed] [Google Scholar]
- 23.Scott P. IFN-γ modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–55. [PubMed] [Google Scholar]
- 24.Keane-Myers A, Nickell SP. Role of IL-4 and IFN-γ in modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;155:2020–8. [PubMed] [Google Scholar]