Abstract
Serotonin (5-hydroxytryptamine, 5-HT) is one of the most extensively studied neurotransmitters of the central nervous system. It also has been identified in constituents of the immune system. Therefore serotonin has been suggested to serve as a mediator of bidirectional interactions between the nervous system and the immune system. We investigated this interaction in experimental autoimmune encephalomyelitis (EAE), a well-defined animal model of autoimmune disease of the central nervous system (CNS) mimicking features of the human disease multiple sclerosis. EAE was induced by immunization with the autoantigens myelin basic protein (MBP) or the immunodominant peptide of myelin oligodendrocyte glycoprotein (MOG) spanning amino acids 35–55 (MOGp 35–55). We studied EAE in knockout (KO) mice lacking the 5-HT transporter (5-HTT) on a C57.BL/6 background, in comparison with wild-type C57.BL/6 animals. After immunization with MOGp 35–55, or with rat MBP, the disease courses of the 5-HTT knockout mice were attenuated as compared to wildtype control mice. This difference was more pronounced in female animals. To dissect potential immune mechanisms underlying this phenomenon, histological studies of the CNS and cytokine measurements in mononuclear cells from the spleens of 5-HTT KO mice and wild-type controls were performed. We found a reduction of the inflammatory infiltrate in the CNS and of the neuroantigen-specific production of IFN-γ in splenocytes, again accompanied by a gender difference. These findings suggest a potential role of extracellular 5-HT homeostasis in the fine-tuning of neuroantigen-specific immune responses.
Keywords: experimental allergic encephalomyelitis, 5-hydroxytryptamine, myelin oligodendrocyte glycoprotein, autoimmune T-cell regulation, cytokines
Introduction
Antigen-specific cytokine production is one of the primary effector functions by which autoreactive CD4 T cells are thought to mediate autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE), which serves as a model for the human disease multiple sclerosis. Effector T cells specific for myelin autoantigens can produce either pro-inflammatory cytokines, e.g. IFN-γ, or anti-inflammatory mediators, e.g. interleukin 5. However, other, not primarily immune-related factors are also involved in modulating the initiation of an autoimmune T cell response and regulating course and severity of clinical autoimmune disease. These include neurotrophic factors like brain-derived neurotrophic factor (BDNF [1]), or ciliary neurotrophic factor (CNTF [2]), as well as hormones [3,4] or neurotransmitters in the peripheral and central nervous system (for review see [5]). Two decades ago, it was shown that 5-HT receptors are existent on leucocytes [6] and also the 5-HT transporter (5-HTT) was found to be present on both macrophages [7], mononuclear leucocytes [8] and B cells [9]. Due to these experiments, 5-HT together with its transporter and receptors has since then been regarded as a critical player at the interface between the nervous system and the immune system (for review [10]). By means of removing 5-HT from the extracellular space, the 5-HTT has a central position in determining duration of action of 5-HT. Its expression and modulation of function are therefore crucial parameters in 5-HT-mediated effects on the immune system and its cellular constituents. It has been shown that inflammatory mediators like IL-1β or TNF-α can influence 5-HTT activity [10,11]. Therefore, the 5-HTT seems to influence 5-HT homeostasis not only in physiological conditions, but also under inflammatory circumstances like an ongoing immune reaction.
Concerning autoimmunity, it has been reported that blockade of 5-HT receptors can suppress the development of EAE [12,13]. On the other hand, blockage of the 5-HTT by a selective 5-HT reuptake inhibitor (SSRI), which is expected to yield increased extracellular levels of 5-HT, has been shown to suppress experimental autoimmune neuritis (EAN [14]). Therefore, only limited (and partially conflicting) information has been accumulated so far about the involvement of 5-HT and the 5-HTT in T-cell mediated neurological autoimmune disease.
In the present study we investigated MOG- and rat-MBP-induced EAE in mice in which the 5-HTT is genetically inactivated (5-HTT KO mice) in comparison with wild-type control animals. EAE is the best studied and most commonly used model of experimentally induced T-cell mediated autoimmune disease (for review see [15]) and in contrast to other autoimmune disease models, it permits the study of T-cell autoimmunity directed towards an immune-privileged organ, the central nervous system (CNS). Here the potential influence of 5-HT and its homeostasis-regulating transporter, the 5-HTT, on clinical symptoms, neuroantigen-specific T cell activity in general and the type 1/type 2 cytokine signature of the disease-driving T cells were examined.
Materials and methods
Animals, antigens, and treatments
Breeding of 5-HTT KO mice and wildtype control mice on a C57.BL/6 background was performed at the local animal facilities under specific pathogen-free conditions. Animals were injected at 2–5 months of age with 200 µg MOG peptide amino acids 35–55 (MOGp 35–55, Biotrend, Cologne, Germany) or 200 µg rat MBP (prepared as described in [16]) in complete Freund's adjuvant (CFA). Pertussis toxin (400 ng, Sigma, Deisenhofen, Germany) was injected twice i.p., in 500 µl saline, 24 and 72 h after the immunization. IFA was purchased from Gibco BRL (Grand Island, NY, USA) and CFA was made by mixing M. tuberculosis H37RA (Difco Laboratories, Detroit, MI, USA) at 2 mg/ml into IFA. Antigens were mixed with the adjuvant to yield a 1 mg/ml emulsion of both antigen and CFA, of which 2 × 100 µl were injected subcutaneously at two different sites of the trunk. Animal experiments were approved by the Bavarian state authorities for animal experimentation.
Evaluation of disease
Mice were monitored daily for signs of clinical disease. The severity of disease was recorded according to the following scale: grade 0, no abnormality; grade 1, limp tail; grade 2, moderate hind limb weakness; grade 3, complete hind limb paralysis; grade 4, quadriplegia or premoribund state; grade 5, death. If necessary, food was provided on the cage floor.
Cell preparations from the organs tested
Single cell suspensions from the spleen were prepared as previously described [17]. The cells were counted by trypan blue exclusion and plated with antigen at the concentrations indicated in presence or absence of antigen.
Tritium-thymidine proliferation assay
For spleen-cell proliferation assays, single cell suspensions of spleens from MOGp 35–55 immunized 5-HTT KO and wild type control mice were prepared at day 15–17 after immunization of the animals. 2 × 105 cells were seeded in 96-well microtiter plates (Nunc, Wiesbaden, Germany) in 100 µl medium with or without addition of MOGp 35–55 in a final concentration of 20 µg/ml. Triplicate cultures were maintained at 37 °C and 5% CO2 in a humidified atmosphere for 56 h and harvested following a 16 h pulse with 0·2 µCi/well 3H-dT (tritiated thymidine, Amersham-Buchler, Braunschweig, Germany). The cells were collected on fibreglass filter paper with a 96-well harvester (Pharmacia, Freiburg, Germany) and radioactivity was quantified with a 96-well Betaplate liquid scintillation counter (Pharmacia).
Cytokine measurements by ELISPOT and computer-assisted ELISPOT image analysis
ELISPOT assays were performed as described [17]. Briefly, ImmunoSpot M200 plates (Cellular Technology, Cleveland OH, USA) were coated overnight with the capture antibodies in sterile PBS. R46A2, at 4 µg/ml (Pharmingen, San Diego, CA, USA) was used for IFN-γ, JES6–1A12, 4 µg/ml (Pharmingen) for IL-2, 11B11, at 4 µg/ml (Pharmingen) was used for IL-4, and TRFK5, at 2 µg/ml (Pharmingen) was used for IL-5. The plates were blocked for 1 h with sterile PBS 1% BSA and washed 3× with sterile PBS. Spleen cells (5 × 105 per well) were plated in HL-1 medium (BioWhittaker, Walkersville, MD, USA) together with and without MOGp 35–55 in triplicate cultures each. Subsequently plates were incubated at 37 °C, 5% CO2 for 20 h (IFN-γ and IL-2) or for 48 h (IL-4, IL-5). After washing with PBS followed by PBS 0·025% Tween, detection antibodies were added overnight. XMG1·2-biotin (Pharmingen) at 2 µg/ml was used for IFN-γ, rat anti-mouse IL-4-biotin (BVD6–24G2, Pharmingen) at 2 µg/ml was used for IL-4, and rat anti-mouse IL-2-biotin (JES6–5H4, Pharmingen) at 2 µg/ml was used for IL-2, and biotinylated TRFK4 (Pharmingen) at 2 µg/ml was used for IL-5. The plate bound second antibody was then visualized by adding streptavidin-alkaline phosphatase (SAV-AP, DAKO, Hamburg, Germany) and NBT/BCIP substrate (Bio-Rad, München, Germany). Image analysis of ELISPOT assays was performed with the ImmunoSpot™ Analysis Software after scanning the plates with an ImmunoSpot™ Analyser (Cellular Technologies, Cleveland, OH, USA). In brief, digitized images of individual wells of the ELISPOT plates were analysed for cytokine spots, based on the comparison of experimental wells (containing T cells and APC with antigen) and control wells (T cells and APC, no antigen). After separation of spots that touched or partially overlapped, nonspecific noise was gated out by applying spot size and circularity analysis as additional criteria. Spots that fell within the accepted criteria were highlighted and counted. Single wells which could not be enumerated because of confluence phenomena due to the large number of cytokine-producing cells were assessed by using the highest numbers of cytokine-producing cells which could be regularly counted in other wells in the same assay for estimation.
Immunohistochemistry
Animals were anaesthesized with pentobarbital and transcardially perfused with saline followed by fixation in 4% of paraformaldehyde. Spinal cord and brain were carefully removed and further processed for routine paraffin embedding. These were subjected to haematoxylin/eosin staining to assess inflammation using the inflammatory index described in [18]. Immunohistochemistry was performed on 5 µm paraffin sections. If necessary, antigen unmasking was achieved by heat pretreatment of sections for 30 min in 1 mM EDTA buffer (CD3 staining) or 10 mM citric acid buffer (Mac-3H staining) in a microwave oven (850 W). After inhibition of unspecific binding with 10% BSA, sections were incubated overnight at 4 °C with the appropriate primary antibody in 1% BSA. Secondary antibodies were used as indicated below. After blocking of endogenous peroxidase with H2O2, the peroxidase-based ABC detection system (DAKO, Hamburg, Germany) was employed with DAB as the chromogenic substrate. Specificity of staining was confirmed by omitting the primary antibody as a negative control. Inflammation was immunohistochemically detected using a monoclonal rat anti-human CD3 antibody crossreacting with mouse CD3 (1 : 200, Serotec, via Biozol, Eching, Germany) for T-cells and a monoclonal rat anti-mouse Mac-3 antibody for macrophages (1 : 50, Pharmingen, Heidelberg, Germany) with an anti-rat IgG secondary antibody. Quantitative evaluation was essentially performed as described in [18]. Briefly, studies were done on cross-sections from 7 animals per experimental groups.
Coded sections were counted by an observer who was masked as to the experimental groups by means of overlaying a stereological grid onto the sections. The inflammatory reaction in situ was grossly assessed using the inflammatory index, where the number of perivascular or intraparenchymal inflammatory infiltrates per mm2 cross section was assessed on haematoxylin/eosin stained sections over several levels of spinal cord. Furthermore inflammatory infiltrates (by the identifiable cells), CD3-positive cells or Mac-3 positive cells were counted per mm2 white matter.
Statistical analysis
For statistical analysis, ‘Graph Pad Prism’ 3·0 (Graph Pad Software, San Diego, USA) was used.
Results
5-HTT KO mice display EAE with reduced clinical severity compared to wild-type control animals
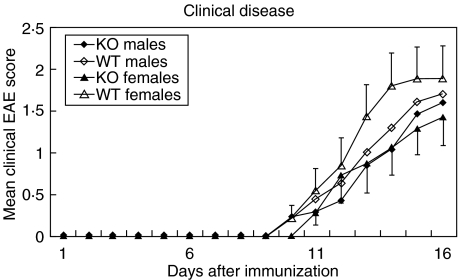
Clinical disease was induced as described in materials and methods. As shown in Table 1, disease tended to be reduced in 5-HTT KO mice both after immunization with MOGp 35–55 in CFA and MBP in CFA (disease courses for the latter group are not shown). Figure 1 demonstrates that 5-HTT KO animals display a reduced severity of clinical symptoms of EAE. This difference was significant in female animals (P = 0·0055, two-way anova).
Table 1. Summary of clinical characteristics of EAE in 5-HTT KO and wild-type mice. Note that in mean maximum severity only those mice who developed EAE are included.
| 5-HTT KO | Wild-type | |||||
|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | |
| MOGp 35–55/CFA | 21/29 | 13/18 | 8/11 | 22/28 | 13/16 | 9/12 |
| Incidence | 72 | 72 | 73 | 79 | 81 | 75 |
| Mean maximum severity | 2.1 | 2.2 | 1.9 | 2.2 | 2.0 | 2.5 |
| Mean day of onset | 11.6 | 11.6 | 11.6 | 10.5 | 10.1 | 10.9 |
| MBP/CFA | 3/5 | 5/5 | ||||
| Incidence | 60 | 100 | ||||
| Mean maximum severity | 1.2 | 2.4 | ||||
| Mean day of onset | 14.0 | 12.0 | ||||
Fig. 1.
Clinical disease courses of male and female 5-HTT KO mice and wild type control mice after immunization with MOGp 35–55 in CFA. All animals were included in this graph, also the mice which did not develop clinical symptoms of EAE. 5-HTT KO mice display an attenuated clinical EAE course with a more pronounced difference in female than in male mice. The graphs shown summarize the data of 11–18 mice for each of the four different groups tested in altogether five independent experiments. Error bars indicate standard error of the mean (SEM) for the female mice.
5-HTT KO mice show differences in CNS infiltration compared to wild-type control mice during acute EAE
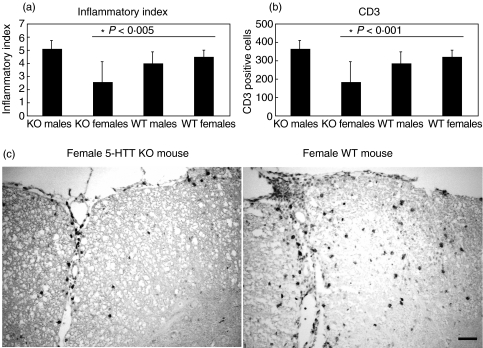
For assessment of the effects of extracellular 5-HT homeostasis on cellular infiltration in situ, histological staining for cellular infiltrates as well as immunohistochemistry for CD3-positive cells were performed on spinal cord sections (Fig. 2a,b). Strong T cell infiltration was seen in clinically affected female control animals as compared to female 5-HTT ko mice (Fig. 2c). Female 5-HTT KO mice displayed lower degree of inflammatory infiltrates (P < 0·005) and CD3-positive cells as compared to wild-type control animals. (P < 0·001).
Fig. 2.
Histological analysis of infiltration (a) and of CD3-positive cells (b) by immunohistochemistry in the CNS during acute EAE at day 14 after immunization with MOGp 35–55 in CFA. Female 5-HTT KO mice show a tendency towards reduction of general infiltration and presence of CD3 positive cells. The difference in CD3 staining is significant at P < 0·001. Shown are the cumulative histological data of 3–4 individual animals per group. Error bars indicate the standard error of the mean. (c) shows a representative CD3 staining of EAE spinal cord from a female 5-HTT KO mouse (left) in comparison with a female WT mouse (right).Bar represents 50 µm.
Proliferative capacity of MOGp-specific T cells is identical in 5-HTT KO mice and wild-type control C57.BL/6 mice
Average proliferative capacity as measured by 3H-dT proliferation assay was nearly identical in both male and female 5-HTT KO animals and wild-type C57.BL/6 as shown in Table 2. Therefore, T-cells of 5-HTT KO mice do not seem to be restricted in their capacity to expand and to mount a T-cell-mediated immune response after initial priming.
Table 2. Proliferation of spleen cells of 5-HTT KO and wild type control animals in response to MOGp 35–55 measured by 3H-dT-proliferation assay 15—17 days after immunization and MOGp 35–55 specific cytokine production in spleen cells during acute EAE at day 14 or 15 after immunization as measured by ELISPOT.
| KO males | KO females | WT males | WT females | |
|---|---|---|---|---|
| SI (prolif.) | 6.31 ± 1.15 | 6.11 ± 1.62 | 4.99 ± 1.58 | 6.74 ± 1.15 |
| IFN-γ | 137 ± 24 | 100 ± 27* | 130 ± 28 | 165 ± 33 |
| IL-2 | 117 ± 16 | 126 ± 36 | 122 ± 34 | 119 ± 35 |
| IL-4 | 73 ± 4 | 84 ± 22 | 64 ± 24 | 81 ± 20 |
| IL-5 | 7 ± 1 | 11 ± 4 | 6 ± 2 | 16 ± 5 |
The numbers represent the MOGp 35–55-specific response of 7–14 animals tested individually. For cytokine assays, the background of the negative control was subtracted. Each single mouse was tested in triplicate cultures. Numbers indicate the mean ± standard error of the mean (SEM).
Reduction of MOGp 35–55-specific IFN-γ production in 5-HTT KO mice.
Fourteen or 15 days after injection with MOGp 35–55 in CFA, single-spleen-cell suspensions were prepared as described in Materials and methods. ELISPOT cytokine measurements are summarized in Table 2. High frequencies of antigen-specific IFN-γ- and IL-2-producing cells were seen in both 5-HTT KO and wild-type control animals, corresponding to a Th1-like phenotype of the overall T cell response. Only negligible frequencies of MOGp-specific IL-5-producing cells were seen, and the high numbers of IL-4 producing cells detected did not differ as well. Therefore, injection of MOGp 35–55 in CFA into mice with genetically changed 5-HT metabolism did not result in any form of tolerance, splenic anergy or Th2-like immune deviation explaining the observed clinical disease amelioration. Concerning the frequencies of cytokine-producing cells, female 5-HTT KO mice showed in average a reduction of the precursor frequency of MOGp 35–55-specific IFN-γ-producing cells. There was no pronounced difference between KO and WT animals regarding the average number of IL-2- and IL-4-producing cells. Frequencies of MOGp 35–55-specific IL-5-producing cells were very low in both groups.
Discussion
The aim of this study was to investigate if changes in 5-HT homeostasis can influence the organism's disposition for the induction of an encephalitogen-specific type-1-autoimmune response in EAE. We show that there is a difference in susceptibility to MOGp 35–55-induced EAE in 5-HTT KO mice in comparison to wild-type animals: female but not male 5-HTT KO mice were significantly less severely affected than female wild-type mice. Reduced immune cell infiltration in the inflamed CNS and a reduced number of MOGp 35–55 specific IFN-γ-producing cells in female 5-HTT KO mice were detected.
Assessment of neuroantigen-specific T cell proliferative capacity and T cell cytokine production revealed a strong MOGp 35–55-specific response with type-1 cytokine production in mononuclear cells isolated from the spleen of both KO and WT mice. However, production of the inflammatory cytokine IFN-γ was on average 50% higher in wild type mice than in 5-HTT KO mice. This difference of the frequency of cells producing one particular type-1 cytokine is a striking observation. IFN-γ has been classically regarded as the paradigmatic pro-inflammatory cytokine indicating destructive autoimmune T cell activity including activation of the innate compartment of the immune system [19]. The reduction of this cytokine therefore could explain the attenuation in clinical disease observed in 5-HTT KO mice, in particular, since the gender difference in clinical disease was also paralleled by a gender difference in MOGp 35–55-specific IFN-γ-production. On the histological level, female 5-HTT KO mice exhibited a significant reduction of inflammatory infiltrates and of CD3-positive cells. This could reflect the reduced effector cell recruiting capability of the less IFN-γ-biased MOGp 35–55-specific T cell repertoire in 5-HTT KO mice. Since the lack of reuptake of 5-HT in the absence of the 5-HTT leads to increased extracellular levels of 5-HT, the observed effects on EAE severity and IFN-γ-production are potentially a consequence of the increased extracellular 5-HT levels, with subsequent changes in signalling via the 5-HT receptors known to be located on immunocompetent cells.
It is commonly accepted that men are less susceptible to MS than women (for review see [20]). Testosterone and the pregnancy hormone oestriol are implicated as protective factors responsible for the clinical differences observed. Gender differences in EAE parallel those seen in MS and develop during the induction of the immune response to encephalitogenic antigens [21]. Both androgens and oestrogens have been shown to be able to down-regulate neuroantigen-specific inflammatory cytokine production [22]. In our work, a gender difference was demonstrated in C57.BL/6 mice, which is in contrast to work by others [23,24]. This might be caused by individual genetic properties of the background on which the 5-HTT KO mice and their wild-type counterparts are generated.
Our results suggest that 5-HT and its homeostasis-regulating system, the 5-HTT, may play a similar gender-specific role in autoimmune T cell regulation. The results of this study therefore indicate a novel mechanism operating neurological autoimmune disease regulation and modulation of inflammatory cytokine production in vivo. These observations may lead to a better understanding of the influence of primarily nonimmunological factors on the development of neurological autoimmune diseases like multiple sclerosis and may ultimately help to develop suitable therapeutic intervention strategies.
Acknowledgments
We thank Gabi Köllner and Verena Wörtmann for excellent technical assistance and Helga Brünner for steady animal care. In addition, we thank Dr C.W. Ip for his expert advice regarding the photomicrographs. The study was supported by the Deutsche Forschungsgemeinschaft (SFB 581, TP A1 and TP B9).
References
- 1.Kerschensteiner M, Gallmeier E, Behrens L, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–70. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linker RA, Maurer M, Gaupp S, et al. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med. 2002;8:620–4. doi: 10.1038/nm0602-620. [DOI] [PubMed] [Google Scholar]
- 3.Bebo BF, Schuster JC, Vandenbark AA, Offner H. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol. 1999;162:35–40. [PubMed] [Google Scholar]
- 4.Ito A, Bebo BF, Matejuk A, et al. Estrogen treatment down-regulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol. 2001;167:542–52. doi: 10.4049/jimmunol.167.1.542. [DOI] [PubMed] [Google Scholar]
- 5.Straub RH, Westermann J, Scholmerich J, Falk W. Dialogue between the CNS and the immune system in lymphoid organs. Immunol Today. 1998;19:409–13. doi: 10.1016/s0167-5699(98)01297-3. [DOI] [PubMed] [Google Scholar]
- 6.Eliseeva LS, Stefanovich LE. Specific Binding of Serotonin by Blood Leukocytes and Peritoneal-Cells of Mice. Biochemistry-Moscow. 1982;47:675–8. [PubMed] [Google Scholar]
- 7.Jackson JC, Walker RF, Brooks WH, Roszman TL. Specific uptake of serotonin by murine macrophages. Life Sci. 1988;42:1641–50. doi: 10.1016/0024-3205(88)90443-2. [DOI] [PubMed] [Google Scholar]
- 8.Finocchiaro LME, Arzt ES, Fernandezcastelo S, et al. Serotonin and melatonin synthesis in peripheral-blood mononuclear-cells – stimulation by interferon-gamma as part of an immunomodulatory pathway. J Interferon Res. 1988;8:705–16. doi: 10.1089/jir.1988.8.705. [DOI] [PubMed] [Google Scholar]
- 9.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 10.Mossner R, Lesch KP. Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav Immun. 1998;12:249–71. doi: 10.1006/brbi.1998.0532. [DOI] [PubMed] [Google Scholar]
- 11.Ramamoorthy S, Ramamoorthy JD, Prasad PD, et al. Regulation of the human serotonin transporter by interleukin-1 beta. Biochem Biophys Res Commun. 1995;216:560–7. doi: 10.1006/bbrc.1995.2659. [DOI] [PubMed] [Google Scholar]
- 12.Bebo BF, Yong T, Orr EL, Linthicum DS. Hypothesis: a possible role for mast cells and their inflammatory mediators in the pathogenesis of autoimmune encephalomyelitis. J Neurosci Res. 1996;45:340–8. doi: 10.1002/(SICI)1097-4547(19960815)45:4<340::AID-JNR3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Linthicum DS. Development of acute autoimmune encephalomyelitis in mice: factors regulating the effector phase of the disease. Immunobiology. 1982;162:211–20. doi: 10.1016/S0171-2985(11)80001-X. [DOI] [PubMed] [Google Scholar]
- 14.Bengtsson BO, Zhu J, Thorell LH, et al. Effects of Zimeldine and Its Metabolites, Clomipramine, Imipramine and Maprotiline in Experimental Allergic Neuritis in Lewis Rats. J Neuroimmunol. 1992;39:109–22. doi: 10.1016/0165-5728(92)90180-s. [DOI] [PubMed] [Google Scholar]
- 15.Kuchroo VK, Anderson AC, Waldner H, et al. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Ann Rev Immunol. 2002;20:101–23. doi: 10.1146/annurev.immunol.20.081701.141316. [DOI] [PubMed] [Google Scholar]
- 16.Eylar EH, Jackson JJ, Kniskern PJ. Suppression and Reversal of Allergic Encephalomyelitis in Rhesus-Monkeys with Basic-Protein and Peptides. Neurochem Res. 1979;4:249–58. doi: 10.1007/BF00964148. [DOI] [PubMed] [Google Scholar]
- 17.Hofstetter HH, Shive CL, Forsthuber TG. Pertussis toxin modulates the immune response to neuroantigens injected in incomplete Freund's adjuvant. Induction of Th1 cells and experimental autoimmune encephalomyelitis in the presence of high frequencies of Th2 cells. J Immunol. 2002;169:117–25. doi: 10.4049/jimmunol.169.1.117. [DOI] [PubMed] [Google Scholar]
- 18.Bachmann R, Eugster HP, Frei K, et al. Impairment of TNF-receptor-1 signaling but not Fas signaling diminishes T-cell apoptosis in myelin oligodendrocyte glycoprotein peptide-induced chronic demyelinating autoimmune encephalomyelitis in mice. Am J Pathol. 1999;154:1417–22. doi: 10.1016/S0002-9440(10)65395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–41. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 20.Voskuhl RR. Gender issues and multiple sclerosis. Curr Neurol Neurosci Report. 2002;2:277–86. doi: 10.1007/s11910-002-0087-1. [DOI] [PubMed] [Google Scholar]
- 21.Bebo BF, Adlard K, Schuster JC, et al. Offner H. Gender differences in protection from EAE induced by oral tolerance with a peptide analogue of MBP-Ac1–11. J Neurosci Res. 1999;55:432–40. doi: 10.1002/(SICI)1097-4547(19990215)55:4<432::AID-JNR4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Whitacre CC, Dowdell K, Griffin AC. Neuroendocrine influences on experimental autoimmune encephalomyelitis. Ann NY Acad Sci. 1998;840:705–16. doi: 10.1111/j.1749-6632.1998.tb09609.x. [DOI] [PubMed] [Google Scholar]
- 23.Papenfuss TL, Rogers CJ, Gienapp I, et al. Sex differences in experimental autoimmune encephalomyelitis in multiple murine strains. J Neuroimmunol. 2004;150:59–69. doi: 10.1016/j.jneuroim.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Okuda Y, Okuda M, Bernard CC. Gender does not influence the susceptibility of C57BL/6 mice to develop chronic experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein. Immunol Lett. 2002;81:25–9. doi: 10.1016/s0165-2478(01)00339-x. [DOI] [PubMed] [Google Scholar]