Abstract
Diagnosis of infection with Mycobacterium tuberculosis (MTB) using tuberculin skin testing (TST) is often hampered by prior Bacille Calmette–Guérin (BCG) vaccination. ESAT-6 is a protein that is expressed by MTB but absent in BCG. It has been postulated that it might be useful in distinguishing MTB-specific immune responses. This study measured CD4 T cell responder frequencies specific for ESAT-6 and the TST reagent purified protein derivative (PPD) in patients with tuberculosis (n = 16), controls with non-tuberculous pneumonia (n = 8) and normal subjects (n = 7). Responses were identified using the intracellular cytokine staining technique and flow cytometry on whole blood samples, and performed blinded to the patient condition. Antigen-specific CD4 cells were defined by CD69 positivity and one or more cytokine [interleukin (IL)-2, IL-4, IL-10, interferon (IFN)-γ] and/or CD40L positivity. With ESAT-6 stimulation it was found that TB patients had significantly higher frequencies of IFN-γ and CD40L-positive CD4 T cells compared to the normal group, while no significant differences were measured with PPD stimulation. A responder frequency of 0·01% or higher for at least one of the measured cytokines/CD40L was defined as a positive response. Using this criterion to compare the two patient groups, PPD had 100% sensitivity but 0% specificity while ESAT-6 had 100% sensitivity and 88% specificity. Use of MTB-specific proteins such as ESAT-6 in combination with intracellular cytokine staining and flow cytometry has the potential to identify individuals with MTB infection.
Keywords: diagnosis, ESAT-6, flow cytometry, Mycobacterium tuberculosis
Introduction
The incidence of tuberculosis is increasing worldwide as a result of the HIV pandemic, lack of sufficient public health resources in the developing world and the breakdown of health-care delivery in Eastern Europe and the former USSR. The World Health Organization estimates that one billion people will be newly infected with Mycobacterium tuberculosis (MTB) during the next 20 years [1]. While the diagnosis of active tuberculosis is relatively straightforward, diagnosis of latent infection remains difficult due to the lack of a simple, reliable test for M. tuberculosis infection. Tuberculin skin testing (TST) with purified protein derivative (PPD) is not specific in people vaccinated with Bacille Calmette–Guérin (BCG) [2].
One solution to this problem is the identification and purification of MTB-specific antigens. ESAT-6 is a secreted antigen of both MTB and wild-type M. bovis but is absent from BCG. Investigation of immune responses to this antigen in cattle has shown that it can differentiate between infected and vaccinated animals [3]. Although the immune response that it elicits is approximately only a tenth of that of PPD [4], human studies have shown similar promising results [5,6]. The measurement of these responses has generally relied on the detection of interferon (IFN)-γ production by ESAT-6-specific CD4 T cells [7,8]. Released IFN-γ can be measured by assessment of the supernatant of the stimulated cells [8] or by using the enzyme-linked immunospot (ELISPOT) method [9]. Recently the technique of intracellular cytokine staining (ICC) using multicolour immunofluorescent labelling and flow cytometry has been used to identify ESAT-6 specific CD4 T cells [10,11].
M. tuberculosis is an intracellular pathogen. The usual immune response follows a T helper type 1 phenotype − the signature cytokine of which is IFN-γ − and so other studies have concentrated on measuring responses to ESAT-6 with this cytokine [8,9]. However, mycobacterial infections may also be associated with Th2 responses under certain circumstances. Lepromatous and tuberculoid leprosy are examples of dichotomous immune responses to the same pathogen, resulting in different patterns of disease [12,13]. Recently, a similar phenomenon has been described with M. ulcerans (MU): patients who have had an ulcer are more likely to respond to MU antigen with Th-2 type cytokine production than healthy exposed controls [14]. Furthermore, there is some evidence in animal and human investigations of MTB infection that Th-2 responses can also be observed with this disease [15,16], and so measurement of these markers could aid diagnosis further.
In this study we investigated whether the ICC technique could distinguish patients with tuberculous pneumonia from non-tuberculous pneumonia on the basis of CD4 T cell responses to ESAT-6 and PPD. Besides IFN-γ we measured interleukin (IL)-2, the Th2 cytokines IL-4 and IL-10 and also the activation marker to CD40L to determine whether it could increase the sensitivity and specificity of this assay in diagnosing MTB infection.
Methods
Study groups
The protocol for this study was passed by the Ethics Committee of Monash Medical Centre and was in accordance with the Helsinki Declaration of 1975, as revised in 1983. Informed consent was obtained from all subjects prior to enrolment.
Subjects in the control and TB case groups used for this study were patients at Monash Medical Centre, a 646-bed tertiary referral hospital located in Melbourne, Australia. The hospital has a weekly out-patient tuberculosis clinic and notifies approximately 50 new cases of tuberculosis per year. The normal donor group consisted of volunteers (four male/three female) with an age range similar to the two patient groups (27–56 years). All were healthy and under no medication at the time of testing.
All adult patients with newly diagnosed, active TB and normal immune function were invited to participate in this study. The diagnosis was generally confirmed by culture; however, patients were not excluded if they had a history, signs and investigation results strongly suggestive of TB and responded to TB therapy. Blood was drawn from all patients within 2 weeks of the commencement of treatment.
Exclusion criteria were haematological malignancy, HIV, other immunosuppressive disease, current or previous chemotherapy and immunosuppressive treatments, including oral corticosteroids.
To serve as controls, adult patients aged less than 50 years with non-tuberculous pneumonia and with a low risk of prior exposure to MTB, and subject to the same exclusion criteria as the cases, were invited to participate. Low exposure risk was defined as being born in Australia or other low-incidence country and without other recognized risk factors for MTB exposure. These risk factors were working in the health-care industry, prolonged overseas travel and household contact with tuberculosis. The age cut-off was chosen to reduce further the risk of controls having had prior exposure to MTB.
The diagnosis of non-tuberculous pneumonia was based on microbiological or clinical criteria. Microbiological diagnosis was made when a respiratory pathogen was isolated from clinical specimens and chest X-ray showed consolidation in a patient with an infective syndrome of less than 2 weeks’ duration. Clinical pneumonia without microbiological confirmation was defined as a short history of illness (<2 weeks), consolidation on chest X-ray, investigations in keeping with an acute inflammatory state, response to conventional antibiotics and complete resolution after a month. All controls were followed-up by telephone after at least 1 month to ensure that pneumonia had resolved and no alternative diagnosis had been made.
In vitro antigen stimulation
The intracellular cytokine (ICC) method used was adapted from Suni et al. [17], with the assays performed blind. Briefly, 6 ml of blood was collected in Li heparin tubes and transported to the laboratory within 6 h to ensure lymphocyte viability. Samples were divided into three 2 ml aliquots. One was incubated with PPD (Cellestis, Melbourne, Australia), one with ESAT-6 (a gift from Jim Rothel, Cellestis) and the final aliquot served as a non-stimulated control. Co-stimulatory antibodies CD28 [1 µl ascites/1 ml blood (CLB, the Netherlands)] and CD49d [1 µg/ml (Cymbus Biotechnology, Southampton, UK)] were added to all samples. An ESAT-6 dose–response experiment found that a concentration of 5 µg/ml achieved optimal stimulation (data not shown). The PPD was used at the dose recommended by the manufacturer for in vitro use. The blood was cultured at 37°C for 6 h, with the Golgi transport inhibitor brefeldin A (10 µg/ml) added for the last 5 h. After 6 h, ethylenediamine tetra-acetic acid (EDTA) (2 m M) was added to detach any adherent cells.
Four-colour immunofluorescence labelling
Red blood cells were lysed with a NH4Cl solution. Leucocytes were then pelleted and resuspended in 2% paraformaldehyde for fixation (30 min, on ice). Following this the cells were washed and left in FACS wash [phosphate-buffered saline (PBS)/1% fetal calf serum (FCS)/0·01% NaAzide] overnight at 4°C. The following day, cells were pelleted and pre-permeablized in 0·2% saponin (5 min, on ice). The cells were spun down and again resuspended in saponin, this time divided into three aliquots, and the following combination of fluorochrome-conjugated antibodies added: anti-IL-2/FITC (fluoroscein) + anti-IL-4/PE (phycoerythrin) + CD69/PECy5 (phycoerythrin–cyanine 5) + CD4/APC (allophycocyanin); anti-IFN-γ/FITC + anti-IL-10/PE + CD69/PECy5 + CD4/APC; and anti-IFN-γ/FITC + CD40L/PE + CD69/PECy5 + CD4/APC. All antibodies were obtained from Becton Dickinson Immunocytometry Systems (San Jose, CA, USA) except for CD4/APC, purchased from Exalpha (Boston, MA, USA). Isotype control antibodies were not used in this study, as previous work had found the same level of background staining in both antigen-stimulated and unstimulated samples.
Cells were incubated (30 min, on ice) and then washed once in saponin. They were then resuspended in FACS wash and left at 4°C until flow cytometric analysis.
Flow cytometry
Antibody labelled cells were analysed on a Mo-Flo flow cytometer (Cytomation, Fort Collins, CO, USA). The instrument was equipped with a 488 nm argon ion laser for excitation of the FITC, PE and PECy5 fluorochromes and a 633 nm HeNe laser for excitation of APC-labelled antibodies. The four fluorescences along with forward and side light-scatter were collected and analysed using the Summit Software program (Cytomation).
The lymphocytes were gated using forward and side light-scatter and their CD4 and CD69 labelling displayed. Addition of the co-stimulatory antibodies without antigen did not result in any induced positive cytokine and/or CD40L staining in the subjects studied here. Gating on the CD4+ CD69+ cells and measuring their cytokine and/or CD40L staining then determined antigen reactive cells. Because of the low frequency of cells expected at least 40 000 CD4 cells were counted, with >60 000 counted, in most cases, for each sample tested.
The frequency of antigen-specific cells was defined as the percentage of CD4 cells positive for CD69 and cytokine/CD40L minus background staining from the non-stimulated sample for each subject. As in a previous study of CD4 T cell responses to TB antigens [10], an arbitrary cut-off of 0·01% CD4 T cells was classified as a positive response.
Statistical analysis
anova analysis of the normal, control and case groups results was used to determine statistically significant differences. Significance was defined as P < 0·05. The analysis was executed by the GraphPad Prism software program (GraphPad Software, San Diego, CA, USA).
Results
Subject details
Cases
Seventeen patients with newly diagnosed active tuberculosis were recruited between May and September 2000. Patient details are presented in Table 1. Sixteen had pulmonary tuberculosis and the remaining patient had a tuberculous psoas abscess. Three patients had extra-pulmonary involvement in addition to pulmonary disease. All the TB patients were overseas-born, the majority from South-east Asia and the Indian subcontinent. The average age was 38 years. One subject was excluded from the study because there were not sufficient T cells to allow ICC analysis.
Table 1. Tuberculosis patients' clinical details.
| Patient | Sex | Age | Country of birth | Diagnosis | Pulmonary site | Cavitation | Extrapulmonary site |
|---|---|---|---|---|---|---|---|
| 1 | M | 25 | China | Micro. | Apical RLL | No | |
| 2 | F | 48 | Vietnam | Micro. | RUL,RML,RLL | No | |
| 3 | M | 24 | India | Micro. | RUL | No | |
| 4 | F | 19 | Vietnam | Micro. | RUL | Yes | |
| 5 | M | 41 | Vietnam | Micro. | LUL | Yes | Ileum |
| 6 | M | 23 | Vietnam | Micro. | RUL | No | |
| 7 | M | 45 | Philippines | Clin. | RUL | Yes | |
| 8 | F | 32 | India | Micro. | LUL | Yes | |
| 9 | F | 57 | Sri Lanka | Histol. | RUL | No | |
| 10 | M | 79 | Holland | Micro. | RUL,LUL | No | Pleura |
| 11 | F | 39 | Somalia | Micro. | No | Psoas | |
| 12 | M | 22 | Hong Kong | Micro. | Apical RLL | Yes | |
| 13 | M | 39 | Philippines | Micro. | RUL | No | Peritoneum |
| 14 | M | 58 | Vietnam | Micro. | RUL | Yes | |
| 15 | F | 29 | Cambodia | Micro. | LUL | Yes | |
| 16 | F | 30 | India | Micro. | LUL | Yes |
Diagnosis was confirmed by culture in 15 cases, one patient was diagnosed on clinical and histological criteria, and one on clinical criteria alone. The diagnosis made on clinical and histological grounds was a 57-year-old woman with a past history of treated pulmonary tuberculosis who presented with a chronic illness associated with fevers, loss of weight and raised inflammatory markers. Chest x-ray (CXR) showed an area of increased density in the right upper lobe. A needle biopsy of this area revealed granulomatous inflammation but was smear- and culture-negative for MTB. She commenced anti-tuberculous chemotherapy and symptoms and inflammatory markers improved. The clinical diagnosis of tuberculosis was made in a 45-year-old Filipino man who was investigated after an immigration chest X-ray demonstrated an area of consolidation in his right upper lobe. Computerized tomography (CT) of the chest confirmed consolidation in this area as well as some cavitation. His tuberculin skin test was strongly positive and he underwent bronchoscopy with washings of the right upper lobe (RUL) before commencing anti-tuberculous chemotherapy. Although cultures were negative, a repeat chest CT scan at 2 months showed significant improvement and he completed a 6-month course of treatment.
Controls and normal subjects
Eight patients with non-tuberculous pneumonia were recruited. Details are presented in Table 2. The average age was 32 years. A single control patient was born in New Zealand and the remainder were Australian-born (the current annual incidence of tuberculosis in Australia 5·2/100 000). The majority had been vaccinated previously with BCG and none had risk factors for prior TB exposure. Respiratory pathogens were isolated from three that were considered responsible for their pneumonia. Despite thorough investigation, aetiology of pneumonia could not be established in the remaining five controls.
Table 2. Control patients’ clinical details.
| Control | Sex | Age | Country of birth | Cough | Sputum | SOB | Temp (°C) | WCC (109/l) | CRP | Consolidation | Microbiology |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 29 | Australia | Yes | Yes | Yes | 38·7 | 16·1 | 186 | LLL | Streptococcus pneumoniae |
| 2 | F | 27 | Australia | No | No | Yes | 38·9 | 23·3 | 236 | LLL | Streptococcus pneumoniae |
| 3 | M | 23 | Australia | Yes | Yes | Yes | 37·2 | 15·9 | 475 | RLL | |
| 4 | F | 35 | Australia | No | No | Yes | 38·6 | 30·4 | 173 | Basal RUL | |
| 5 | M | 30 | Australia | No | No | Yes | 39·5 | 11·2 | 262 | RLL,LLL | |
| 6 | F | 33 | Australia | Yes | No | Yes | 39·2 | 19·0 | 45 | RUL | |
| 7 | F | 40 | New Zealand | Yes | No | No | 38·4 | 9·1 | 28 | RLL,LLL | Influenza B |
| 8 | F | 38 | Australia | Yes | No | No | 38·3 | 10·8 | 317 | RML,RLL |
Seven normal volunteers were recruited for testing. All were Australian-born, with an average age of 43 years. All had been vaccinated previously with BCG.
Measurement of antigen-specific CD4 T cell frequencies
PPD-specific CD4 responses
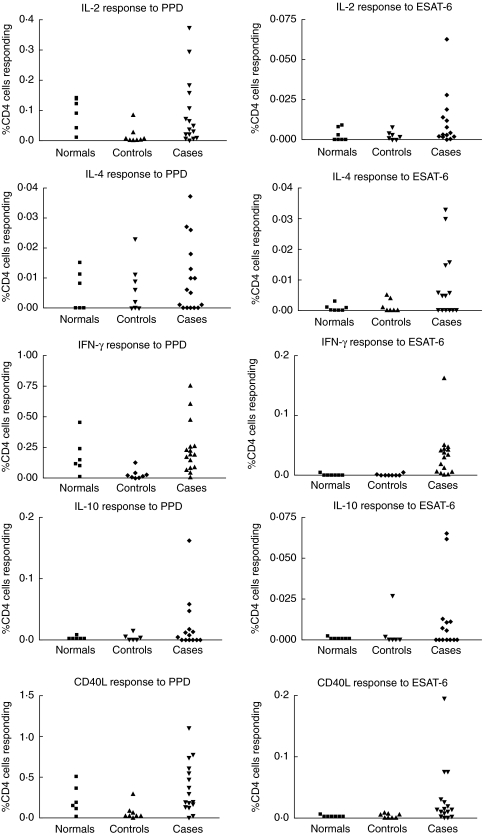
The proportion of cytokine/CD40L positive CD4 T cells following PPD stimulation for each subject tested is presented in the left-hand column of Fig. 1. Representative histograms detailing PPD-induced IFN-γ and CD40L staining in CD4 T cells from subjects in both patient groups are shown in Fig. 2. Overall, no significant differences were found between the normal subjects, controls and cases for percentage of PPD-responding CD4 cells for any of the cytokines/CD40L measured.
Fig. 1.
Each of the cytokine/CD40L responses to purified protein derivative (PPD) (left column) and ESAT-6 (right column) for the three groups studied; normal subjects, controls and cases. Note that responses are seen to each cytokine/CD40L but none produces a response in all subjects.
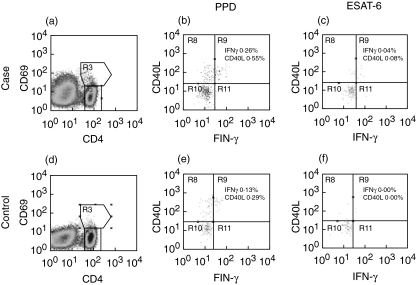
Fig. 2.
Representative histograms detailing purified protein derivative (PPD) and ESAT-6 induced interferon (IFN)-γ and CD40L staining in CD4 T cells from subjects in the tuberculosis patient group (a–c) and control patient group (d–f). Histograms (b, c, e and f) were gated on CD4+ CD69+ lymphocytes (R3) in histograms (a) and (b), respectively. The percentage in each histogram is the frequency of CD4 cells positive for CD69 and IFN-γ or CD40L after subtracting staining from the non-stimulated control cells.
Using a positive response threshold of at least 0·01% CD4 T cells it was found that all six normal subjects tested had positive CD40L and IL-2 responses following PPD stimulation. Five of six were positive for IFN-γ, and two of six for IL-4. No positive IL-10 responses were detected in this group. In the control group, applying the 0·01% positive response threshold to the PPD stimulation data resulted in at least one positive cytokine and/or CD40L measurement in all subjects. Seven of eight non-TB patients had a CD40L positive response, with four of these seven also positive for IFN-γ, two for IL-2, two for IL-4 and one for IL-10. The control that did not have a positive response for CD40L did have positive responses with IFN-γ and IL-10. Again, as for the controls, all TB cases had at least one positive response for the cytokines/CD40L measured. More of the TB patients had a positive result for each of the cytokines/CD40L tested than the controls: 15 of 16 for CD40L, 15 of 16 for IFN-γ, 12 of 16 for IL-2, seven of 16 for IL-4 and six of 14 for IL-10. The sole TB patient negative for the CD40L response was positive for IFN-γ, IL-2 and IL-4. If the positive threshold was increased to 0·05% it was found that all TB patients had at least one positive cytokine/CD40L response, while three controls of the eight and five of the six normal subjects tested still scored positive. The positive PPD reactions detected among the control and normal groups indicates that enumerating PPD-specific CD4 T cells is not a useful means to discriminate patients with tuberculous pneumonia from non-tuberculous pneumonia. The PPD reactivity in the non-TB groups is due most probably to previous vaccination with BCG.
ESAT-6 specific CD4 responses
The proportion of cytokine/CD40L positive CD4 T cells following ESAT-6 stimulation for each subject tested is presented in the right-hand column of Fig. 1. Representative histograms detailing ESAT-6-induced IFN-γ and CD40L staining in CD4 T cells from subjects in both patient groups are shown in Fig. 2. The antigen specificity of the ESAT-6 response was confirmed by complete blocking of the induced cytokine/CD40L staining by preincubation with anti-HLA-DR, DP and DQ antibodies. Overall, responses to ESAT-6 were approximately a tenth of those with PPD. Taken as a whole, the responses for the TB patient group was significantly higher for IFN-γ (P < 0·001) and CD40L (P < 0·05) compared to the normal group. No significant differences for any of the cytokines/CD40L were found between the control and case groups. Individual TB cases did have strong CD40L, IL-2 and IL-10 responses, with one of the control patients also having a higher IL-10 response.
Using the 0·01% positive threshold it was found that no normal subjects and only one subject in the control group had a positive response to ESAT-6, and this was only for IL-10. For the TB cases it was found that 11 of the 16 patients tested had a positive IFN-γ response, IL-2 five of 14, IL-4 four of 14, IL-10 five of 14 and CD40L 11 of 16. Every patient in this group had at least one cytokine or CD40L-positive response. If the test focused on the two most strongly defined markers, IFN-γ and CD40L, no controls and 14 of 16 patients had a detectable response to the ESAT-6 protein. The two remaining TB patients negative for these markers were strikingly positive for IL-10 alone (both 0·06%), indicating perhaps that these patients had a Th2-type immune response against this antigen. Unlike the PPD results, the measured ESAT-6 responses clearly delineated the TB cases from the control and normal groups, indicating the usefulness of this assay in detecting active TB infection.
Discussion
After approximately 100 years, TST remains the most widely accepted and used method for the diagnosis of latent MTB infection. Newer serological and cytokine-based assays have recently become available, but have yet to demonstrate clearly enhanced sensitivity and specificity compared with TST [18,19]. In people who have received prior BCG vaccination (the majority of the world's population) false positive results occur with all these tests, and false negative tests due to anergy or immune deficiency are widely recognized.
In previous work we have shown that the specificity of immune-based diagnosis can be improved by replacing PPD with an MTB-specific protein such as ESAT-6 [4]. In this investigation we used flow cytometry and the intracellular cytokine staining technique [17] to detect PPD and ESAT-6 specific CD4 T cells in a group of patients who presented with pneumonia at our hospital. Other studies that have investigated ESAT-6 specific T cell responses have generally concentrated on IFN-γ as the reporter protein [7,8]. In contrast to these reports, the study presented here examined a range of cytokine expression, including those associated with T helper type 2 responses interleukins-4 and -10, as well as the activation-related co-stimulatory molecule CD40L.
Using the ICC technique we were able to detect clearly responses to both PPD and ESAT-6 in the subjects we studied. With PPD stimulation it was found that there was no significant difference between the TB and non-TB groups in the overall CD4 responder frequencies for CD40L and the cytokines measured, and that all individuals tested had a positive response on the basis of the 0·01% responder frequency threshold. Even if the threshold was raised fivefold it was still unable to distinguish clearly between MTB and non-MTB infected subjects. This positive response in the normal and control groups is due most probably to the prior exposure to BCG, and reflects similar results to that found with the TST [2].
Unlike PPD, we found that ESAT-6 stimulation was able to distinguish the TB and non-TB populations studied, with only one of the eight controls and no normal subjects having a response above the 0·01% threshold, but all 16 TB patients having a positive response to at least one of the cytokines/CD40L measured. The specificity of this test was increased to 100% if only the IFN-γ and CD40L results were taken into consideration, but at a corresponding loss of sensitivity. Interestingly, the two TB patients who had no detectable IFN-γ and CD40L response to ESAT-6 did have clear IL-10 positivity. This could indicate in these patients that the immune response directed at the MTB infection was of a Th2 type, or at least was not the usual Th1 response expected with intracellular pathogen infection [13].
Our results are similar to other published studies that investigated the use of the ICC technique to enumerate TB antigen-specific CD4 T cells. Tesfa et al. compared normal donors and known TB patients using the ICC assay [10]. They found that all patients and 10 of 11 normal subjects had a positive IFN-γ CD4 T cell response to PPD as defined by the same 0·01% cut-off that we used. With ESAT-6 stimulation they found 13 of 15 of the TB patients had an IFN-γ-positive response while none of the normal subjects did. Unlike our study, they did not investigate other cytokines besides IL-2, which they found was of no greater sensitivity than IFN-γ, and did not use patients with non-TB pneumonia as a control group. Interestingly, two of the 15 patients they could not identify as ESAT-6 responders corresponds well with two of the 16 patients we detected who were IL-10-positive but negative for IFN-γ, indicating the advantage of using other markers besides IFN-γ for detecting CD4 responses. Sester et al. investigated PPD and ESAT-6 responses in normal subjects and haemodialysis patients using the whole-blood ICC technique [11]. They also found that IFN-γ responses could be detected in both groups, with the number of ESAT-6 responders less than PPD. Unlike our investigation, they did not try to correlate the ESAT-6 response with TB infection.
A single control patient in our study exhibited a high IL-10 response to ESAT-6 compared to the rest of the control group. One possible explanation for this result was that this subject was indeed infected with TB, although this seems unlikely, as we went to some effort to select controls without latent infection. A second explanation is that the IL-10 response to ESAT-6 is non-specific, but this is also unlikely in view of other evidence that immune responses to ESAT-6 are specific (although ESAT-6 analogues have been identified in some other species of mycobacteria, including M. kansasii[20]). We therefore attribute this finding to a technical limitation in our methodology as employed, but a true positive response due to exposure to MTB or another ESAT-6 containing mycobacterium cannot be excluded.
Studies have shown that a minority of patients with MTB infection have Th2 responses [16]. These patients are unlikely to be detected using strategies that rely on the measurement of Th1 cytokines such as IFN-γ. The current study suggests that these patients may be detected by assessing Th2 cytokine responses. In this study both IFN-γ and CD40L-negative patients were detected with the inclusion of these cytokines. However, one control was positive for IL-10 in response to ESAT-6. It is difficult to be certain of the true and false positive rates of ESAT-6-induced IL-10 with a study of this size, but the results do indicate that larger studies exploring the diagnostic value of Th2 cytokines, especially IL-10, should be considered. The investigation of additional markers such as the cell activation surface marker CD40L also allowed us to increase the sensitivity of the assay further. CD40L plays a role in a wide range of cellular signalling activities and interactions [21–23]. It performs similar functions to a cytokine but remains fixed to the cell surface. CD40L has also been described as a marker of T cell activation in an ICC study identifying T cells responding to CMV [24].
Using the results from this successful pilot study, there is considerable scope to streamline this method of diagnosing MTB infection. While positive responses were seen with all cytokines, we found that measurement of IL-4 and IL-2 responses were redundant and could be excluded from future study. The combination of CD40L and IFN-γ alone was highly sensitive and specific with ESAT-6 stimulation. Additional cytokines such as tumour necrosis factor (TNF)-α could also be measured to possibly increase the assay's usefulness. The addition of other MTB-specific proteins such as CFP10 have already demonstrated their usefulness in increasing sensitivity and specificity in the IFN-γ release assay [25] and so offer the same potential with the ICC assay presented here. It has been found that higher frequencies of TB-specific T cells exist in fluids at the sites of TB disease [26] and it may be possible to characterize further these cells with the ICC technique.
The flow cytometric technique was convenient and rapid and allowed us to test a range of cytokine responses simultaneously. It offers the potential for improved accuracy in diagnosis of MTB infection in individuals, especially those with positive TSTs due to past BCG vaccination, and may enable characterization of the immune response occurring during infection.
Acknowledgments
The authors thank Jim Rothel for supply of PPD, Peter Andersen for supply of the ESAT-6, Di Olden for help with initial testing and Elmer Villanueva for statistical analysis.
References
- 1.Dye C, Scheele S, Dolin P, et al. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. WHO Global Surveillance Monitoring Project. [DOI] [PubMed] [Google Scholar]
- 2.Huebner RE, Schein MF, Bass JB., Jr The tuberculin skin test. Clin Infect Dis. 1993;17:968–75. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 3.Vordermeier HM, Cockle PC, Whelan A, et al. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin Diagn Lab Immunol. 1999;6:675–82. doi: 10.1128/cdli.6.5.675-682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson PD, Stuart RL, Grayson ML, et al. Tuberculin-purified protein derivative-, MPT-64-, and ESAT-6-stimulated gamma interferon responses in medical students before and after Mycobacterium bovis BCG vaccination and in patients with tuberculosis. Clin Diagn Lab Immunol. 1999;6:934–7. doi: 10.1128/cdli.6.6.934-937.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravn P, Demissie A, Eguale T, et al. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999;179:637–45. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 6.Pathan AA, Wilkinson KA, Klenerman P, et al. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol. 2001;167:5217–25. doi: 10.4049/jimmunol.167.9.5217. [DOI] [PubMed] [Google Scholar]
- 7.Lalvani A, Pathan AA, McShane H, et al. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am J Respir Crit Care Med. 2001;163:824–8. doi: 10.1164/ajrccm.163.4.2009100. [DOI] [PubMed] [Google Scholar]
- 8.Arend SM, Engelhard AC, Groot G, et al. Tuberculin skin testing compared with T-cell responses to Mycobacterium tuberculosis-specific and non-specific antigens for detection of latent infection in persons with recent tuberculosis contact. Clin Diagn Lab Immunol. 2001;8:1089–96. doi: 10.1128/CDLI.8.6.1089-1096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalvani A, Pathan AA, Durkan H, et al. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specifc T cells. Lancet. 2001;357:2017–21. doi: 10.1016/S0140-6736(00)05115-1. [DOI] [PubMed] [Google Scholar]
- 10.Tesfa L, Koch FW, Pankow W, et al. Confirmation of Mycobacterium tuberculosis infection by flow cytometry after ex vivo incubation of peripheral blood T cells with an ESAT-6-derived peptide pool. Cytometry. 2004;60B:47–53. doi: 10.1002/cyto.b.20007. [DOI] [PubMed] [Google Scholar]
- 11.Sester M, Sester U, Clauer P, et al. Tuberculin skin testing underestimates a high prevalence of latent tuberculosis infection in hemodialysis patients. Kidney Int. 2004;65:1826–34. doi: 10.1111/j.1523-1755.2004.00586.x. [DOI] [PubMed] [Google Scholar]
- 12.Salgame P, Abrams JS, Clayberger C, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–82. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 13.Yamamura M, Uyemura K, Deans RJ, et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 14.Gooding TM, Johnson PD, Campbell DE, et al. Immune response to infection with Mycobacterium ulcerans. Infect Immun. 2001;69:1704–7. doi: 10.1128/IAI.69.3.1704-1707.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orme IM, Roberts AD, Griffin JP, et al. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–25. [PubMed] [Google Scholar]
- 16.Surcel HM, Troye-Blomberg M, Paulie S, et al. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Suni MA, Picker LJ, Maino VC. Detection of antigen-specific T cell cytokine expression in whole blood by flow cytometry. J Immunol Meth. 1998;212:89–98. doi: 10.1016/s0022-1759(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 18.Pottumarthy S, Morris AJ, Harrison AC, et al. Evaluation of the tuberculin gamma interferon assay: potential to replace the Mantoux skin test. J Clin Microbiol. 1999;37:3229–32. doi: 10.1128/jcm.37.10.3229-3232.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Streeton JA, Desem N, Jones SL. Sensitivity and specificity of a gamma interferon blood test for tuberculosis infection. Int J Tuberc Lung Dis. 1998;2:443–50. [PubMed] [Google Scholar]
- 20.Arend SM, van Meijgaarden KE, de Boer K, et al. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J Infect Dis. 2002;186:1797–807. doi: 10.1086/345760. [DOI] [PubMed] [Google Scholar]
- 21.Howland KC, Ausubel LJ, London CA, et al. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J Immunol. 2000;164:4465–70. doi: 10.4049/jimmunol.164.9.4465. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T, Rao SP, Meylan PR, et al. Role of CD40 ligand in Mycobacterium avium infection. Infect Immun. 1999;67:3558–65. doi: 10.1128/iai.67.7.3558-3565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campos-Neto A, Ovendale P, Bement T, et al. CD40 ligand is not essential for the development of cell-mediated immunity and resistance to Mycobacterium tuberculosis. J Immunol. 1998;160:2037–41. [PubMed] [Google Scholar]
- 24.Rentenaar RJ, Gamadia LE, van DerHoek N, et al. Development of virus-specific CD4(+) T cells during primary cytomegalovirus infection. J Clin Invest. 2000;105:541–8. doi: 10.1172/JCI8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arend SM, Ottenhoff TH, Andersen P, et al. Uncommon presentations of tuberculosis: the potential value of a novel diagnostic assay based on the Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10. Int J Tuberc Lung Dis. 2001;5:680–6. [PubMed] [Google Scholar]
- 26.Wilkinson KA, Wilkinson RJ, Pathan A, et al. Ex vivo characterization of early secretory antigenic target 6-specific T cells at sites of active disease in pleural tuberculosis. Clin Infect Dis. 2005;40:184–7. doi: 10.1086/426139. [DOI] [PubMed] [Google Scholar]