Abstract
Type 1 IFN is thought to be implicated in the autoimmune process of SLE. Plasmacytoid dendric cells (DC), which are natural IFN-α producing cells, play a pivotal epipathogenic role in SLE. The present study was undertaken to investigate the phenotypic characteristics of peripheral blood DC in SLE patients in comparison with those of healthy controls. Samples from 20 SLE patients and 18 healthy controls were studied. Three-colour flow cytometry was performed to identify myeloid DC, as CD11c+ lineage marker−, and HLA-DR+ cells and plasmacytoid DC, as BDCA-2+ linage marker−, and HLA-DR+ cells. We used the whole blood ‘lyse/no-wash’ procedure, which allows precise counting of peripheral blood DC. BDCA-2+ plasmacytoid DC and CD11c+ myeloid DC were reduced in SLE patients compared with controls. Similarly, BDCA-3+ DC were reduced in SLE patients. These results indicated that SLE patients had a reduced number of both BDCA-2+ plasmacytoid DC and CD11c+ myeloid DC. These alternations of the DC subset may drive the autoimmune response in SLE.
Introduction
SLE is a multisystem autoimmune disease characterized by the production of auto-antibody against DNA and nucleosome [1]. Although the pathogenesis of the disease is largely unknown, both genetic and environmental factors are involved in this disease [2]. The first documented cytokine abnormality of the immune system in SLE patients was increased serum levels of IFN-α[3,4]. The levels of IFN-α were correlated with disease activity, and it was suggested that IFN-α is involved in the SLE disease process [5]. The observation that IFN-α treatment of patients with nonautoimmune disease may cause the development of antinuclear antibody (ANA), anti-dsDNA and, occasionally, an SLE syndrome also suggests that IFN-α may play a pivotal role in initiation of the autoimmune process in SLE [6,7].
Dendritic cells (DC) are bone marrow-derived antigen-presenting cells (APC) that have an important role in the induction of immunity and maintenance of tolerance against self antigens [8]. DC lack certain lineage (Lin) specific markers and express high levels of MHC class II molecules; thus, phenotypic definition of DC relies on Lin− HLR-DR+ cells [9,10]. Their relatively low frequency in leucocyte preparations has hampered their investigation. Recently, at least two distinct human DC subsets, myeloid DC and plasmacytoid DC, have been characterized [11]. Recent studies with purified precursor of plasmacytoid DC have demonstrated that natural IFN-α producing cells (NIPC) share the phenotype of plasmacytoid DC (PDCs) [12,13]. It was reported that SLE patients have a reduced number of functionally normal NIPC in the peripheral blood [14].
The identification and enumeration of the DC subset among circulating PBL is challenging, because these cells cannot be positively identified by a specific known marker. Recently, Dzionek et al. [15] identified a novel marker for PDCs, BDCA-2, which enables direct identification of PDCs in blood.
In this study, we investigated the populations of plasmacytoid DC (BDCA-2+) and myeloid DC in the peripheral blood of SLE patients using a new ‘lyse no-wash’ method that allows direct DC counting without conventional gradient-density separation procedures.
Patients and methods
Patients and controls
A total of 20 patients with SLE (2 males and 18 females, age; 39·1 ± 12·6 years) were enrolled in the study (Table 1), in addition to 18 healthy volunteers (3 males and 15 females, age 37·7 ± 8·1 years) and 12 rheumatoid arthritis patients (2 males and 10 females, age 50·9 ± 11·4 years) as controls. Consecutive patients entering the rheumatology clinic who fulfilled the American College of Rheumatology 1987 revised classification criteria for SLE were selected for this investigation. Among the 20 patients, 15 received prednisolone as monotherapy (mean dosage 16·5 mg/day, range 7·5–60 mg/day). The remaining 4 patients were treated with both prednisolone (mean dosage 21·1 mg/day, range 7–50 mg/day) and cyclophosphamide (intermittent intravenous cyclophosphamide therapy) and one patient received predonisolone (5 mg/day) and azathioprine (50 mg/day). Disease activity was scored in all SLE patients by a SLE Disease Activity Index (SLEDAI) [16]. The study protocol was approved by the Ethics Committees of both NHO Nagasaki Medical Centre and informed consent was obtained from each of the individuals.
Table 1. Data on the individual SLE patients.
| Patient no. | BDCA-2+ DC (%) | CD11c+ DC (%) | BDCA-3+ DC (%) | Leukocyte (×106/l) | Lymphocyte (×106/l) | Age (years) | SLEDI† | Anti-dsDNA‡ (IU/ml) | CH50 (IU/ml) | Prednisolone (mg/day) | Cyclophosphamide | Azathioprine | Serum IFN-α (U/ml) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.174 | 0.067 | 0.0095 | 4500 | 1161 | 49 | 6 | 141 | 30 | 25 | 15.1 | ||
| 2 | 0.511 | 0.171 | 0.0126 | 4400 | 1030 | 25 | 3 | 5 | 47 | 9 | |||
| 3 | 0.062 | 0.049 | 0.011 | 9800 | 1147 | 54 | 10 | 4 | 37 | 7.5 | 17.1 | ||
| 4 | 0.063 | 0.057 | 0.023 | 6400 | 902 | 27 | 4 | 26 | 30 | 11 | 13.3 | ||
| 5 | 0.078 | 0.120 | 0.0106 | 5900 | 873 | 55 | 6 | 14 | 36 | 10 | 32.1 | ||
| 6 | 0.591 | 0.146 | 0.044 | 5300 | 2094 | 29 | 9 | 17 | 12 | 14 | 15.8 | ||
| 7 | 0.125 | 0.316 | 0.029 | 4100 | 906 | 19 | 5 | 5 | 36 | 5 | + | 23.2 | |
| 8 | 0.169 | 0.197 | 0.0064 | 6700 | 791 | 27 | 8 | 21 | 29 | 10 | |||
| 9 | 0.129 | 0.120 | 0.0163 | 6400 | 1114 | 41 | 4 | 5 | 32 | 7.5 | 20.9 | ||
| 10 | 0.315 | 0.065 | 0.0109 | 7700 | 1217 | 24 | 14 | 30 | 15 | 30 | 13.1 | ||
| 11 | 0.055 | 0.321 | 0.0388 | 6500 | 527 | 57 | 4 | 5 | 38 | 7 | + | 11.4 | |
| 12 | 0.033 | 0.127 | 0.0076 | 6900 | 428 | 45 | 12 | 33 | 25 | 12.5 | + | 21.7 | |
| 13 | 0.0065 | 0.124 | 0.0082 | 4400 | 818 | 25 | 16 | 14 | 26 | 60 | 13.4 | ||
| 14 | 0.126 | 0.09 | 0.0072 | 7800 | 1630 | 33 | 12 | 19 | 23 | 50 | + | 19.2 | |
| 15 | 0.183 | 0.391 | 0.0347 | 4200 | 521 | 42 | 5 | 5 | 31 | 17.5 | 25.2 | ||
| 16 | 0.0807 | 0.208 | 0.0084 | 9200 | 984 | 56 | 8 | 16 | 38 | 19 | 35.3 | ||
| 17 | 0.373 | 0.25 | 0.0329 | 5800 | 2610 | 34 | 6 | 16 | 30 | 13 | |||
| 18 | 0.111 | 0.195 | 0.0371 | 4100 | 1701 | 38 | 2 | 17 | 38 | 5 | 37.0 | ||
| 19 | 0.132 | 0.141 | 0.013 | 11000 | 759 | 55 | 10 | 32 | 41 | 15 | + | 16.1 | |
| 20 | 0.0848 | 0.302 | 0.00991 | 7700 | 200 | 47 | 4 | 5 | 26 | 9 |
SLEDAI, systemic lupus erythematosus disease activity index;
anti-dsDNA, anti-double-stranded DNA (normal 0.0-7.0 IU/ml).
Reagents
Fluorescein isothiocyanate (FITC)-conjugated lineage cocktail (CD3-FITC, CD14-FITC, CD16-FITC, CD19-FITC, CD20-FITC, CD56-FITC) was purchased from Beckton Dickinson (BDIS, Erembodegem, Belgium). Phycoerythrin (PE)-conjugated CD11c and PC-5-conjugated anti-HLA-DR were purchased from Coulter (Fullerton, CA, USA). PE-conjugated BDCA-2 and BDCA-3 were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany).
Flow cytometry
The Upham method was performed as described previously [17], with minor modifications. In brief, 200 µl of blood sample was stained with 20 µl of FITC-conjugated antibody cocktail (lineage marker), PC5-conjugated anti-HLA-DR, and PE-conjugated DC markers (anti-CD11c, anti-BDCA-2, anti-BDCA-3) for 15 min at room temperature in the dark, with the proper isotype controls in parallel. RBC lysis was done with 2·0 ml of FACS lysing solution (BDIS) for 15 min at room temperature. After one wash, the cells were resuspended in 400 µl of fixation buffer (BDIS), stored at 4 °C in the dark, and analysed within 24 h by flow cytometry. For the protocol proposed by BDIS, samples were stained with lineage-specific antibodies (CD3-FITC, CD14-FITC, CD16-FITC, CD19-FITC, CD20-FITC, CD56-FITC), CD11c-PE, and anti-HLA-DR-PC5, and processed strictly as prescribed by the manufacturer. Samples were analysed on an EPCS XL (Coulter, Fullerton, CA, USA) flow cytometer with 488-nm argon laser and ADC software (Coulter). Total leucocyte and differential white cell counts were determined on a separate sample with a Sysmex SF-3000 automated haematology analyser (Toa Medical Electronics, Kobe, Japan).
Serum IFN-α measurement
The serum was stored at −30°C until use. The serum IFN-α levels were then measured using a sensitive sandwich enzyme-linked immunosorbent assay (ELISA) kit (PBL Biomedical Laboratories, NJ, USA). All measurements were made in duplicate, and the average values were used in the statistical analyses.
Statistical analysis
Differences between groups were analysed using the Mann–Whitney U-test. Correlation analyses were performed using Spearman's rank correlation test. All analysis was performed using Statview 5·0 software (SAS Institute, Cary, NC, USA).
Results
Flow cytometric profiles of DCs and absolute DC counts
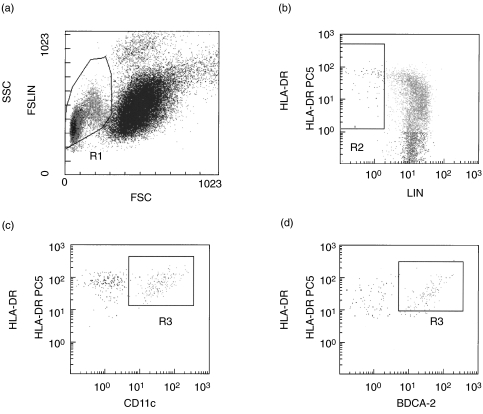
The numbers of CD11c+ (myeloid) and BDCA-2+ (plasmacytoid) DC were assessed by flow cytometry (Fig. 1). The R1 gate (Fig. 1a) containing lymphocytes and monocytes was combined with an R2 gate (Fig. 1b) excluding cells strongly positive for lineage-specific molecules (CD3, CD14, CD19, CD16). Therefore, the HLA-DR+ lin− populations (R2) contained plasmacytoid DC and myeloid DC. Among these DC populations, cells expressing CD11c (Fig. 1c) were characterized as myeloid DC, whereas the cells expressing BDCA-2 (Fig. 1d) were characterized as plasmacytoid DC. Also, BDCA-3+ DC were determined from R3 lesions. Absolute DC counts were calculated using the percentage of DC in R3 lesions and the mononuclear cell count determined by the automated haematology blood analysis.
Fig. 1.
Gating strategy used to define BDCA-2+ and CD11c+ DCs by flowcytometry. Peripheral blood mononuclear cells were selected in the R1 gate excluding debris and polynuclear cells (a). Lineage-negative and HLA-DR-positive cells were selected in the R2 gate (b). In this combined R1 and R2 gate, CD11c+, HLA-DR+ (c)(R3) or BDCA-2+, HLA-DR+ (d)(R3) events were quantified.
C11c+, BDCA-2+ and BDCA-3+ DC numbers are decreased in SLE patients
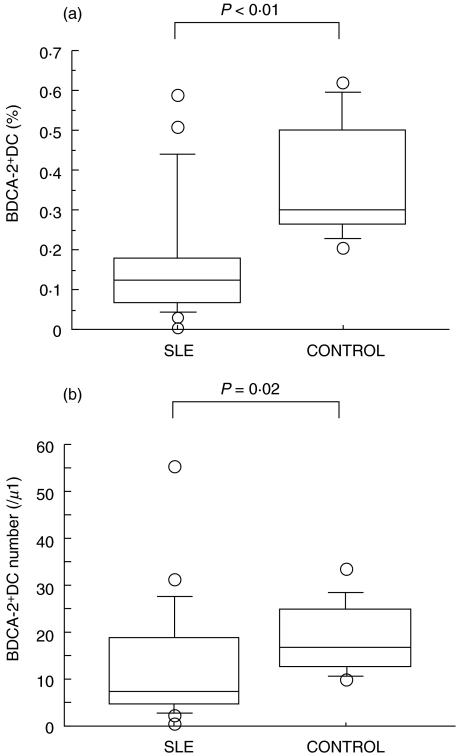
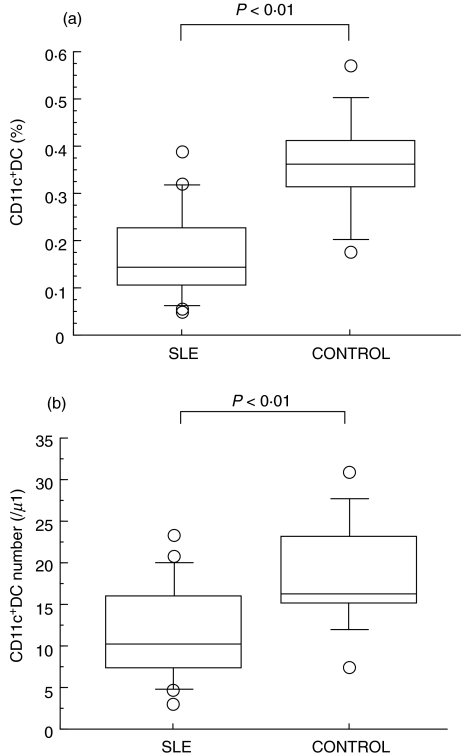
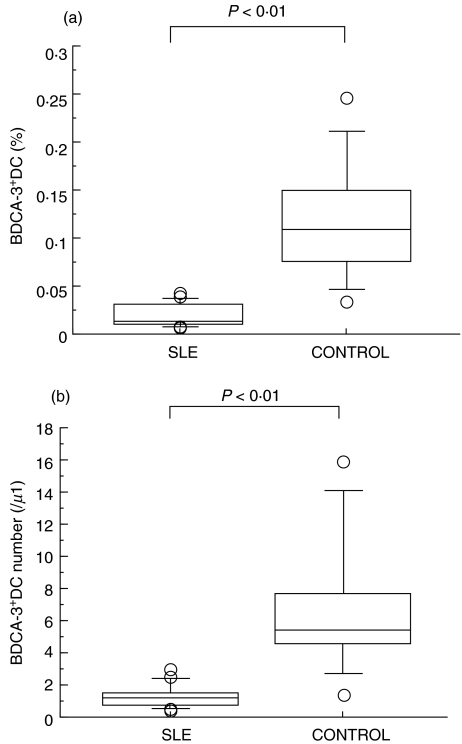
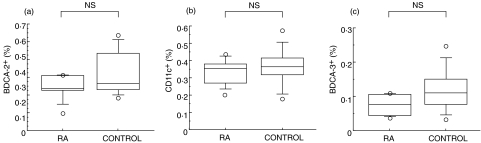
We evaluated the percentage of BDCA-2+ DC, identified as a Lin−HLA-DR+ BDCA-2+ population, in SLE patients and healthy controls. As shown in Fig. 2a, the percentage of BDCA-2+ DC was significantly reduced in SLE patients compared to those of healthy controls (0·170 ± 0·158% in SLE versus 0·369 ± 0·148% in control). Indeed, the absolute number of BDCA-2+ DC was decreased in SLE patients (Fig. 2b). As shown in Fig. 3a, the percentage of CD11c+ myeloid DC was also reduced in SLE patients compared to those of healthy controls (0·173 ± 0·099% in SLE versus 0·359 ± 0·109% in control). In addition as shown in Fig. 4, the percentage of BDCA-3+ DCs was greatly decreased in SLE patients (0·019 ± 0·013% in SLE versus 0·119 ± 0·063% in control). In contrast, these reduced frequencies of DC subsets were not observed in the patients with RA, another autoimmune disease (Fig. 5).
Fig. 2.
(a) Blood BDCA-2+ DC percentage in SLE patients and healthy controls. Percentages of BDCA-2+ DC were analysed by flow cytometry as shown in Fig. 1. Boxes show the 25th and 75th percentiles. Horizontal lines within the boxes show the median. Bars above and below the boxes show the 10th and 90th percentiles. Significance levels for differences between groups were analysed by the Mann–Whitney U-test. (b) Blood BDCA-2+ DC absolute numbers in SLE patients and healthy controls. Absolute numbers of BDCA-2+ DC were calculated using the percentage of BDCA-2+ DC and the mononuclear cell count determined by the automated haematology blood analysis. Boxes show the 25th and 75th percentiles. Horizontal lines within the boxes show the median. Bars above and below the boxes show the 10th and 90th percentiles. Significance levels for differences between groups were analysed by the Mann–Whitney U-test.
Fig. 3.
(a) Blood CD11c+ DC percentage in SLE patients and healthy controls. Percentages of CD11c+ DC were analysed by flow cytometry as shown in Fig. 1. Boxes show the 25th and 75th percentiles. Horizontal lines within the boxes show the median. Bars above and below the boxes show the 10th and 90th percentiles. Significance levels for differences between groups were analysed by the Mann–Whitney U-test. (b) Blood CD11c+ DC absolute numbers in SLE patients and healthy controls. Absolute numbers of CD11c+ DC were calculated using the percentage of CD11c+ DC and the mononuclear cell count determined by the automated haematology blood analysis. Boxes show the 25th and 75th percentiles. Horizontal lines within the boxes show the median. Bars above and below the boxes show the 10th and 90th percentiles. Significance levels for differences between groups were analysed by the Mann–Whitney U-test.
Fig. 4.
(a) Blood BDCA-3+ DC percentage in SLE patients and healthy controls. Percentages of BDCA-3+ DC were analysed by flow cytometry as shown in Fig. 1. Boxes show the 25th and 75th percentiles. Horizontal lines within the boxes show the median. Bars above and below the boxes show the 10th and 90th percentiles. Significance levels for differences between groups were analysed by the Mann–Whitney U-test. (b) Blood BDCA-3+ DC absolute numbers in SLE patients and healthy controls. Absolute numbers of BDCA-3+ DC were calculated using the percentage of BDCA-3+ DC and the mononuclear cell count determined by the automated haematology blood analysis. Boxes show the 25th and 75th percentiles. Horizontal lines within the boxes show the median. Bars above and below the boxes show the 10th and 90th percentiles. Significance levels for differences between groups were analysed by the Mann–Whitney U-test.
Fig. 5.
Blood DC percentages in RA patients and healthy controls.Percentages of (a) BDCA-2+, (b) CD11c+ and (c) BDCA-3+ DC were analysed by flow cytometry as shown in Fig. 1. Boxes show the 25th and 75th percentiles. Horizontal lines within the boxes show the median. Bars above and below the boxes show the 10th and 90th percentiles. Significance levels for differences between groups were analysed by the Mann–Whitney U-test.
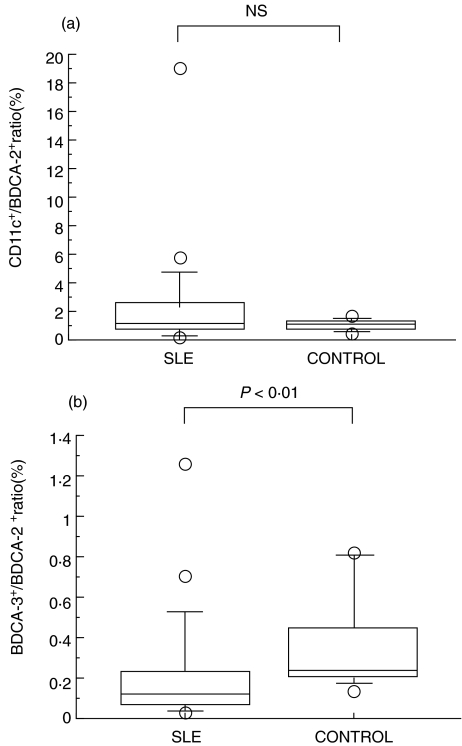
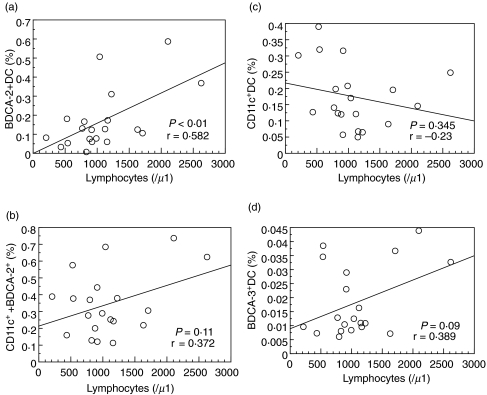
The CD11c+:BDCA-2+ ratio was not significantly different between SLE and healthy control groups (Fig. 6a), whereas the ratio of BDCA-3+:BDCA-2+ was significantly reduced in SLE patients in comparison with healthy controls (Fig. 6b). We evaluated the correlations of the proportions of each DC subset with clinical parameters. As shown in Fig. 7a, a positive correlation was found between percentage of BDCA-2+ DC and the peripheral blood lymphocyte counts (r = 0·582 P < 0·01). However, there was no significant correlation between the peripheral blood lymphocyte counts and total DC (Fig. 7b, BDCA-2+ plus CD11c+ DC), myeloid DC (Fig. 7c, CD11c+ DC), or BDCA-3+ DC (Fig. 7d).
Fig. 6.
(a) The CD11c+: BDCA-2+ DC ratio in SLE patients (n = 20) and healthy controls (n = 18). (b) The BDCA-3+: BDCA-2+ DC ratio in SLE patients (n = 20) and healthy controls (n = 18).
Fig. 7.
(a) Correlation between BDCA-2+ DC and peripheral blood lymphocyte counts. The percentages of BDCA-2+ DC were determined by flow cytometry. Peripheral blood lymphocyte counts were determined by the automated haematology blood analysis. (b) Correlation between BDCA-2+ plus CD11c+ DC and peripheral blood lymphocyte counts. (c) Correlation between CD11c+ DC and peripheral blood lymphocyte counts. (d) Correlation between BDCA-3+ DC and peripheral blood lymphocyte counts.
Discussion
Dendritic cells (DC) have been shown to represent the most potent antigen-presenting cell (APC) and to be crucial for activation of naive T cells, and therefore may play a key role in triggering the induction of an autoimmune response [8]. It has been recently hypothesized that an imbalance of dendritic cell subsets could be responsible for the pathogenesis of autoimuune disease [18]. However, the relatively low frequency of DC in leucocyte preparations has hampered their investigation.
Two distinct DC populations within blood and lymphoid organs have been identified [11]. Both populations are distinguished from other lymphoid and myeloid cells by their high levels of HLA-DR expression and lack of lineage marker (CD3, CD19, CD14, and CD16) expression. CD11c+ DC (also termed DC1, myeloid) differ considerably from CD11c− DC (also termed DC2, plasmacytoid DC) in their maturation requirements, cytokine production, and functional capacities [9,10].
Previous reports have demonstrated that the frequencies of DC in peripheral blood are consistently reduced in SLE patients as compared with healthy controls [19]. However, conflicting data concerning the frequency of DC1 and DC2 have been reported. Scheinecker et al. [20] demonstrated that the CD11c− DC subset was almost normal and CD11c+ DCs were reduced by 80% in SLE patients. In contrast, Blanco et al. [21] reported that CD123+ CD11c− plasmacytoid DC population was reduced in paediatric SLE patients. The identification of DC1 or DC2 among circulating peripheral blood leucocytes is challenging, because these rare cells cannot be positively identified by one or two specific markers. To resolve the variety and complexity of published procedures to determine DC counts, we measured circulating DC1 and DC2 directly in fresh blood by ‘lyse/no-wash’ flow cytometry protocol to eliminate the potential cell loss during the washing steps [22]. Further, a new surface marker for DC (BDCA-2 and BDCA-3 mAb) has been developed [15]. We investigated the population of DC1 (CD11c+) and DC2 (BDCA-2+) in peripheral blood of SLE patients.
The data presented here show a profound reduction of BDCA-2+ DC2 number in SLE patients. These findings are in line with the recent observation that IFN-α-producing BDCA-2 positive cells, which were identified by double staining IFN-α and BDCA-2, were decreased in SLE patients [23]. The BDCA-2+ PDCs are reduced in number in the blood of SLE patients, although increased plasmacytoid DC were demonstrated in the pathogenic skin lesions, suggesting a general recruitment of plasmacytoid DC to tissues in SLE patients [24]. In our data, a reduced number of peripheral blood lymphocytes was correlated with the frequency of BDCA-2+ DC in SLE patients. However, there was no significant correlation between lymphocyte counts and CD11c+, BDCA-3+, or total (CD11c+ plus BDCA-2+) DCs. Our results are inconsistent with the previous report demonstrating the positive correlation between the total lymphocyte counts and the frequency of lineage marker−, HLA-DR+, CD4+ DC [20]. Although lineage marker−, HLA-DR+, CD4+ DC population may represent the total DC [20], this population may not completely equal to the CD11c+ plus BDCA-2+ DC subset. Mature plasmacytoid DC are capable of activating T cells and promoting B cells’ maturation differentiation [25]. Although we did not determine the functions, such as IFN-α production, in plasmacytoid DC, the above results would suggest that plasmacytoid DC reduction might fail to promote T- and B-lymphocytes differentiation.
Our finding of decreased numbers of CD11c+ DC1 in peripheral blood of SLE patients is in agreement with those of Scheinecker et al. [20]. The authors reported an 80% reduction of the CD11c+ DC subset, in contrast to almost normal frequency of CD11c− DC [20]. Furthermore, they demonstrated the impaired T cell-stimulating capacity of DC-enrich antigen presenting cells (APC) in SLE patients as compared to those of healthy controls [20]. Although we did not perform the functional analysis, the reduced CD11c+ myeloid DC could be implicated in these impaired DC functions. Alternatively, the observed reduction of circulating DC in SLE patients could be explained by a redistribution into the sites of active disease as previously reported [26].
In our data, the ratio of BDCA-2+ DC/CD11c+ DC in SLE patients was not significantly different compared to that in healthy controls suggesting numerical decreases in both the DC1 and DC2 components of peripheral blood of SLE patients. However, the BDCA-3+ DC subset was more profoundly reduced than was BDCA-2+ DC2 in SLE patients, given that the ratio of BDCA-3+ DC/BDCA-2+ DC was significantly lower in SLE than in healthy controls.
It became obvious that two myeloid-derived DC populations (CD11c+ BDCA-3−, CD11c− BDCA-3+) are present in human blood [27]. Recently, it has been hypothesized that unabated DC differentiation leads to activation of auto-reactive T cells [28]. IFN-α induces the activation of mature DC, and the increase of mature DC and the decrease of immature DC leads to the activation of auto-reactive T cells [29]. The BDCA-3+ DC are considered to be a subpopulation of CD11c+ myeloid DC, and they share the immunophenotypic features with CD11c+ myeloid DC [27]. However, the lack of Fc receptor expression indicates that BDCA-3+ DC do not have the capability of Ig-mediated Ag uptake [15,30]. Although the differentiation phenotype of BDCA-3+ DC is unclear, it is possible that the reduction of BDCA-3+ DC could be related to the inappropriate DC state in SLE patients.
In summary, we have shown that SLE patients are characterized by a decrease in both BDCA-2+ DC2 and CD11c+ DC1. Although these diminished DC subpopulations were not found to be related to SLE disease activity, this feature appears to be involved in the immunological abnormality of SLE patients.
References
- 1.Liossis SN, Vassilopoulos D, Kovacs B, Tsokos GC. Immune cell biochemical abnormalities in systemic lupus erythematosus. Clin Exp Rheumatol. 1997;15:677–84. [PubMed] [Google Scholar]
- 2.Ruiz-Irastorza G, Khamashta MA, Castellino G, Hughes GR. Systemic lupus erythematosus. Lancet. 2001;357:1027–32. doi: 10.1016/S0140-6736(00)04239-2. [DOI] [PubMed] [Google Scholar]
- 3.Ronnblom L, Alm GV. An etiopathogenic role for the type I IFN system in SLE. Trends Immunol. 2001;22:427–31. doi: 10.1016/s1471-4906(01)01955-x. [DOI] [PubMed] [Google Scholar]
- 4.Ronnblom L, Alm GV. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J Exp Med. 2001;194:59–63. doi: 10.1084/jem.194.12.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ytterberg SR, Schnitzer TJ. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum. 1982;25:401–6. doi: 10.1002/art.1780250407. [DOI] [PubMed] [Google Scholar]
- 6.Ehrenstein MR, McSweeney E, Swane M, Worman CP, Goldstone AH. Isenberg DA. Appearance of anti-DNA antibodies in patients treated with interferon-alpha. Arthritis Rheum. 1993;36:279–80. doi: 10.1002/art.1780360224. [DOI] [PubMed] [Google Scholar]
- 7.Ronnblom LE, Alm GV, Oberg KE. Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. J Intern Med. 1990;227:207–10. doi: 10.1111/j.1365-2796.1990.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 9.Mohty M, Jarrossay D, Lafage-Pochitaloff M, et al. Circulating blood dendritic cells from myeloid leukemia patients display quantitative and cytogenetic abnormalities as well as functional impairment. Blood. 2001;98:3750–6. doi: 10.1182/blood.v98.13.3750. [DOI] [PubMed] [Google Scholar]
- 10.Pacanowski J, Kahi S, Baillet M, et al. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98:3016–21. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Vandenabeele S, Georgopoulos K. Derivation of dendritic cells from myeloid and lymphoid precursors. Int Rev Immunol. 2001;20:117–35. doi: 10.3109/08830180109056726. [DOI] [PubMed] [Google Scholar]
- 12.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 13.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 14.Ronnblom L, Alm GV. The natural interferon-alpha producing cells in systemic lupus erythematosus. Hum Immunol. 2002;63:1181–93. doi: 10.1016/s0198-8859(02)00757-7. [DOI] [PubMed] [Google Scholar]
- 15.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 16.Urowitz MB, Gladman DD. Measures of disease activity and damage in SLE. Baillieres Clin Rheumatol. 1998;12:405–13. doi: 10.1016/s0950-3579(98)80027-7. [DOI] [PubMed] [Google Scholar]
- 17.Upham JW, Lundahl J, Liang H, Denburg JA, O'Byrne PM, Snider DP. Simplified quantitation of myeloid dendritic cells in peripheral blood using flow cytometry. Cytometry. 2000;40:50–9. doi: 10.1002/(sici)1097-0320(20000501)40:1<50::aid-cyto7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 19.Gill MA, Blanco P, Arce E, Pascual V, Banchereau J, Palucka AK. Blood dendritic cells and DC-poietins in systemic lupus erythematosus. Hum Immunol. 2002;63:1172–80. doi: 10.1016/s0198-8859(02)00756-5. [DOI] [PubMed] [Google Scholar]
- 20.Scheinecker C, Zwolfer B, Koller M, Manner G, Smolen JS. Alterations of dendritic cells in systemic lupus erythematosus: phenotypic and functional deficiencies. Arthritis Rheum. 2001;44:856–65. doi: 10.1002/1529-0131(200104)44:4<856::AID-ANR142>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 21.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–3. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 22.Ma L, Scheers W, Vandenberghe P. A flow cytometric method for determination of absolute counts of myeloid precursor dendritic cells in peripheral blood. J Immunol Meth. 2004;285:215–21. doi: 10.1016/j.jim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Blomberg S, Eloranta ML, Magnusson M, Alm GV, Ronnblom L. Expression of the markers BDCA-2 and BDCA-4 and production of interferon-alpha by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Rheum. 2003;48:2524–32. doi: 10.1002/art.11225. [DOI] [PubMed] [Google Scholar]
- 24.Blomberg S, Eloranta ML, Cederblad B, Nordlin K, Alm GV, Ronnblom L. Presence of cutaneous interferon-alpha producing cells in patients with systemic lupus erythematosus. Lupus. 2001;10:484–90. doi: 10.1191/096120301678416042. [DOI] [PubMed] [Google Scholar]
- 25.Shreedhar V, Moodycliffe AM, Ullrich SE, Bucana C, Kripke ML, Flores-Romo L. Dendritic cells require T cells for functional maturation in vivo. Immunity. 1999;11:625–36. doi: 10.1016/s1074-7613(00)80137-5. [DOI] [PubMed] [Google Scholar]
- 26.Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon-alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237–43. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabbe S, Kampgen E, Schuler G. Dendritic cells: multi-lineal and multi-functional. Immunol Today. 2000;21:431–3. doi: 10.1016/s0167-5699(00)01694-7. [DOI] [PubMed] [Google Scholar]
- 28.Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–50. doi: 10.1016/s1074-7613(04)00108-6. [DOI] [PubMed] [Google Scholar]
- 29.Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–53. [PubMed] [Google Scholar]
- 30.Kwakkenbos MJ, Chang GW, Lin HH, Pouwels W, de Jong EC, van Lier RA, Gordon S, Hamann J. The human EGF-TM7 family member EMR2 is a heterodimeric receptor expressed on myeloid cells. J Leukoc Biol. 2002;71:854–62. [PubMed] [Google Scholar]