Abstract
The objective of this study was to detect autoantibodies against granzyme B cleavage products in sera from patients with primary Sjögren's syndrome (SS). Cell lysates derived from human salivary gland (HSG) cell lines were incubated with granzyme B. The susceptibility to the generation of cleavage fragments of SS autoantigens was assayed by immunoblotting using sera from 57 primary SS patients, 17 primary SS patients with malignant lymphoma (ML), 28 systemic lupus erythematosus (SLE) patients, and 20 healthy controls. A 27 kD protein was recognized by serum autoantibodies in 8 (14·0%) of 57 primary SS patients, 5 (29·4%) of 17 SS patients with ML, 2 (7·1%) of 28 SLE patients, but not in 20 normal subjects. This protein was recognized by anti-SSB (La) monoclonal antibodies. Granzyme B-treated recombinant La protein was also shown to migrate as a discrete 27 kD protein by SDS PAGE. Blocking studies demonstrated the existence of an apoptosis-specific B cell epitope present in sera from 2 of 8 primary SS patients and in 2 of 5 primary SS patients with ML which recognized the 27 kD protein. Granzyme B-induced La fragments are generated during cytotoxicity in vitro. This is the first report describing autoantibodies in sera from primary SS patients that specifically recognize fragments of the La protein that are produced by the granzyme B protease.
Keywords: apoptosis, autoantibody, granzyme B, La (SS–B), Sjögren's syndrome, malignant lymphoma
Introduction
Primary Sjögren's syndrome (SS) is an autoimmune disease defined by keratoconjunctivitis sicca (dry eye), xerostomia (dry mouth) and other extraglandular abnormalities. One of the unique immunological manifestations of SS is a polyclonal activation of lymphocytes resulting in hypergammaglobulinemia and autoantibody production, including anti-SS-A (Ro) and anti-SS-B (La) antibodies [1,2]. In the salivary glands from SS patients, lymphocytes including T and B cells can be seen infiltrating acinar and ductal epithelial cells. Many autoantibody- producing cells are present within glands [3], suggesting that the immunological responses may occur in target tissues.
Studies during the last decade suggest that apoptotic processes may provide a source of autoantigen [4–6]. After induction of apoptotic stimuli, autoantigens are redistributed in apoptotic cells, for example, into small ‘blebs’ containing Ro 52, Fodrin, and Golgins, and into ‘apoptotic bodies’ containing Ro 60, La, Ku/DNA-PK, PARP, and NuMA [7]. It is thought that these autoantigens are efficiently presented to macrophages and dendritic cells, leading to the initiation of inflammatory immune responses. Cryptic epitopes produced during apoptosis are candidates for antigens which are recognized by autoantibodies. One of the main enzymes known to recognize autoantigens as substrates is caspase 3, which is a final effector molecule for apoptosis [8]. However, caspase 3 is expressed in a wide variety of cells, suggesting that the immune system should already be tolerant to many caspase 3 substrates.
Granzyme B is a serine protease found in the lytic granules of cytotoxic T-lymphocytes (CTL) and NK cells. When granzyme B and perforin are secreted into the interspace between the cytotoxic cell and the target cell, granzyme B finds its way into the nucleus and cytoplasm of the target cell, where it triggers apoptotic cell death [9–11]. As apoptotic epithelial cells and CTL containing granzyme B have been detected in the salivary glands from SS patients [12], it is possible that CTL may induce apoptosis in epithelial cells in a perforin/granzyme B dependent manner, leading to autoimmune destruction of glands. As many autoantigens are known to be cleaved by granzyme B at sites that generate a different pattern of proteolytic fragments than seen with caspase 3-mediated cell death [5,13], we speculated that autoantibodies that specifically recognize granzyme B autoantigen cleavage fragments are produced in the salivary glands and can be detected in sera from SS patients. In the present study, we screened sera for their ability to recognize cleavage fragments produced by granzyme B, using as substrates cell lines derived from salivary glands exposed to this protease.
We report the discovery of granzyme B-generated proteolytic fragments of the prominent SS autoantigen La (SS-B) as targets of the immune response. Implications of these findings to our understanding of SS pathogenesis will be discussed.
Materials and methods
Patients and controls
We studied 57 patients diagnosed as having primary SS (55 females, 2 males; mean age 55·6 years, range 19–79 years), 17 primary SS with ML (13 females, 4 males; mean age 68·9 years, range 53–85 years) and 28 SLE (27 females, 1 male; mean age 37·7years, range 16–67 years), as well as 20 healthy controls. Classification of the 17 ML patients in Ann Arbor stages were as follows: pathological findings were all non-Hodgkin lymphoma (8 diffuse large B-cell lymphoma, 4 marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type, 2 mantle cell lymphoma, 2 angioimmunoblastic T-cell lymphoma, 1 peripheral T-cell lymphoma), 5 patients were found to be stage I, 1 stage II, 3 stage III and 8 stage IV. All patients were followed at our out-patient rheumatology facilities at the Nagasaki University Hospital and Kanazawa Medical University Hospital. Healthy control subjects were recruited from the medical staff. The protocol was approved by Nagasaki University Ethics Committee, and informed consent was obtained from all patients.
Antibodies and reagents
Anti-La mAb (SW5) was kindly provided by Dr Walther van Venrooij (University of Nijmegen, the Netherlands) [14]. Granzyme B was purchased from Kamiya Biomedical Co. (Seattle, WA, USA). Recombinat La and Ro proteins were purchased from DIARECT AG (Freiburg, Germany). Both proteins are tagged with a hexahistidine tag, which is used for IMAC (immobilized metal ion affinity chromatography) purification of the recombinant proteins.
Cells
HSG cells were kindly provided by Dr Yoshio Hayashi (Tokushima University School of Dentistry, Tokushima, Japan) and cultured in 6-well tissue culture plates, Costar 3516 (Costar, Cambridge, MA, USA) in RPMI 1640 containing 10% fetal calf serum (FCS). YT, HeLa, Huh7, MCF7, A549, and Jurkat cell lines were purchased from ATCC and cultured in RPMI 1640 containing 10% FCS.
Preparation of cell-free extracts
Cytoplasmic extracts were prepared essentially as previously described by Martin et al. [15,16]. Briefly, 1 × 108 HSG cells were harvested and washed twice with 50 ml of phosphate-buffered saline (PBS), pH 7·2, followed by a single wash with 5 ml of cell extract buffer (CEB; 50 mM PIPES, pH 7·4, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol (DTT), 10 µM cytochalasin B and 1 mM phenylmethylsulphonyl fluoride (PMSF)). Cells were pelleted, the supernatant was aspirated, and the supernatant was transferred to a 5 ml glass Dounce homogenizer. Cells were then allowed to swell by addition of an equal volume of CEB to the volume occupied by the packed cell pellet, followed by incubation on ice for 20 min. Cells were gently lysed with 20 strokes of a B-type pestle. Lysis was monitored by staining a small aliquot of the lysate with trypan blue and examining under the light microscope. The cell lysate was then transferred to a 1 ml Eppendorf tube and centrifuged at 4°C for 20 min at 14000 g. The supernatant was diluted to 7·5–15 mg/ml with extract buffer (EDB; 10 mM HEPES, 50 mM NaCl, 2 mM MgCl2, 5 mM EGTA, 1 mM DTT). Extracts were frozen at −80 °C for later use.
Western blotting
Six hundred nanograms of recombinant granzyme B was incubated with 18 µg of HSG cell lysates or 0·5 µg of recombinant La protein in Eppendorf tubes for 1 h at 37 °C. After treatment with granzyme B, HSG cell lysates were added to SDS loading buffer with 1 mM DTT and boiled for 5 min. Samples were then subjected to 5–20% gradient sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE, PAGEL, ATTO, Tokyo, Japan). Proteins were transferred to a polyvinylidene difluoride (PVDF) filter (ATTO), and the filter was blocked for 1 h using 5% nonfat dried milk in PBS containing 0·1% Tween 20 (PBS-T) at room temperature (RT), and incubated for 1 h at RT in the presence of each serum sample diluted 1 : 1000 in PBS using a multiscreen blotter (POSTBLOT, ATTO). The filter was washed with PBS-T and incubated with a 1 : 5000 dilution of goat anti-human IgG coupled to horseradish peroxidase (MBL). The enhanced chemiluminescence system (Amersham Biosciences, Piscataway, NJ, USA) was used for detection. In some experiments, the following changes in protocols were made: (1) in blocking experiments, HSG cell lysate incubated with or without granzyme B was immunoblotted in the presence or absence of 2 µg/ml of recombinant La protein blocking antigen, which was preincubated in each serum (1 : 10 000 dilution) for one hour at 4 °C; (2) in cytotoxicity experiments, 1 × 105 YT cells were incubated with adherent HSG cells (1 × 105 in a 6 well plate). After 6, 12 or 24 h, plates were washed once with PBS to remove the YT cells. HSG cells were then lysed with 1%NP40 buffer (1%NP40, 50 mM Tris, Cl, pH 8·0, 150 mM NaCl, 0·1% SDS, 0·02% sodium azide) containing 2 mM ZnSO4, resuspended in SDS loading buffer with 1 mM DTT, and boiled for 5 min [17].
ELISA Sera from the study subjects were collected and stored at −20 °C. Anti-SS-A (Ro) and anti-SS-B (La) antibodies were measured using ELISA kits (kindly provided from MBL) according to the manufacturer's instructions. Each index above 30 or 25 was considered positive for anti-Ro or anti-La antibodies, respectively.
Results
Serum autoantibodies recognize proteins derived from HSG cell lysates incubated with granzyme B
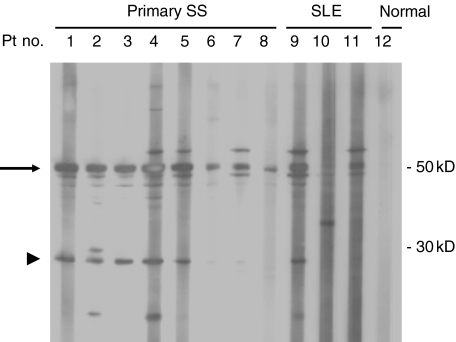
Figure 1 shows that the cell free lysates of HSG cells treated with granzyme B contained several proteins detected by Western blotting when probed with serum IgG antibodies derived from patients with primary SS and SLE. Major detected proteins were 50 kD and 60 kD in size (patient nos. 1, 2, 3, 4, 5, 6, 7, 9 have a 50 kD protein and patient nos. 4, 5, 7, 9 and 11 have a 60 kD protein), which are likely to be La and Ro, respectively. In addition to these proteins, a 27 kD protein was detected by Western blotting using sera from 8 (14·0%) of 57 primary SS patients (sera from patient nos. 1, 3 and 5 recognized a 27 kD protein, and nos. 6, 7 and 8 were negative), 5 (29·4%) of 17 SS patients with ML (patient nos. 2 and 4 were positive) and 2 (7·1%) of 28 SLE patients (patient no. 9 was positive and nos. 10 and 11 were negative) and from 20 normal subjects (patient no. 12 was negative). This experiment was repeated using the same sera three times with identical results.
Fig. 1.
Screening of sera from patients with primary Sjögren's syndrome using HSG cell lysates treated with granzyme B. Lysates from HSG cells after treatment of granzyme B were prepared and subjected to 5–20% gradient SDS-PAGE and proteins were transferred to a PVDF filter. The filter was incubated in the presence of each serum sample. The serum was diluted 1 : 1000 in PBS using a multiscreen blotter after blocking with 5% nonfat dried milk in PBS-T. The filter was washed with PBS-T and incubated with goat anti-human IgG coupled to horseradish peroxidase. The enhanced chemiluminescence system was used for detection. The positions of the molecular weight markers are indicated on the right (in kD). Representative results are shown for 8 primary SS patients, 3 SLE patients, and 1 normal control. Fifty kD and 27 kD proteins are indicated by arrow and arrow head, respectively.
Recombinant SS-B (La) protein is cleaved after treatment with granzyme B
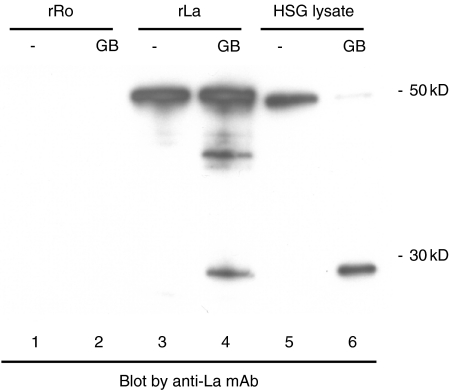
As a 27 kD protein exists together with a 50 kD protein in our Western blots, we speculated that the 27 kD protein may be a granzyme B cleavage product of La. Casciola-Rosen et al. [18] reported that the La protein (408 amino acids) is cleaved between Asparadic acid D (position 220) and Alanine A (position 221) by granzyme B, suggesting that the predicted size of the large fragment of La protein after cleavage by granzyme B should be 27 kD. To determine whether the 27 kD protein is a La cleavage product, recombinant La protein treated with granzyme B was subjected to SDS-PAGE followed by blotting with a monoclonal antibody specific for the La protein. Figure 2 shows that a 27 kD protein was detected by Western blotting in the granzyme B treated recombinant La preparations (lane 4) as well as in the granzyme B treated HSG cell lysate preparations (lane 6) using anti-La mAbs, demonstrating that the 27 kD protein is likely to represent a La cleavage fragment. The size of the recombinant La protein (lanes 3 and 4) is slightly larger than native La protein derived from HSG cells (lanes 5 and 6) due to the presence of a hexahistidine tag (Fig. 2). In some experiments, an additional band corresponding to a ∼40 kD protein was observed following exposure to granzyme B (e.g. compare Fig. 2 lanes 3 and 4, and Fig. 4). This band may represent a partial cleavage product of La or a cross-reactive epitope from an unrelated polypeptide that is liberated upon cleavage by granzyme B. An ELISA platform was also used to study these sera, demonstrating La reactivity in sera from 13 (22·8%) of 57 primary SS patients, 5 (29·4%) of 17 primary SS patients with ML, 3 (10·7%) of 28 SLE patients, and 0 (0%) of 20 normal subjects (Table 1). A 50 kD protein (the intact form of La) was detected by Western blotting using sera from all 18 primary SS patients with or without ML, and 3 SLE patients which were also positive by ELISA.
Fig. 2.
Recombinant La protein is cleaved after treatment with granzyme B. Recombinant Ro protein, recombinant La protein, and HSG cell lysate were treated with granzyme B and were subjected to SDS-PAGE followed by Western blotting with a monoclonal antibody specific for the La protein. The positions of the molecular weight markers are indicated on the right (in kD).
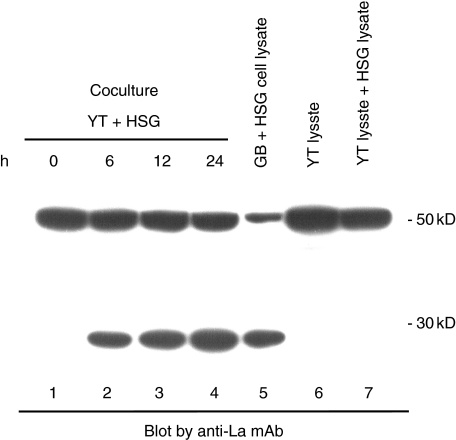
Fig. 4.
Granzyme B-specific La fragments are generated during cytotoxicity in vitro. 1 × 105 YT cells were incubated with adherent HSG cells (1 × 105 in a 6 well plate). After 6, 12, or 24 h, HSG cells were lysed with 1% NP40 buffer containing 2 mM ZnSO4, subjected to SDS-PAGE and electropheretic transfer, and immunoblotted using a monoclonal antibody specific for the La protein. The positions of the molecular weight markers are indicated on the right (in kD).
Table 1. Anti-Ro and anti-La antibodies in serum derived from patients with autoimmune diseases and from health controls, as detected by ELISA.
| SS-A (Ro) | SS-B (La) | |
|---|---|---|
| Primary SS (n = 57) | 37/57 (64·9%) | 13/57 (22·8%) |
| Primary SS + ML (n = 17) | 10/17 (58·8%) | 5/17 (29·4%) |
| SLE (n = 28) | 16/28 (57·1%) | 3/28 (10·7%) |
| Normal (n = 6) | 0/20 (0%) | 0/20 (0%) |
Anti-Ro and anti-La antibodies were measured by enzyme-linked immunosorbent assay (ELISA). Patients were diagnosed as having primary Sjögren's syndrome (primary SS; n = 57), primary Sjögren's syndrome with malignant lymphoma (primary SS + ML; n = 17), and systemic lupus erythematosus (SLE; n = 28).
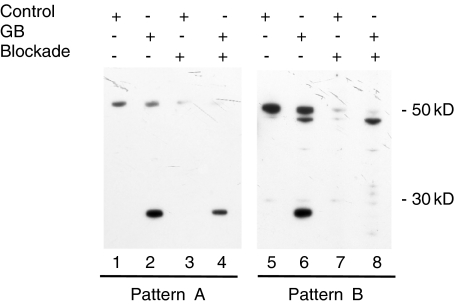
A B-cell epitope present on granzyme B-treated La autoantigens
To determine whether recognition of the 27 kD fragment of La is attributable (1) to epitopes common to the intact and cleaved forms of La or (2) to granzyme B-specific epitopes, Western blotting of intact and granzyme B treated lysates was performed in the presence of competing recombinant La protein. Figure 3 displays two representative patterns that were observed (pattern A, serum no. 3, 65-year-old female, SS + ML; pattern B, serum no. 2, 70-year-old female, SS + ML in Fig. 1). In 4 of 13 sera in which the 27 kD cleavage fragment was detected (2 of 8 sera from primary SS patients and 2 of 5 sera from primary SS patients with ML), blockade of recognition of the 27 kD La cleavage fragment by recombinant La protein was either partial or did not occur, despite the disappearance of the 50 kD intact La (pattern A, Fig. 3, left panel, lane 4). When using other sera, recombinant La protein completely blocked recognition of the 27 kD La fragment, as well as the 50 kD intact, native La protein (pattern B, Fig. 3, right panel, lane 8). These results suggest that granzyme B-specific B cell epitopes of the La autoantigen exist and are detected by sera from 4 patients with primary SS. To confirm the appearance of La neoepitopes after granzyme B cleavage, and the recognition of neoepitopes by anti-La antibodies from 4 sera (pattern A), we further examined whether the recombinant 27 kD La cleavage fragment revealed by granzyme B treatment inhibited the antibody response to native 27 kD La fragment. The antibody response to the 27 kD protein was decreased but still remained after incubation with sera and recombinant La treated with granzyme B (data not shown), suggesting that native La and recombinant His tagged La may have distinct conformations following exposure to granzyme B. We noted the smaller size of the La protein in pattern B in Fig. 3 (lanes 6 and 8). We were unable to identify this protein, although we determined that it is not a La fragment, because no inhibition was seen in blocking experiments (lane 8). We performed chart reviews about clinical and immunological features in 4 patients. Three of 4 patients developed malignant lymphoma.
Fig. 3.
Presence of apoptosis-specific La antibodies. HSG cell lysates incubated with or without granzyme B (GB+ or Control+, respectively) were immunoblotted in the presence or absence of 2 µg/ml of recombinant La protein (Blockade + or –, respectively). Pattern A, A representative immunoblot (serum no. 3 in Fig. 1: 65-year-old female, SS + ML) using serum (1 : 10 000 dilution) recognizing both intact (50 kD) and cleaved La (27 kD) in the absence of blockade; in the presence of blockade, recognition of intact La was diminished to a greater extent than was recognition of cleaved La. Pattern B, A representative immunoblot (serum no. 2 in Fig. 1: 70-year-old female, SS + ML) using serum recognizing both intact and cleaved La in the absence of blockade; recognition of both the intact and cleaved La was equally diminished in the presence of blockade.
Granzyme B-specific La fragments are generated during cytotoxicity in vitro
To confirm that similar autoantigen fragments are generated in intact cells during granzyme B-induced cell death, we exposed HSG cells to YT cells, which express large amounts of granzyme B, and have strong killing activity in vitro. When YT cells are lysed with a general lysing buffer, such as 1% NP40 or PIPA buffer, granzyme B cleaves substrates during processing of lysates, leading to artifactual proteolytic processing [17]. In this study, 1% NP40 buffer containing 2 mM ZnSO4 was used in this in vitro analysis, which eliminated the false proteolytic processing (data not shown). After 6 h, a 27 kD La fragment appeared in the case of cocultivation with HSG cells and YT cells (Fig. 4, lanes 2–4), identical in size to fragments in granzyme B-treated HSG free cell preparations (Fig. 4, lane 5). No fragment was seen in YT cell lysates only, or in a mixture of YT cell lysate and HSG cell lysate (Fig. 4, lanes 6 or 7, respectively).
Discussion
In this study we have identified novel autoantibodies that specifically recognize granzyme B substrate fragments derived from salivary gland cell lines. A 27 kD fragment was detected by Western blotting using sera from 13 of 74 primary SS patients (16·9%). This fragment was recognized by anti-La monoclonal antibodies. Blocking studies using recombinant La protein revealed that an apoptosis-specific B cell epitope was revealed and detected by serum antibodies from 4 of 13 primary SS patients. This represents the first report of detection of autoantibodies directed specifically against a granzyme B-induced cleaved form of the La autoantigen in sera from primary SS patients.
We and many investigators have reported that apoptosis may play a critical role in the pathogenesis of SS [19,20]. Apoptosis of the acinar and ductal epithelial cells of the salivary and lacrimal glands is one of the mechanisms for the destruction of these tissues [20,21]. Infiltrating lymphocytes are thought to induce apoptosis in the acinar and ductal epithelial cells via either perforin/granzyme B dependent killing, or by Fas ligand–Fas interaction [21]. Moreover, increased Bcl2 expression may account for resistance to apoptosis in infiltrating lymphocytes [12], resulting in a prolonged production of cytokines and autoantibodies. An immunohistochemical report has been published demonstrating that CD8+CD103(αEβ7)+ T cells that contain granzyme B adhere to acinar epithelial cells, leading to induction of apoptosis [22]. The infiltrating T cells stained for granzyme B in the minor salivary glands [12], suggesting that granzyme B may play a crucial role in the pathogenesis of SS.
Recently the hypothesis that autoimmunity arises when cryptic determinants are revealed to the immune system has been proposed [4–6]. Granzyme B is a unique protease that efficiently cleaves many prominent autoantigens. Some of these autoantigens are substrates for granzyme B but not caspase 3 (e.g. CENP-B, fibrillarin, B23, PMS1 and M3R), while other autoantigens are cleaved by both proteases (e.g. La, golgins, PARP, NuMA and fodrin). Importantly, the cleavage sites for granzyme B and caspase 3 are distinct [5,18]. Since caspase 3 is expressed ubiquitously in cells and is activated by a variety of apoptotic stimuli, and since thymocytes are exposed to these fragments during development, it is likely that tolerance to caspase 3 fragments exists in vivo. On the other hand, granzyme B is mainly expressed in cytotoxic cells, and granzyme B only encounters substrate in target cells when they encounter cytotoxic T cells, which release granule contents into the cytoplasm of target cells. It is unclear if granzyme B cleavage fragments are present in the thymus during the education of T cells.
La is a highly abundant nuclear phosphoprotein that associates with all newly synthesized RNA polymerase III transcripts and also binds a number of virally encoded RNAs [23]. La is the only autoantigen identified to date that undergoes dephosphorylation during apoptosis. La is specifically dephosphorylated at Ser366 and loses its nuclear localization signal upon cleavage during apoptosis [24,25]. La is also an excellent substrate for granzyme B and is cleaved at sites that generate a different pattern of proteolytic fragments than seen with caspase-mediated cell death [18]. Little is known about the function of the N terminus of La, which consists of a La motif and a single RNA recognition motif (RRM) [23,26]. Further studies are needed to determine the role of the 27 kD La fragment that is produced by granzyme B. Because La is an important molecule for cells, 5 cell lines (HeLa, Huh7, MCF7, A549 and Jurkat) derived from different tissues (cervical uterus, liver, mammary gland, lung and lymphocyte, respectively) were analysed and found to have equivalent amounts of La and cleaved La fragments after treatment of granzyme B (data not shown), suggesting that granzyme B-specific La fragments are not salivary gland specific. Nevertheless, future investigations directed at the mechanisms of antibody production for La autoantigen, especially in patients with SS that mainly suffered from destruction of salivary glands, are clearly indicated.
Lymphoma is an important complication in SS, with an estimated prevalence of 4–8% in SS [27]. Lymphoma is thought to be generated by a multistep process. In the salivary gland, infiltrating lymphocytes interact, ultimately leading to B cell clonal proliferation and autoantibody production. Chronic continuous inflammation may induce neoplastic transformation of lymphocytes, especially B cells [19]. In this study, a higher prevalence of antibodies specific for the 27 kD cleavage fragment was observed in primary SS patients with ML (29·4%) when compared with primary SS patients without ML (14·0%), although these results were not statistically different given the small sample sizes (P > 0·05).
In addition to our results, autoantibodies directed against granzyme B-specific neoepitopes were recently reported for the U1-70 kD protein [28] and for centromere protein [29] in sera from patients with SLE and scleroderma, respectively. Interestingly, Kohsaka et al. [30] previously reported that some sera from Japanese SS patients recognize a fragment of La (amino acids 179–220), which contains the granzyme B cleavage site. In summary, this represents the first study describing the existence of a humoral immune response targeting epitopes of the La autoantigen that are produced by proteolysis by granzyme B.
Acknowledgments
This research was supported in part by a grant-in-aid (16590986) from the Ministry of Education, Science, Sport and Culture, Japan (to H.I). N.O and S.S. were supported by a grant from the Ministry of Health, Labor and Welfare, Japan. P.J.U. was supported by the Dana Foundation, the Stanford Program in Molecular and Genetic Medicine (PMGM), NIH Grants DK61934, AI50854, AI50865, AR49328, NHLBI Proteomics Contract N01-HV-28183, the Floren Family Foundation and by a Baxter Foundation Award. H.I and T.O. are fellows of the Japanese Society of Internal Medicine.
References
- 1.Moutsopoulos HM, Chused TM, Mann DL, et al. Sjogren's syndrome (Sicca syndrome): current issues. Ann Intern Med. 1980;92:212–26. doi: 10.7326/0003-4819-92-2-212. [DOI] [PubMed] [Google Scholar]
- 2.Vitali C, Moutsopoulos HM, Bombardieri S. The European Community Study Group on diagnostic criteria for Sjogren's syndrome. Sensitivity and specificity of tests for ocular and oral involvement in Sjogren's syndrome. Ann Rheum Dis. 1994;53:637–47. doi: 10.1136/ard.53.10.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tengner P, Halse AK, Haga HJ, Jonsson R, Wahren-Herlenius M. Detection of anti-Ro/SSA and anti-La/SSB autoantibody-producing cells in salivary glands from patients with Sjogren's syndrome. Arthritis Rheum. 1998;41:2238–48. doi: 10.1002/1529-0131(199812)41:12<2238::AID-ART20>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Utz PJ, Anderson P. Posttranslational protein modifications, apoptosis, and the bypass of tolerance to autoantigens. Arthritis Rheum. 1998;41:1152–60. doi: 10.1002/1529-0131(199807)41:7<1152::AID-ART3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Utz PJ, Gensler TJ, Anderson P. Death, autoantigen modifications, and tolerance. Arthritis Res. 2000;2:101–14. doi: 10.1186/ar75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen A, Casciola-Rosen L. Autoantigens as substrates for apoptotic proteases. implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 1999;6:6–12. doi: 10.1038/sj.cdd.4400460. [DOI] [PubMed] [Google Scholar]
- 7.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–31. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Andrade F, Casciola-Rosen LA, Rosen A. Granzyme B-induced cell death. Acta Haematol. 2004;111:28–41. doi: 10.1159/000074484. [DOI] [PubMed] [Google Scholar]
- 10.Lord SJ, Rajotte RV, Korbutt GS, Bleackley RC. Granzyme B: a natural born killer. Immunol Rev. 2003;193:31–8. doi: 10.1034/j.1600-065x.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 11.Trapani JA, Sutton VR. Granzyme B. pro-apoptotic, antiviral and antitumor functions. Curr Opin Immunol. 2003;15:533–43. doi: 10.1016/s0952-7915(03)00107-9. [DOI] [PubMed] [Google Scholar]
- 12.Polihronis M, Tapinos NI, Theocharis SE, Economou A, Kittas C, Moutsopoulos HM. Modes of epithelial cell death and repair in Sjogren's syndrome (SS) Clin Exp Immunol. 1998;114:485–90. doi: 10.1046/j.1365-2249.1998.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrade F, Roy S, Nicholson D, Thornberry N, Rosen A, Casciola-Rosen L. Granzyme B directly and efficiently cleaves several downstream caspase substrates: implications for CTL-induced apoptosis. Immunity. 1998;8:451–60. doi: 10.1016/s1074-7613(00)80550-6. [DOI] [PubMed] [Google Scholar]
- 14.Pruijn GJ, Thijssen JP, Smith PR, Williams DG, Van Venrooij WJ. Anti-La monoclonal antibodies recognizing epitopes within the RNA-binding domain of the La protein show differential capacities to immunoprecipitate RNA-associated La protein. Eur J Biochem. 1995;232:611–9. doi: 10.1111/j.1432-1033.1995.611zz.x. [DOI] [PubMed] [Google Scholar]
- 15.Martin SJ, Newmeyer DD, Mathias S, et al. Cell-free reconstitution of Fas-, UV radiation- and ceramide-induced apoptosis. EMBO J. 1995;14:5191–200. doi: 10.1002/j.1460-2075.1995.tb00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin SJ, Amarante-Mendes GP, Shi L, et al. The cytotoxic cell protease granzyme B initiates apoptosis in a cell-free system by proteolytic processing and activation of the ICE/CED-3 family protease, CPP32, via a novel two-step mechanism. EMBO J. 1996;15:2407–16. [PMC free article] [PubMed] [Google Scholar]
- 17.Ida H, Nakashima T, Kedersha NL, et al. Granzyme B leakage-induced cell death: a new type of activation-induced natural killer cell death. Eur J Immunol. 2003;33:3284–92. doi: 10.1002/eji.200324376. [DOI] [PubMed] [Google Scholar]
- 18.Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med. 1999;190:815–26. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto K. Pathogenesis of Sjogren's syndrome. Autoimmun Rev. 2003;2:13–8. doi: 10.1016/s1568-9972(02)00121-0. [DOI] [PubMed] [Google Scholar]
- 20.Manganelli P, Fietta P. Apoptosis and Sjogren syndrome. Semin Arthritis Rheum. 2003;33:49–65. doi: 10.1053/sarh.2003.50019. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura H, Koji T, Tominaga M, et al. Apoptosis in labial salivary glands from Sjogren's syndrome (SS) patients: comparison with human T lymphotropic virus-I (HTLV-I)-seronegative and — seropositive SS patients. Clin Exp Immunol. 1998;114:106–12. doi: 10.1046/j.1365-2249.1998.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujihara T, Fujita H, Tsubota K, et al. Preferential localization of CD8+ alpha E beta 7+ T cells around acinar epithelial cells with apoptosis in patients with Sjogren's syndrome. J Immunol. 1999;163:2226–35. [PubMed] [Google Scholar]
- 23.Wolin SL, Cedervall T. The La protein. Annu Rev Biochem. 2002;71:375–403. doi: 10.1146/annurev.biochem.71.090501.150003. [DOI] [PubMed] [Google Scholar]
- 24.Rutjes SA, Utz PJ, van der Heijden A, Broekhuis C, van Venrooij WJ, Pruijn GJ. The La (SS-B) autoantigen, a key protein in RNA biogenesis, is dephosphorylated and cleaved early during apoptosis. Cell Death Differ. 1999;6:976–86. doi: 10.1038/sj.cdd.4400571. [DOI] [PubMed] [Google Scholar]
- 25.Ayukawa K, Taniguchi S, Masumoto J, et al. La autoantigen is cleaved in the COOH terminus and loses the nuclear localization signal during apoptosis. J Biol Chem. 2000;275:34465–70. doi: 10.1074/jbc.M003673200. [DOI] [PubMed] [Google Scholar]
- 26.Raats JM, Roeffen WF, Litjens S, et al. Human recombinant anti-La (SS-B) autoantibodies demonstrate the accumulation of phosphoserine-366-containing la isoforms in nucleoplasmic speckles. Eur J Cell Biol. 2003;82:131–41. doi: 10.1078/0171-9335-00304. [DOI] [PubMed] [Google Scholar]
- 27.Tonami H, Matoba M, Kuginuki Y, et al. Clinical and imaging findings of lymphoma in patients with Sjogren syndrome. J Comput Assist Tomogr. 2003;27:517–24. doi: 10.1097/00004728-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Greidinger EL, Foecking MF, Ranatunga S, Hoffman RW. Apoptotic U1-70 kd is antigenically distinct from the intact form of the U1-70-kd molecule. Arthritis Rheum. 2002;46:1264–9. doi: 10.1002/art.10211. [DOI] [PubMed] [Google Scholar]
- 29.Schachna L, Wigley FM, Morris S, Gelber AC, Rosen A, Casciola-Rosen L. Recognition of Granzyme B-generated autoantigen fragments in scleroderma patients with ischemic digital loss. Arthritis Rheum. 2002;46:1873–84. doi: 10.1002/art.10407. [DOI] [PubMed] [Google Scholar]
- 30.Kohsaka H, Yamamoto K, Fujii H, et al. Fine epitope mapping of the human SS-B/La protein. Identification of a distinct autoepitope homologous to a viral gag polyprotein. J Clin Invest. 1990;85:1566–74. doi: 10.1172/JCI114606. [DOI] [PMC free article] [PubMed] [Google Scholar]