Abstract
Resveratrol, a natural polyphenolic phytoalexin, has been considered as a potential anti-inflammatory agent because of its suppressive effect on nuclear factor-κB (NF-κB). However, we recently found that treatment of glomerular mesangial cells with resveratrol significantly and dose-dependently enhanced NF-κB activation triggered by proinflammatory cytokines. This finding was evidenced by different reporter assays as well as by expression of an endogenous NF-κB-dependent gene, intercellular adhesion molecule-1. The NF-κB promoting effect of resveratrol was also observed in renal tubular LLCPK1 cells, but not in HepG2 hepatoma cells. In all cell types tested, treatment with resveratrol alone did not affect NF-κB activity. The enhanced activation of NF-κB by resveratrol progressed for at least 24 h and was accompanied by sustained down-regulation of an endogenous NF-κB inhibitor, IκBβ, but not IκBα. Although expression of inducible nitric oxide synthase was suppressed by resveratrol, nitric oxide, a negative regulator of NF-κB, was not involved in the regulation of NF-κB by resveratrol. These data elucidated, for the first time, that resveratrol may enhance activation of NF-κB under certain circumstances.
Keywords: resveratrol, inflammation, mesangial cell, NF-κB, IκB
Introduction
Nuclear factor-κB (NF-κB) is a family of transcription factors that regulate a wide range of cellular functions [1]. NF-κB, classically composed of p50 and p65, remains sequestered in the cytoplasm as an inactive complex with its inhibitory counterpart IκB protein. In response to external stimuli, IκB is phosphorylated by IκB kinase complexes, ubiquitinated and degraded by the proteasome system. After the degradation of IκB, free NF-κB dimers expose their nuclear localization sequence and translocate into the nucleus, where they bind to the κB consensus sequences and activate transcription of target genes [1].
NF-κB is activated by proinflammatory stimuli including cytokines, growth factors, bacterial toxins and viral products [1]. Activation of NF-κB contributes to transcriptional induction of target genes with inflammatory, immunoregulatory and/or mitogenic functions and is involved in the development of inflammation [1]. In glomerulonephritis, activation of NF-κB is observed in glomerular cells [2], leading to expression of proinflammatory genes including chemokines, adhesion molecules, inducible nitric oxide synthase (iNOS) and cyclooxygenases (COX) [3]. Based on these previous findings, inhibition of NF-κB is a possible strategy for therapeutic intervention in glomerulonephritis [4,5].
Resveratrol (3,5,4′-trihydroxystilbene) is a natural polyphenolic phytoalexin that is abundantly contained in grapes and red wine. Resveratrol modifies multiple cellular targets, exerting immunosuppressive and anticancer activities in various cells and animal models. Its biological activities include;
effects on oxidative stress, carcinogen metabolism and DNA-adduct formation;
anti-inflammatory and anticancer potential;
induction of apoptosis;
regulation of cell cycle progression [6].
In particular, suppressive effects of resveratrol on the NF-κB signalling cascades are widely recognized. In various situations, resveratrol suppresses IKK activity, phosphorylation and degradation of IκBα, and subsequent nuclear translocation and DNA binding of NF-κB subunits. Because of this pharmacological potential, resveratrol can inhibit expression of NF-κB-dependent, proinflammatory genes and is thereby considered as a therapeutic agent for inflammatory diseases [6].
Based on the current knowledge described above, we started experiments to investigate the utility of resveratrol for therapeutic intervention in glomerulonephritis. Using reporter mesangial cells, we first examined how this agent affects activity of NF-κB in glomerular cells under inflammatory circumstances. Unexpectedly, resveratrol did not inhibit but enhanced activation of NF-κB in cytokine-stimulated mesangial cells. In contrast to a number of previous reports showing suppressive effects of resveratrol on NF-κB [6], enhancement of NF-κB activity by this agent has never been reported. The present study was performed to investigate this novel observation.
Materials and methods
Cells and reagents
Mesangial cells (SM43) were established from isolated renal glomeruli of a male Sprague-Dawley rat and identified as being of the mesangial cell phenotype as described before [7]. The porcine renal proximal tubular cell line LLCPK1 and the human hepatoma cell line HepG2 were obtained from the American Type Culture Collection (Manassas, VA, USA). Culture media supplemented with 1% fetal bovine serum (FBS) were generally used for studies. Resveratrol and forskolin were purchased from Sigma-Aldrich Japan (Tokyo, Japan), and nitric oxide (NO) synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME) and NO donor S-nitroso-N-acetylpenicillamine (SNAP) were obtained from Sigma (St. Louis, MO, USA). Human recombinant IL-1β and human recombinant tumour necrosis factor-α (TNF-α) were gifts of Otsuka Pharmaceutical Co. Ltd (Tokushima, Japan) and Dr Katsuo Noguchi (Teikyo University School of Medicine, Tokyo), respectively.
Establishment of reporter cells
Using a calcium-phosphate coprecipitation method, SM43 rat mesangial cells were transfected with pNFκB-SEAP (5 µg; BD Biosciences, Palo Alto, CA, USA) together with pcDNA3·1 (1 µg; Invitrogen, Carlsbad, CA, USA) that encodes neomycin phosphotransferase [8]. pNFκB-SEAP encodes secreted alkaline phosphatase (SEAP) under the control of 4 copies of the κB enhancer element. Stable transfectants were selected by G418, and SM/NFκB-SEAP5 cells were established. Another reporter clone SM/SV-SEAP28 was similarly established by cotransfection of SM43 cells with pSEAP2-Control (5 µg; BD Biosciences) that codes for SEAP under the control of the simian virus 40 (SV40) early promoter and enhancer, together with pcDNA3·1 (1 µg). It is known that the SV40 promoter/enhancer contains functional NF-κB responsive sequences and is significantly activated by IL-1β and TNF-α[9]. As a negative control, SM/CRE-SEAP15 cells [10] that secrete SEAP under the control of the cAMP response element (CRE) were used.
Transient transfection
LLCPK1 cells (6 × 106) and HepG2 cells (5 × 106) were transiently transfected with 10 µg of pNFκB-SEAP by electroporation. After 48 h, the cells were treated with or without resveratrol (25–50 µM) and stimulated by TNF-α (250 U/ml) or IL-1β (10 ng/ml) for 24 h, respectively. The culture media were then subjected to SEAP assay to evaluate NF-κB activity.
Pharmacological treatment
Reporter cells (SM/NFκB-SEAP5, SM/SV-SEAP28 and SM/CRE-SEAP15) were treated with resveratrol (0–75 µM) for 1 h and stimulated by TNF-α (250 U/ml), IL-1β (10 ng/ml) or forskolin (10 µM) for 6 h in Northern and Western blot analyses and for 24 h in SEAP assay. Fifty micromolar resveratrol was generally used for experiments. To examine involvement of NO in the effect of resveratrol, SM/NFκB-SEAP5 cells were stimulated for 24 h with TNF-α or IL-1β in the presence or absence of resveratrol together with or without L-NAME (200 µM) or SNAP (100 µM).
SEAP assay
Activity of SEAP was evaluated by a chemiluminescent method using Great EscAPe SEAP detection kit (BD Biosciences), as described previously [8]. In brief, 5 µl of culture medium was mixed with 15 µl of 1 × dilution buffer and incubated at 65 °C for 30 min. After the incubation, the samples were mixed with 20 µl of assay buffer containing l-homoarginine, left at room temperature for 5 min and then added with 20 µl of chemiluminescent enhancer containing 1·25 mM CSPD chemiluminescent substrate. After incubation in dark for 30 min, the samples were analysed using a luminometer.
Northern blot analysis
Northern blot analysis was performed as described before [11]. cDNAs for monocyte chemoattractant protein 1 (MCP-1) [12], intercellular adhesion molecule-1 (ICAM-1) and iNOS (gifted from Dr Javier Lucio-Cazana, University of Alcala, Spain) were used to prepare radio-labelled probes. Expression of glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
Western blot analysis
Equal amounts (20 µg) of extracted protein were separated by 10% SDS-polyacrylamide gels and electrotransferred onto 0·4 µM polyvinylidene difluoride membranes. Western blot analysis was performed by the enhanced chemiluminescence system (Amersham Biosciences, Buckinghamshire, UK), as described before [10]. Primary antibodies used were; anti-IκBα antibody (1 : 200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-IκBβ antibody (1 : 200 dilution; Santa Cruz Biotechnology). As a loading control, the level of β-actin was evaluated using anti-β-actin antibody (1 : 30000 dilution; Sigma). The intensity of individual bands was evaluated by densitometric analysis using Scion Image Software (Scion Corporation, Frederick, MD, USA).
Statistical analysis
All assays were performed in quadruplicate. Data were presented as mean ± SE. Statistical analysis was performed using the nonparametric Mann–Whitney U-test to compare data in different groups. P-value < 0·05 was considered to indicate a statistically significant difference.
Results
Enhancement of cytokine-triggered NF-κB activation by resveratrol
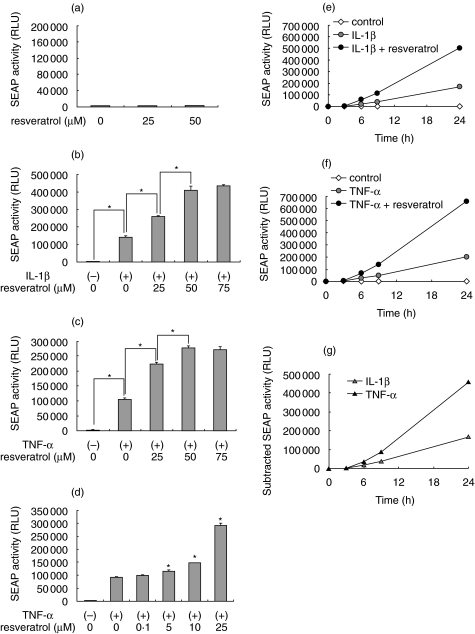
To examine effects of resveratrol on the activity of NF-κB, we used reporter mesangial cells SM/NFκB-SEAP5 that secrete SEAP under the control of the κB enhancer elements [8]. The cells were treated with resveratrol (0–50 µM) for 24 h, and activity of SEAP in culture medium was evaluated. In unstimulated mesangial cells, treatment with resveratrol did not activate NF-κB (Fig. 1a). However, treatment with resveratrol significantly enhanced NF-κB activation triggered by a proinflammatory cytokine IL-1β (Fig. 1b). This effect was dose-dependent, and the maximum effect was observed at 50–75 µM. Similarly, resveratrol dose-dependently enhanced NF-κB activation triggered by another proinflammatory cytokine, TNF-α (Fig. 1c). Significant enhancement of NF-κB was observed at concentrations ≥5 µM (Fig. 1d). Time-lapse experiments revealed that the enhanced activation of NF-κB by resveratrol was observed for at least 24 h in IL-1β-stimulated cells (Fig. 1e) and TNF-α-stimulated cells (Fig. 1f). Subtraction of the SEAP activity in resveratrol-untreated cells from that in resveratrol-treated cells revealed that the enhancement of NF-κB by resveratrol was reinforced progressively during the course of the incubation (Fig. 1g).
Fig. 1.
Effects of resveratrol on the activity of nuclear factor-κB (NF-κB) in SM/NFκB-SEAP5 reporter mesangial cells. (a) Effects on basal NF-κB activity. SM/NFκB-SEAP5 cells were treated with 0–50 µM resveratrol for 24 h, and activity of secreted alkaline phosphatase (SEAP) in culture media was evaluated by chemiluminescent assay. (b–d) Effects on cytokine-inducible NF-κB activation. SM/NFκB-SEAP5 cells were treated with 0–75 µM resveratrol for 1 h, stimulated with (+) or without (–) IL-1β (10 ng/ml) or tumour necrosis factor-α (TNF-α; 250 U/ml) for 24 h and subjected to SEAP assay. (e–g) Kinetics of NF-κB activity in IL-1β (e) or TNF-α (f)–stimulated cells in the presence or absence of 50 µM resveratrol. In (g), the SEAP activity in resveratrol-treated cells was subtracted by that in resveratrol-untreated cells. All assays were performed in quadruplicate. Data are presented as mean ± SE, and asterisks indicate statistically significant differences (P < 0·05). RLU, relative light unit.
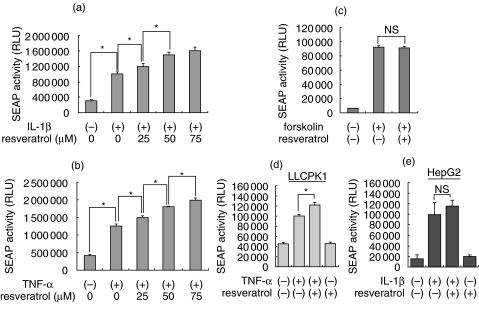
We confirmed the effect of resveratrol using another reporter mesangial cell SM/SV-SEAP28. The SV40 promoter/enhancer is known to contain functional NF-κB responsive sequences and is activated by IL-1β and TNF-α[9]. Consistent with the results observed in SM/NFκB-SEAP5 cells, the NF-κB-responsive SV40 promoter was activated significantly by IL-1β and TNF-α, and the activation was further enhanced by the treatment with resveratrol in a dose-dependent manner (Fig. 2a,b).
Fig. 2.
Effects of resveratrol on the activity of NF-κB in other reporter cells. (a,b) SM/SV-SEAP28 reporter mesangial cells were treated with 0–75 µM resveratrol for 1 h, stimulated with IL-1β (10 ng/ml) (a) or TNF-α (250 U/ml) (b) for 24 h, and subjected to SEAP assay. (c) SM/CRE-SEAP15 reporter mesangial cells were treated with (+) or without (–) 50 µM resveratrol for 1 h, stimulated with forskolin (10 µM) for 24 h and subjected to SEAP assay. (d,e) LLCPK1 cells and HepG2 cells were transiently transfected with pNFκB-SEAP, treated with (+) or without (–) resveratrol (25–50 µM) and stimulated by cytokines for 24 h. Culture media were subjected to SEAP assay to evaluate NF-κB activity. Data are presented as mean ± SE, and asterisks indicate statistically significant differences (P < 0·05). NS, not statistically significant.
In this SEAP reporter system, resveratrol might induce a false-positive response through nonspecific promotion of SEAP secretion, attenuation of SEAP degradation and/or enhancement of SEAP activity. To exclude these possibilities, SM/CRE-SEAP15 cells [10] that secrete SEAP under the control of CRE were stimulated by forskolin in the presence or absence of resveratrol, and activity of SEAP was examined. In SM/CRE-SEAP cells, activity of SEAP was markedly induced by the treatment with forskolin, a cAMP elevating agent. In contrast to SM/NFκB-SEAP5 and SM/SV-SEAP28 cells, however, treatment with resveratrol did not affect the induction of SEAP activity in SM/CRE-SEAP15 cells (Fig. 2c).
To examine whether the effect of resveratrol on NF-κB is cell-type specific, the porcine renal tubular cell line LLCPK1 and the human hepatoma cell line HepG2 were tested. These cells were transiently transfected with pNFκB-SEAP, treated with or without resveratrol and stimulated by cytokines for 24 h. Consistent with the result in mesangial cells, resveratrol significantly enhanced activation of NF-κB in TNF-α-triggered LLCPK1 cells (Fig. 2d). In contrast, this effect was not observed in IL-1β-stimulated HepG2 cells (Fig. 2e). As in mesangial cells, resveratrol alone did not induce activation of NF-κB in both cell types. These results indicated that the enhancement of NF-κB activity by resveratrol may be restricted to particular cell types.
Effects of resveratrol on the levels of endogenous NF-κB inhibitors, IκBα and IκBβ
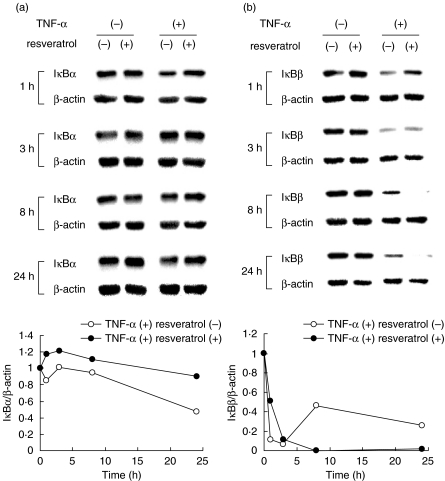
Under unstimulated conditions, NF-κB locates in the cytoplasm as complexes consisting of NF-κB subunits (e.g. p65 and p50) and its inhibitory counterpart IκB. When cells are stimulated, IκB kinases phosphorylate IκB and cause its rapid degradation by proteasomes [1]. The decrease in the level of IκB protein is therefore a marker for NF-κB activation. To investigate mechanisms involved in the enhancement of NF-κB activity by resveratrol, we examined the levels of IκBα and IκBβ. Mesangial cells were treated with or without resveratrol and stimulated by TNF-α for up to 24 h. Western blot analysis revealed that, following the stimulation with TNF-α, the level of IκBα was not obviously altered within 8 h but decreased to approximately 50% of the original level after 24 h. This suppression was abrogated by the treatment with resveratrol (Fig. 3a). In contrast, the level of IκBβ was rapidly down-regulated within 1 h following exposure to TNF-α. Although the level was partially recovered after 8 h, sustained suppression IκBβ was observed for at least 24 h (Fig. 3b). Treatment with resveratrol attenuated the early down-regulation of IκBβ whereas markedly enhanced the suppression of IκBβ at the later phase (8−24 h) (Fig. 3b). This result indicated a possibility that the effect of resveratrol may be biphasic; i.e. in the very early phase, resveratrol delays activation of NF-κB, but in the later phase, it enhances and sustains NF-κB activation triggered by cytokines.
Fig. 3.
Effects of resveratrol on the levels of IκBα and IκBβ in cytokine-exposed mesangial cells. Mesangial cells were treated with (+) or without (–) 50 µM resveratrol for 1 h, stimulated by TNF-α for 1–24 h and subjected to Western blot analyses of IκBα (a) and IκBβ (b). The level of β-actin is shown as a loading control. Densitometric analysis of individual data was presented as relative intensity of IκB against β-actin (IκB/β-actin).
Effects of resveratrol on the expression of endogenous NF-κB-dependent genes
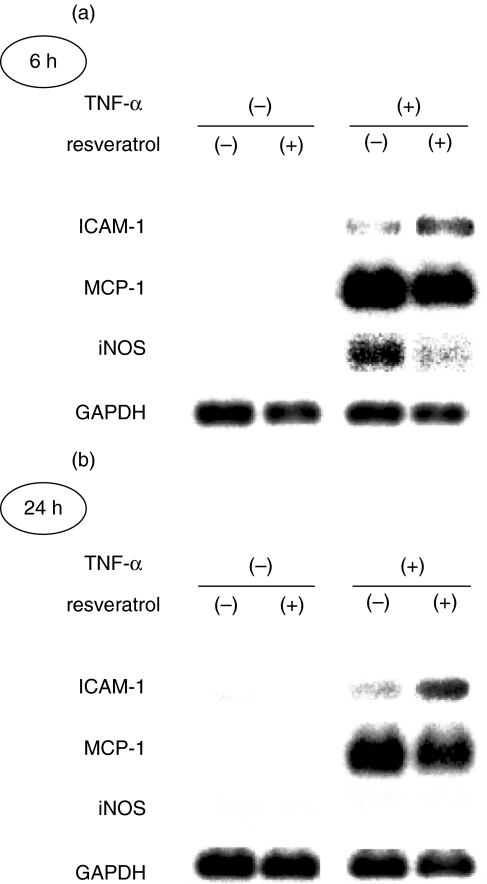
We examined effects of resveratrol on the expression of endogenous, NF-κB-dependent genes, ICAM-1, MCP-1 and iNOS [1]. Mesangial cells were treated with or without resveratrol and stimulated by TNF-α. After 6 h and 24 h, the cells were harvested and subjected to Northern blot analyses. As shown in Fig. 4, expression of ICAM-1 was modestly induced by the treatment with TNF-α at both time points. This induction was substantially enhanced by the treatment with resveratrol at both 6 h and 24 h. However, induction of iNOS mRNA by TNF-α (6 h) was attenuated by the treatment with resveratrol (Fig. 4a). Induction of MCP-1 mRNA by TNF-α was not obviously affected by resveratrol.
Fig. 4.
Effects of resveratrol on the expression of endogenous NF-κB-dependent genes, intercellular adhesion molecule-1 (ICAM-1), monocyte chemoattractant protein 1 (MCP-1) and inducible nitric oxide synthase (iNOS). Mesangial cells were treated with (+) or without (–) 50 µM resveratrol for 1 h, stimulated by TNF-α for (a) 6 h or (b) 24 h and subjected to Northern blot analyses. Expression of glyceraldehydes-3-phosphate dehydrogenase (GAPDH) is shown at the bottom as a loading control.
Lack of involvement of NO in the regulation of NF-κB by resveratrol
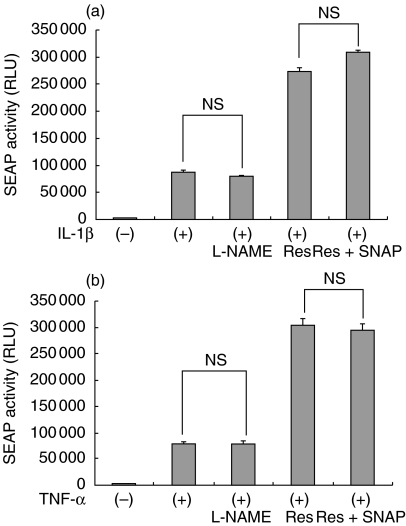
Previous reports demonstrated that NO is a negative regulator of NF-κB in some tissues and cells [13,14]. Because the induction of iNOS by TNF-α was suppressed by the treatment with resveratrol, we speculated that the enhanced NF-κB activity by resveratrol may be caused by suppression of the iNOS – NO signalling pathway. To examine this possibility, SM/NFκB-SEAP5 cells were treated with a NOS inhibitor L-NAME or a NO donor SNAP, and the effect of resveratrol was retested. As shown in Fig. 5, inhibition of NOS by L-NAME (200 µM) did not mimic the effect of resveratrol on the IL-1β- and TNF-α-induced activation of NF-κB. Furthermore, the enhancement of NF-κB activity by resveratrol in cytokine-stimulated mesangial cells was not inhibited in the presence of the NO donor SNAP (100 µM). Of note, the concentrations of L-NAME and SNAP used were effective in modulating NO-dependent gene expression in SM43 mesangial cells, as we previously reported [10]. These results excluded the possibility that resveratrol enhanced NF-κB activity via inhibition of NO.
Fig. 5.
Lack of involvement of the iNOS-NO signalling pathway in the effect of resveratrol. SM/NFκB-SEAP5 cells were treated with NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME; 200 µM), resveratrol (Res: 50 µM), or resveratrol + NO donor S-nitroso-N-acetylpenicillamine (SNAP; 100 µM) for 24 h in the presence of IL-1β (a) or TNF-α (b) and subjected to SEAP assay. Assays were performed in quadruplicate. Data are presented as mean ± SE. NS, not statistically significant.
Discussion
Resveratrol has been widely recognized as an inhibitor of NF-κB and regarded as a potential therapeutic agent for inflammatory diseases [6]. In the present investigation, however, we found that resveratrol enhanced activation of NF-κB in mesangial cells exposed to proinflammatory cytokines. It was accompanied by sustained down-regulation of IκBβ, but not IκBα. To our knowledge, this is the first to demonstrate enhancement of cytokine-triggered NF-κB activation by resveratrol.
Activity of NF-κB is regulated by its inhibitory counterparts, IκB proteins. In response to external stimuli, IκB is phosphorylated by IκB kinases and degraded by the proteasome system, leading to translocation of free NF-κB dimers into the nucleus and their binding to the κB sites [1]. Our current data showed that IκBβ, but not IκBα, was down-regulated by resveratrol in cytokine-stimulated mesangial cells. This result suggests that resveratrol enhances NF-κB activity via selective, sustained phosphorylation and degradation of IκBβ. Alternatively, resveratrol may suppress expression and/or synthesis of IκBβ, leading to enhanced NF-κB activity. The lack of involvement of IκBα might be relevant with a previous report showing that IκBα did not form a negative regulatory loop for NF-κB in mesangial cells [15]. A previous report also showed that NF-κB was dissociated from IκBs and activated without degradation of IκB proteins [16]. We cannot exclude a possibility that IκBα could mediate the effect of resveratrol without altering its protein level. Of note, in contrast to IκBβ, expression of IκBα is NF-κB-dependent [17]. Enhanced synthesis of IκBα by resveratrol via NF-κB activation might have masked the degradation of IκBα, resulting in the unaltered level of IκBα protein.
The NF-κB promoting effect of resveratrol was observed not only in rat mesangial cells but also in the porcine renal tubular cell line LLCPK1. It is contrastive to a number of previous reports that showed suppressive effects of resveratrol on NF-κB [6]. Currently, the reason for the discrepancy is unclear. It should be noted, however, that the majority of previous studies were based on malignant cells or cells of lymphoid/myeloid lineages [6]. Little information is available regarding effects of resveratrol on cytokine-triggered normal cells and cells other than leukocytes. The discrepancy between current data and previous reports may be simply ascribed to cell lineage-specific differences, and particular machinery sensitive to resveratrol could be present preferentially in normal cells. Indeed, the human hepatoma cell line HepG2 was insensitive to the effect of resveratrol, as shown in this report (Fig. 2e).
In this report, we demonstrated that activation of NF-κB in cytokine-stimulated mesangial cells was enhanced by the treatment with resveratrol. However, the kinetics of endogenous, NF-κB-dependent genes was different from molecule to molecule. For example, resveratrol up-regulated ICAM-1, down-regulated iNOS and almost unaffected MCP-1 expression induced by proinflammatory cytokines. A possible explanation is that expression of individual genes is regulated differently by multiple transcriptional factors, some of which might be suppressed by resveratrol. For example, expression of iNOS and MCP-1 is known to be regulated by both NF-κB and activator protein 1 (AP-1) [18,19], and resveratrol is a potent inhibitor of AP-1 in various cell types [6]. Down-regulation of AP-1 might offset the effect of up-regulated NF-κB on the expression of iNOS and MCP-1.
Activity of the κB site is regulated by a variety of NF-κB subunits including p65, p50, Rel, RelB, v-Rel and p52 [1]. Among these subunits, p65 is the prototypic, most important component that mediates NF-κB activation. We examined binding of p65 to the κB consensus sequence using an oligonucleotide-immobilized, ELISA-based system. We found that the binding activity of p65 to the κB site was significantly attenuated by the treatment with resveratrol in cytokine-treated mesangial cells (our unpublished data). It was contrastive to the results from reporter assays (Figs 1 and 2) and Western blot analysis of IκB (Fig. 3), as shown in this report. Two possibilities may explain this discrepancy. One possibility is that resveratrol may regulate individual NF-κB subunits differently, and activation of NF-κB subunits other than p65 might mediate the effect of resveratrol. It is consistent with a recent report showing that resveratrol regulated p65 and Rel differently in THP-1 cells [20]. Among the activated NF-κB dimers, the p50-p65 heterodimers enhance transcription of target genes, whereas the p50–p50 homodimers repress gene transcription. The p65–p65 homodimers can be involved in both transcriptional activation and repression [21–23]. Based on this current knowledge, another possibility is that resveratrol may facilitate conversion of the p65–p65 homodimers to the more potent p65–p50 heterodimers, which has been observed in other experimental systems [22,23]. In this situation, the amount of p65 bound to the κB site is reduced by resveratrol, although activity of NF-κB is up-regulated. Further investigation will be required to examine these possibilities.
Previous studies demonstrated that resveratrol has the in vivo anti-inflammatory potential that may be useful for therapeutic intervention in inflammatory diseases [24,25]. However, currently, information is limited regarding therapeutic utility of resveratrol for glomerulonephritis. One previous report showed that administration of resveratrol attenuated proteinuria in antiglomerular basement membrane glomerulonephritis in rats [26]. Effects of resveratrol on histopathological changes remained to be elucidated. Our current data indicate that, under certain inflammatory situations, resveratrol may enhance cytokine-induced NF-κB activity, leading to exacerbation of tissue inflammation. This effect seems to be cell type-specific, and certain IκB protein and NF-κB subunits may be selectively involved. The dark side of resveratrol, as well as its light side, should be considered carefully if this agent is used for the treatment of inflammation, especially glomerulonephritis.
Acknowledgments
This investigation was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (16390243 to M. K.).
References
- 1.Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 2.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 3.Lopez-Franco O, Suzuki Y, Sanjuan G, et al. Nuclear factor-κB inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am J Pathol. 2002;161:1497–505. doi: 10.1016/s0002-9440(10)64425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomita N, Morishita R, Tomita S, Kaneda Y, Higaki J, Ogihara T, Horiuchi M. Inhibition of TNF-α-induced cytokine and adhesion molecule expression in glomerular cells in vitro and in vivo by transcription factor decoy for NF-κB. Exp Nephrol. 2001;9:181–90. doi: 10.1159/000052610. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai H, Shigemori N, Hisada Y, Ishizuka T, Kawashima K, Sugita T. Suppression of NF-κB and AP-1 activation by glucocorticoids in experimental glomerulonephritis in rats: Molecular mechanisms of anti-nephritic action. Biochim Biophys Acta. 1997;1362:252–62. doi: 10.1016/s0925-4439(97)00068-9. [DOI] [PubMed] [Google Scholar]
- 6.Kundu JK, Surh Y-J. Molecular basis of chemoprevention by resveratrol. NF-κB and AP-1 as potential targets. Mutation Res. 2004;555:65–80. doi: 10.1016/j.mrfmmm.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura M, Taylor S, Unwin R, Burton S, Shimizu F, Fine LG. Gene transfer into the rat renal glomerulus via a mesangial cell vector: Site-specific delivery, in situ amplification, sustained expression of an exogenous gene in vivo. J Clin Invest. 1994;94:497–505. doi: 10.1172/JCI117361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng Y, Kasai A, Hiramatsu N, et al. Real-time monitoring of mesangial cell – macrophage cross-talk using SEAP in vitro and ex vivo. Kidney Int. 2005;68:886–93. doi: 10.1111/j.1523-1755.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- 9.Espel E, Fromental C, Reichenbach P, Nabholz M. Activity and interleukin 1 responsiveness of SV40 enhancer motifs in a rodent immature T cell line. EMBO J. 1990;9:929–37. doi: 10.1002/j.1460-2075.1990.tb08191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao J, Hiramatsu N, Zhu Y, Morioka T, Takeda M, Oite T, Kitamura M. Nitric oxide-mediated regulation of connexin43 expression and gap junctional intercellular communication in mesangial cells. J Am Soc Nephrol. 2005;16:58–67. doi: 10.1681/ASN.2004060453. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura M, Sütö T, Yokoo T, Shimizu F, Fine LG. Transforming growth factor-β1 is the predominant paracrine inhibitor of macrophage cytokine synthesis produced by glomerular mesangial cells. J Immunol. 1996;156:2964–71. [PubMed] [Google Scholar]
- 12.Rollins BJ, Morrison ED, Stiles CD. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci USA. 1988;85:3738–42. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang JL, Park W, Pack IS, Lee HS, Kim MJ, Lim CM, Koh Y. Inhaled nitric oxide attenuates acute lung injury via inhibition of NF-κB and inflammation. J Appl Physiol. 2002;92:795–801. doi: 10.1152/japplphysiol.00202.2001. [DOI] [PubMed] [Google Scholar]
- 14.Xiong H, Zhu C, Li F, Hegazi R, He K, Babyatsky M, Bauer AJ, Plevy SE. Inhibition of interleukin-12 p40 transcription and NF-κB activation by nitric oxide in murine macrophages and dendritic cells. J Biol Chem. 2004;279:10776–83. doi: 10.1074/jbc.M313416200. [DOI] [PubMed] [Google Scholar]
- 15.Auwardt RB, Mudge SJ, Chen C, Power DA. Inhibition with antisense oligonucleotide suggests that IκBα does not form a negative autoregulatory loop for NF-κB in mesangial cells. Exp Nephrol. 2000;8:144–51. doi: 10.1159/000020662. [DOI] [PubMed] [Google Scholar]
- 16.Canty TG, Boyle EM, Farr A, Morgan EN, Verrier ED, Pohlman TH. Oxidative stress induces NF-κB nuclear translocation without degradation of IκBα. Circulation. 1999;100(Suppl. II):361–4. doi: 10.1161/01.cir.100.suppl_2.ii-361. [DOI] [PubMed] [Google Scholar]
- 17.Rupec RA, Poujol D, Grosgeorge J, et al. Structural analysis, expression, and chromosomal localization of the mouse IκBα gene. Immunogenetics. 1999;49:395–403. doi: 10.1007/s002510050512. [DOI] [PubMed] [Google Scholar]
- 18.Chu SC, Marks-Konczalik J, Wu HP, Banks TC, Moss J. Analysis of the cytokine-stimulated human inducible nitric oxide synthase (iNOS) gene: Characterization of differences between human and mouse iNOS promoters. Biochem Biophys Res Commun. 1998;248:871–8. doi: 10.1006/bbrc.1998.9062. [DOI] [PubMed] [Google Scholar]
- 19.Lucio-Cazana J, Nakayama K, Xu Q, Konta T, Moreno-Manzano V, Furusu A, Kitamura M. Suppression of constitutive but not IL-1β-inducible expression of monocyte chemoattractant protein-1 in mesangial cells by retinoic acids: Intervention in the activator protein-1 pathway. J Am Soc Nephrol. 2001;12:688–94. doi: 10.1681/ASN.V124688. [DOI] [PubMed] [Google Scholar]
- 20.Pendurthi UR, Meng F, Mackman N, Rao LV. Mechanism of resveratrol-mediated suppression of tissue factor gene expression. Thromb Haemost. 2002;87:155–62. [PubMed] [Google Scholar]
- 21.Plaksin D, Baeuerle PA, Eisenbach L. KBF1 (p50 NF-κB homodimer) acts as a repressor of H-2Kb gene expression in metastatic tumor cells. J Exp Med. 1993;177:1651–62. doi: 10.1084/jem.177.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knuefermann P, Chen P, Misra A, Shi S-P, Abdellatif M, Sivasubramanian N. Myotrophin/V-1, a protein up-regulated in the failing human heart and in postnatal cerebellum, converts NF-κB p50-p65 heterodimers to p50-p50 and p65-p65 Homodimers. J Biol Chem. 2002;277:23888–97. doi: 10.1074/jbc.M202937200. [DOI] [PubMed] [Google Scholar]
- 23.Tong X, Yin L, Washington R, Rosenberg DW, Giardina C. The p50-p50 NF-κB complex as a stimulus-specific repressor of gene activation. Mol Cell Biochem. 2004;265:171–83. doi: 10.1023/b:mcbi.0000044394.66951.4d. [DOI] [PubMed] [Google Scholar]
- 24.Martin AR, Villegas I, La Casa C, de la Lastra CA. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem Pharmacol. 2004;67:1399–410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Culpitt SV, Rogers DF, Fenwick PS, Shah P, De Matos C, Russell RE, Barnes PJ, Donnelly LE. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax. 2003;58:942–6. doi: 10.1136/thorax.58.11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nihei T, Miura Y, Yagasaki K. Inhibitory effect of resveratrol on proteinuria, hypoalbuminemia and hyperlipidemia in nephritic rats. Life Sci. 2001;68:2845–52. doi: 10.1016/s0024-3205(01)01061-x. [DOI] [PubMed] [Google Scholar]