Abstract
The poor outcome of intraportal islet transplantation may be explained by the instant blood-mediated inflammatory reaction (IBMIR), characterized by islet entrapment in blood clots, leucocyte infiltration and disruption of islet morphology. Here we employ a newly developed in vitro system to identify the blood cells involved in this process. Islets were mixed with ABO-compatible blood in heparinized tubes and incubated for various times up to 6 h. Clots were analysed immunohistochemically for detection of platelets (CD41a), leucocytes/lymphocytes (CD11b), granulocytes (CD16, lysozyme), neutrophilic granulocytes (neutrophil elastase), eosinophilic granulocytes (NaCN + H2O2), macrophages (CD68), dendritic cells (CD209/DC-SIGN), B cells (CD20) and T cells (CD4, CD8). Platelets were rapidly deposited around the islets in contact with the blood, reaching a maximum by 30 min. The first neutrophilic granulocytes appeared in the islets after 15 min, increased at 1 h and peaked at 2 h. Small numbers of macrophages were found infiltrating the islets already after 5 min, with a slight increase over time. However, control stainings of cultured islets and pancreas biopsies identified these cells as being largely of donor origin. No T cells, B cells, dendritic cells or eosinophilic granulocytes were detected during the 6 h observation time. Neutrophilic granulocytes were identified as the main infiltrating blood cell in islets exposed to blood, implying that these cells play a key role in clinical islet transplantation. Because islets are known to be exquisitely susceptible to oxidative stress, development of drugs targeting neutrophilic cytotoxicity could markedly improve the outcome of islet transplantation.
Keywords: IBMIR, islet transplantation, infiltration, inflammation, neutrophilic granulocytes, type 1 diabetes mellitus
Introduction
Type 1 diabetes is an organ specific autoimmune disease in which the immune system attacks the insulin producing pancreatic β cells (islets) [1,2]. Despite marked improvement in diabetes care in recent years, insulin-dependent diabetes is the leading cause of end-stage renal disease, blindness and amputation. The currently available method for restoring endogenous insulin production is to replace the patient's destroyed islets, either by whole pancreas transplantation or by transplanting the islets. Replacement of the whole gland re-establishes long-term normoglycaemia with a success rate of 80%. However, because of the risk of surgical complications, this procedure will never be a viable option for most type 1 diabetic patients. Since the breakthrough made by Shapiro et al. [3], islet transplantation with its minimal surgery has the potential to be the procedure of choice. This procedure can be performed on an almost out-patient basis by infusing the islets into the portal vein. However, one obstacle to bringing this procedure to clinical practice is the consistent observation that islets from multiple donors are needed to achieve normoglycaemia. It has been observed that the metabolic capacity in patients receiving islets corresponds to only 20% of that in healthy subjects [4], indicating that the majority of the transplanted islets are lost in this procedure.
We have previously reported that a thrombotic/inflammatory reaction termed the instant blood-mediated inflammatory reaction (IBMIR) is elicited when islets come into direct contact with ABO-compatible blood [5,6]. Similar reactions have also been observed in clinical islet transplantation [7].
The IBMIR includes coagulation and complement activation, a rapid binding of activated platelets to the islet surface entrapping them in a clot, and recruitment and infiltration of leucocytes within the first hour, culminating in disruption of islet morphology [5,8]. The IBMIR provide a possible explanation for the low success rate and need for large numbers of islets to render a type 1 diabetic patient insulin-independent.
The mechanisms underlying the injurious effects of the IBMIR must be clarified further to find the ideal approach to avoid the destruction of islets in the immediate post-transplantation phase. Researchers in several laboratories, including ours, have studied the IBMIR in the short-term perspective [5,8,9]. However, no detailed characterization has yet been reported concerning blood cells infiltrating human islets after exposure to blood.
In the present study, we have developed a new tube system where islets and ABO-identical blood are mixed for up to 6 h. This experimental system was designed to mimic the first few hours after islet infusion into the portal vein and is particularly suitable for immunohistochemical evaluation. By using this system and collecting samples at various time-points the infiltration pattern of blood cells during the early post-transplantation period was characterized.
Materials and methods
Islet isolation and culture
Islets were isolated from human cadaver donors (using a protocol approved by the local ethical committee), as described previously [10–12]. The organs were obtained from six normoglycemic donors, four males and two females (aged 19–69 years, three with blood group O, two with blood group A and one with blood group B).
The islet preparations were placed into untreated culture dishes and maintained in suspension 2–10 days, cultured at 37°C (5% CO2) in CMRL 1066 (Gibco-BRL, Invitrogen, Paisley, UK) supplemented with 10 mmol/l nicotinamide (Sigma Chemicals, St Louis, MO, USA), 10 mmol/l HEPES, 0·25 µg/ml fungizone, 50 µg/ml gentamicin, 2 mmol/l l-glutamine (all purchased from Gibco-BRL, Invitrogen), 10 µg/ml ciprofloxacin (Bayer AG, Leverkusen, Germany) and 10% (v/v) heat-inactivated human serum. The medium was changed every other day.
The volume and purity of the islets were determined, after staining with diphenylthiocarbazone by microscopic sizing on a grid. The purity of the islet preparations used in this study ranged from 60% to 90% (mean 72 ± 5·2%).
Heparin coating
Materials in contact with fresh human blood were furnished with a Corline heparin surface (Corline, Uppsala, Sweden) according to the manufacturer's recommendation [13]. The surface concentration of heparin was 0·5 µg/cm2, corresponding to approximately 0·1 U/cm2, with an antithrombin binding capacity of 2–4 pmol/cm2. The stability of a similar heparinized surface has been determined by Andersson et al. [14] with no leakage of heparin detected over a period of 1 h.
Preparation of blood
Fresh human blood, obtained from healthy volunteers who had received no medication for at least 14 days was collected in surface-heparinized 60-ml syringes (18-gauge, Microlance; Becton Dickinson, Franklin Lakes, NJ, USA). The cannulae of the syringes were connected to surface-heparinized silicon tubing. During sampling, the syringes were gently rotated continuously.
Experimental design
Heparinized 2·5-ml Ellerman tubes were used to mix 1·5 µl islets (corresponding to ∼1500 IEQ) with 250 µl fresh human ABO-compatible blood (no anticoagulant added). The tubes were incubated on a rocking device from 5 min up to 6 h at 37°C. Samples were collected at 5, 15, 30, 60, 120, 180, 240, 300 and 360 min. For each time-point, one tube with islets and blood was used.
At the time-points where samples were collected, ethylenediamine tetra-acetic acid (EDTA) was added to the tubes to give a final concentration of 4·1 mmol/l, to halt the ongoing clotting reaction. Immediately after the reaction was stopped, islets and macroscopic clots from each tube were recovered on filters, collected in embedding medium (Tissue-Tek; Miles, Eckhart, IN, USA) and snap-frozen in liquid nitrogen for further immunohistochemical analyses. All experiments included a blood control tube that had no islets but contained 1·5 µl serum-free culture medium (the buffer in which the islets were resuspended).
The reaction in the control tube was stopped at the first time-point at which a clot appeared.
Preparation of slides
Cryostat sections (7 µm) of frozen islets and clots were prepared and stored at −70°C until analysed. For each biopsy specimen, sections from four different layers were placed on a slide and each stained for an individual marker.
Immunohistochemistry
The stored slides were fixed in cold 50% acetone (v/v) for 30 s, then transferred to 100% acetone (v/v) for 5 min and air-dried. All subsequent steps were performed at room temperature. The sources, specificities and dilutions of the antibodies used are summarized in Table 1.
Table 1. Antisera used in this study.
| Antisera raisedab | Dilution | Specificity | Code | Source |
|---|---|---|---|---|
| CD41aa | 1 : 20 | Platelets | M 7057 | Dako |
| Tissue factora | 1 : 50 | TF | 4509 | American Diagnostica, Inc. |
| CD11ba | 1 : 50 | Leu/Lymc | M 0741 | Dako |
| CD20a | 1 : 100 | B-cells | M 0755 | Dako |
| CD4a | 1 : 20 | Th2 | 346320 | Becton Dickinson |
| CD8a | 1 : 100 | Th1 | M 0707 | Dako |
| CD16a | 1 : 50 | Gran/Moc | F 7011 | Dako |
| CD68a | 1 : 100 | Mo | M 0718 | Dako |
| CD209 (DC-SIGN) | 1 : 50 | Dendritic | 14–2099 | Bioscience |
| Neutrophil elastasea | 1 : 50 | Neutrophils | M 0752 | Dako |
| Lysozymeb | 1 : 200 | Gran/Mo | A 0099 | Dako |
Antisera was raised in mouse.
Antisera was raised in rabbit.
Leu: leucocytes, Lym: lymphocytes, Gran: granulocytes, Mo: monocytes.
Depending on the origin of the primary antibody, three different sets of reagents and protocols were used: Mouse-Envision (from Dako, Copenhagen, Denmark) for monoclonal antibodies (i.e. tissue factor (TF), CD41a, CD11b, CD20, CD68, CD16 and neutrophil elastase), Rabbit-Envision (Dako) for polyclonal antibodies (lysozyme) and the APAAP technique (alkaline phosphatase anti-alkaline phosphatase) for the CD209/DC-SIGN antibody [15].
The Envision systems comprised the following steps: blocking of endogenous peroxidases with 0·3% H2O2 (Kebo Laboratory AB, Stockholm, Sweden) in phosphate-buffered saline (PBS) for 15 min (except for the TF staining, where the slides were left untreated); incubation with primary antibody for 30 min, followed by Mouse- or Rabbit-Envision (Dako); detection in a solution of 10 mg 3-amino-9-ethylcarbazol in 6 ml of dimethylsulphoxide, 50 ml of 0·02 mmol/l NaAc (pH = 5·5) and 8 µl of 30% (v/v) H2O2 for 15 min; counterstaining with haematoxylin; and finally embedding in glycerol gelatin.
The APAAP technique involved the following steps: blocking with normal rabbit serum [Dako, dilution 1 : 10 in antibody diluent (Dako)] for 15 min; incubation with primary antibody (diluted in antibody diluent) for 30 min; incubation with secondary antibody (rabbit anti-mouse-Ig, Dako, diluted 1 : 25 in antibody diluent); incubation with APAAP complex (mouse, Dako, diluted 1 : 15 in antibody diluent) for 30 min; and finally detection with 5-bromo-4-chloro-3-indoxyl phosphate (BCIP, Dako) for 15 min, followed by counterstaining with haematoxylin and embedding in glycerol.
In all immunohistochemical staining experiments, a slide with no primary antibody was treated in parallel to serve as a negative control.
Eosinophilic granulocytes were detected by histochemical visualization of cyanide-resistant endogenous peroxidase activity [16]. In brief, sections were incubated for 8 min at room temperature in PBS buffer supplemented with 3·3-diaminobenzamidine tetrahydrochloride (Sigma Chemicals), 30% H2O2 and NaCN (120 mg/100 ml). After rinsing in water, slides were counterstained with haematoxylin and mounted in glycerine gelatin.
The blood cells within the clots surrounding the islets served as positive controls for the different antisera and enzymatic staining used. Purified dendritic cells [17] served as a positive control for the CD209/DC-SIGN staining.
Immunohistochemical evaluations
The infiltrating cells with various phenotypical characteristics were divided semiquantitatively into four categories: 0 = no observed infiltrating cells, 1 = few infiltrating cells, 2 = moderate number of infiltrating cells, 3 = massive number of infiltrating cells.
Twenty sections from each time-point (5, 15, 30, 60, 120, 180, 240, 300 and 360 min) were evaluated for each of the various markers and scored blinded with regard to the antibody used.
Results
To study the infiltration of islets in contact with blood over time, islets from six pancreases were mixed with blood, and the presence of blood cells was analysed immunohistochemically using specific markers at 5, 15, 30, 60, 120, 180, 240, 300 and 360 min.
Tissue factor
TF was detected in all islet preparations (n = 6) (Table 2). This is consistent with our recent finding that freshly isolated islets express TF [7]. However, of the six islet preparations, one islet batch was negative for TF up to 60 min, while the other five already stained positive for TF in the 5-min sample.
Table 2. Frequency of infiltration into islets of Langerhans a.
| Marker | 5′ | 15′ | 30′ | 60′ | 120′ | 180′ | 240′ | 300′ | 360′ |
|---|---|---|---|---|---|---|---|---|---|
| TF | 2·6 ± 0·4b | 2·6 ± 0·4 | 2·4 ± 0·4 | 2·75 ± 0·2 | 2·6 ± 0·2 | 2·8 ± 0·2 | 3·0 ± 0·0 | 3·0 ± 0·0 | 3·0 ± 0·0 |
| CD16 | 0·25 ± 0·2 | 1·0 ± 0·4 | 1·2 ± 0·4 | 2·2 ± 0·5 | 2·6 ± 0·4 | 2·75 ± 0·2 | 2·75 ± 0·2 | 2·75 ± 0·2 | 3·0 ± 0·0 |
| Lysozyme | 1·0 ± 0·4 | 2·25 ± 0·2 | 2·25 ± 0·2 | 2·6 ± 0·2 | 2·6 ± 0·2 | 2·6 ± 0·4 | 2·6 ± 0·4 | 3·0 ± 0·0 | 2·8 ± 0·2 |
Staining for CD20, CD4, CD8, eosinophils (detected by cyanide-resistant endogenous peroxidase activity) and CD209 (DC-SIGN) were all negative, i.e. they were not detected in the islets.
Frequency of infiltration scored from 0 to 3, with 3 indicating strongly positive staining. All data are expressed as mean ± s.e.m.
In the initially TF-negative islet batch, TF was first expressed after 60 min in contact with blood; expression reached a maximum at 2 h and remained constant throughout the rest of the observation time (6 h). The apparent up-regulation of TF in contact with blood that is seen in Table 2 is due only to this islet batch that initially stained negative for TF (data represent mean values).
Platelets
The platelets were never seen to penetrate the islets in contact with blood, but rapidly surrounded them. The platelet wreath grew during the initial phase (up to 30 min) then remained constant throughout the observation period (Fig. 1b).
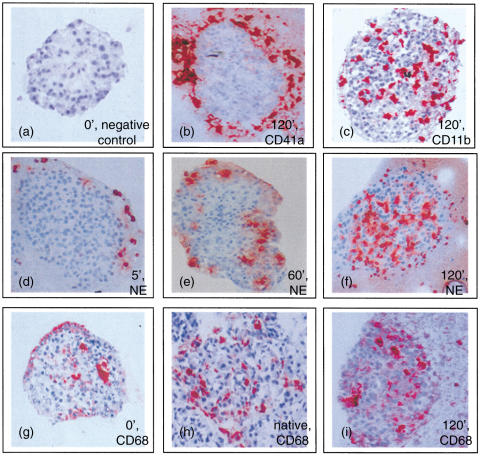
Fig. 1.
Immunohistochemical stainings. Human islets retrieved from the tube model at the time points indicated in the figure (a-g and i) or islets in the native pancreas (h). Sections were stained for the different markers indicated in the figure: platelets (CD41a), leucocytes (CD11b), neutrophilic granulocytes (NE) and macrophages (CD68). Magnification × 20.
Infiltration of white blood cells
The presence of leucocytes and lymphocytes in the islets, as detected immunohistochemically by staining for the specific marker CD11b, was apparent after 15 min and reached a peak at 60 min (Figs 1c and 2). The degree of infiltration by CD11b+ cells varied among the islets found in the same clot. This variation was not limited to CD11b, but was observed for all the markers discussed below.
Fig. 2.
White blood cells infiltrating islets during the 6 h in contact with blood, □, CD11b-positive leucocytes, ▴, neutrophilic granulocytes (neutrophil elastase, NE), ▵, macrophages (CD68). Data represent mean ± s.e.m., n = 6.
Granulocytes
Several markers were used to identify the presence of granulocytes in the islets.
After 5 min neutrophilic granulocytes were seen to gather in close proximity to the islets without infiltrating them (Fig. 1d), and after 15 min the neutrophilic granulocytes were detected in the islets (Fig. 2). The number of infiltrating neutrophilic granulocytes was increased at 60 min (Figs 1e and 2) and peaked at 120 min (Figs 1f and 2). Eosinophilic granulocytes were found in the surrounding clots but were never detected within the islets at any time-point.
Macrophages
Infiltration by macrophages was indicated by staining for CD68. These cells were detected in the islets at the 5-min time-point after exposure to blood (Fig. 2). Additional control stainings of isolated islets that had not been exposed to blood and in pancreas biopsies revealed the presence of macrophages in the islets (Fig. 1g,h). Another macrophage marker, MAC 387, also stained positive in these additional control specimens, further confirming their presence in the non-treated islets and pancreas.
During the 6-h observation period there was a trend towards a slight increase in the number of infiltrating macrophages (not statistically significant, Fig. 1i).
Lymphocytes
Staining for specific markers for B cells (CD20) and T cells (CD8 and CD4) revealed no infiltration of any of these cell types throughout the 6-h observation period. All specific markers stained positive in the surrounding clots with no increase in number over time. Notably, no tendency of clustering around or in the islets was observed.
Dendritic cells
No dendritic cells (CD209/DC-SIGN) were detected in any of the islet preparations during the 6-h observation time.
Discussion
By employing a new experimental system, we were able to examine islets embedded in clots and identify the blood cells involved in the IBMIR at defined time-points. The intention with the tube system used was to mimic the situation during the first few hours after intraportal transplantation of isolated islets. To produce a tube surface that generated very little or no intrinsic activation of the cells and cascade systems present in blood was of particular concern in the design of this tube system. Therefore, surface-heparinization was used to create an ‘endothelial cell-like lining’ of all the artificial surfaces that came into contact with blood. Also, as smaller volumes of blood were used than in the blood loop system described previously [5], islets in clots were easily recovered, and the IBMIR could be studied for a longer time interval.
Using this system, we found that neutrophilic granulocytes were the predominant cell type infiltrating the islets. After 15 min they had already appeared in the islets, and at 60 min this infiltration was increased further and peaked at 2 h. A low number of macrophages, with a slight increase (not significant), were also found within the islets. Control staining of islets not exposed to blood and in pancreas biopsies also revealed the presence of macrophages. We therefore presumed that the majority of these macrophages were of donor origin, although it cannot be excluded that there is a continuous exchange of donor and recipient macrophages.
The macrophages found in the islets may act in a fashion similar to that described previously for antigen-presenting cells (APCs) migrating from a transplanted organ to the tissues of the recipient, thereby directing the adaptive immune system towards the graft. At present, there is an ongoing debate about whether these cells participate in the development of tolerance or initiate rejection of the transplanted organ [18,19].
Variability was observed in the extent to which both granulocytes and macrophages infiltrated different islets from the same donor. This difference is difficult to explain, but may be due to variation of expressed inflammatory mediators between individual islets. Most probably, the islets escaping the IBMIR have an inherent low expression of inflammatory regulators increasing their chances of successful engraftment. This observation also holds promise for in vitro techniques currently being developed to down-regulate the expression of inflammatory mediators in human islets prior to transplantation.
However, platelets were seen to adhere to the islet surface after 5 min, increasing progressively over time. Activated platelets are known to release a number of important growth factors, such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) [20,21]. Hence, in clinical islet transplantation, when islets are embolized in the liver, a wreath of adhering platelets might support revascularization and engraftment in the liver tissue. A balanced IBMIR might be the optimal strategy for maximal engraftment.
Our findings suggest that the neutrophilic granulocyte is the predominant cell type infiltrating the islets. The infiltration pattern in the IBMIR resembles that observed in ischaemic reperfusion injuries. In this context the neutrophilic granulocytes are attracted to the graft due to up-regulation and release of agents by the ischaemia-induced alterations of the endothelial cells and parenchyma cells of the transplanted organ [22]. Similarly, their recruitment to the islets could also be due to induced proinflammatory signals [23] released from the islets that are capable of attracting and activating neutrophilic granulocytes when they are exposed to blood.
Neutrophilic granulocytes fulfil their role by killing pathogens via cytotoxic attack and phagocytosis. After cell activation they generate superoxidase to form reactive oxygen species (ROS) [24,25]. ROS, together with proteases liberated from the granules of the activated neutrophilic granulocytes, are involved in killing the microbes. ROS are short-lived and react largely without specificity; therefore, they cause widespread destruction. In this matter, it is worthwhile mentioning that islet β cells are exquisitely susceptible to oxidative stress because of their insufficient antioxidant pool [26], a situation that points to rapid and direct damage to the islets by the infiltrating granulocytes.
Neutrophilic granulocytes are also known to contain a pool of cytokines that are released upon activation, and there is increasing evidence that cytokines have deleterious effects upon islets [27]. Also, infiltration by neutrophilic granulocytes leads to the release of chemotactic factors for T cells and macrophages, such as tumour necrosis factor α (TNF-α) and macrophage inflammatory protein 1α (MIP-1α). This mobilization of immune effectors could have implications for the specific immune system, inducing and enhancing cellular rejection episodes.
The mechanism(s) by which the islets stimulate neutrophilic granulocyte and macrophage recruitment is (are) unknown. In previous studies we have suggested that CD11b+ leucocyte infiltration is a result of complement activation [5,8]. The anaphylatoxins C3a and C5a, released upon activation, are known to be major mediators for neutrophilic granulocyte migration [28]. In a follow-up study in which melagatran, a direct thrombin inhibitor, was used to inhibit the IBMIR, both coagulation and complement were abrogated. However, infiltration of CD11b+ leucocytes was still found in the islets, although to a lesser extent [8]. Taken together, these observations indicate that complement activation by itself is not the only mechanism by which neutrophilic granulocytes are recruited to the graft; one or more additional mechanism(s) also seem(s) to be involved.
The recruitment of neutrophilic granulocytes as well as macrophages to the islets could also be elicited by proinflammatory signals released by the islets themselves. One such mechanism has been demonstrated by Piemonti and coworkers [23]. Using chemotaxic assays, they found a correlation between the number of infiltrating macrophages and the amount of macrophage chemotactic protein-1 (MCP-1) produced by the islet [23]. Islets have also been shown to express interleukin (IL)-8, a known chemotactic agent for neutrophilic granulocytes [29].
The massive infiltration by neutrophilic granulocytes probably causes direct damage to the islets, not only by functionally impairing or reducing the mass of the implanted islets but probably also by amplifying the subsequent immune response [30].
If tolerance to the transplanted islets is to be achieved, the IBMIR must be controlled. The results presented in this study identify the cells that apparently participate in the destructive processes of the IBMIR. The immuosuppressive regimen applied in clinical islet transplantation at present (tacrolimus, rapamycin and daclizumab) is directed against the specific cellular immune response and the IBMIR is most probably not affected by this medication. The findings in the present study suggest that the next generation of immunosuppressive protocols for islet transplantation should also include drugs to counteract the IBMIR. The tube system employed here could serve as an in vitro system for evaluating the effect of candidate drugs designed to prevent the infiltration part of the IBMIR.
Acknowledgments
We thank Graciela Elgue for much appreciated input during the writing of the manuscript, Selina Parvin for excellent technical assistance and Dr Deborah McClellan for editing the text. This study was supported by grants from the Juvenile Diabetes Foundation International, the Swedish Research Council (16P-13568, 16X-12219, 16X-5674, 72X-06817–19), the Åke Wiberg Foundation, the Nordic Insulin Fund, the Novo Nordisk Foundation, the Torsten and Ragnar Söderbergs Foundation, the Ernfors Family Fund, Barn Diabetes Fonden, the Swedish Diabetes Association, the Clas Groschinsky Foundation, the Knut and Alice Wallenberg Foundation, the National Health Institute and the Swedish Animal Welfare Agency.
References
- 1.Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–36. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 2.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–8. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 4.Ryan EA, Lakey JR, Rajotte RV, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710–9. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 5.Bennet W, Sundberg B, Groth CG, et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907–14. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 6.Bennet W, Sundberg B, Lundgren T, et al. Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: protective effects of sCR1 and heparin. Transplantation. 2000;69:711–9. doi: 10.1097/00007890-200003150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360:2039–45. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 8.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51:1779–84. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 9.Titus TT, Horton PJ, Badet L, et al. Adverse outcome of human islet–allogeneic blood interaction. Transplantation. 2003;75:1317–22. doi: 10.1097/01.TP.0000064517.98252.00. [DOI] [PubMed] [Google Scholar]
- 10.Brandhorst H, Brandhorst D, Brendel MD, Hering BJ, Bretzel RG. Assessment of intracellular insulin content during all steps of human islet isolation procedure. Cell Transplant. 1998;7:489–95. doi: 10.1177/096368979800700508. [DOI] [PubMed] [Google Scholar]
- 11.Lakey JR, Warnock GL, Shapiro AM, et al. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant. 1999;8:285–92. doi: 10.1177/096368979900800309. [DOI] [PubMed] [Google Scholar]
- 12.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–20. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 13.Gong J, Larsson R, Ekdahl KN, Mollnes TE, Nilsson U, Nilsson B. Tubing loops as a model for cardiopulmonary bypass circuits: both the biomaterial and the blood–gas phase interfaces induce complement activation in an in vitro model. J Clin Immunol. 1996;16:222–9. doi: 10.1007/BF01541228. [DOI] [PubMed] [Google Scholar]
- 14.Andersson J, Sanchez J, Ekdahl KN, Elgue G, Nilsson B, Larsson R. Optimal heparin surface concentration and antithrombin binding capacity as evaluated with human non-anticoagulated blood in vitro. J Biomed Mater Res A. 2003;67:458–66. doi: 10.1002/jbm.a.10104. [DOI] [PubMed] [Google Scholar]
- 15.Cordell JL, Falini B, Erber WN, et al. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes) J Histochem Cytochem. 1984;32:219–29. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- 16.Ten RM, Pease LR, McKean DJ, Bell MP, Gleich GJ. Molecular cloning of the human eosinophil peroxidase. Evidence for the existence of a peroxidase multigene family. J Exp Med. 1989;169:1757–69. doi: 10.1084/jem.169.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlsson B, Cheng WS, Totterman TH, Essand M. Ex vivo stimulation of cytomegalovirus (CMV)-specific T cells using CMV pp65-modified dendritic cells as stimulators. Br J Haematol. 2003;121:428–38. doi: 10.1046/j.1365-2141.2003.04300.x. [DOI] [PubMed] [Google Scholar]
- 18.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–82. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones ND, Van Maurik A, Hara M, et al. CD40—CD40 ligand-independent activation of CD8+ T cells can trigger allograft rejection. J Immunol. 2000;165:1111–18. doi: 10.4049/jimmunol.165.2.1111. [DOI] [PubMed] [Google Scholar]
- 20.Jensen RL. Growth factor-mediated angiogenesis in the malignant progression of glial tumors: a review. Surg Neurol. 1998;49:189–95. doi: 10.1016/s0090-3019(97)00218-8. ; discussion 96. [DOI] [PubMed] [Google Scholar]
- 21.Dunn IF, Heese O, Black PM. Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neurooncol. 2000;50:121–37. doi: 10.1023/a:1006436624862. [DOI] [PubMed] [Google Scholar]
- 22.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61:481–97. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Piemonti L, Leone BE, Nano R, et al. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51:55–65. doi: 10.2337/diabetes.51.1.55. [DOI] [PubMed] [Google Scholar]
- 24.Phillis JW. A ‘radical’ view of cerebral ischemic injury. Prog Neurobiol. 1994;42:441–8. doi: 10.1016/0301-0082(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 25.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 26.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–42. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 27.Andersson AK, Flodstrom M, Sandler S. Cytokine-induced inhibition of insulin release from mouse pancreatic beta-cells deficient in inducible nitric oxide synthase. Biochem Biophys Res Commun. 2001;281:396–403. doi: 10.1006/bbrc.2001.4361. [DOI] [PubMed] [Google Scholar]
- 28.Tanoue M, Yoshizawa Y, Sato T, Yano H, Kimula Y, Miyamoto K. The role of complement-derived chemotactic factors in lung injury induced by preformed immune complexes. Int Arch Allergy Immunol. 1993;101:47–51. doi: 10.1159/000236497. [DOI] [PubMed] [Google Scholar]
- 29.Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgren O. Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem Biophys Res Commun. 2003;308:474–9. doi: 10.1016/s0006-291x(03)01392-5. [DOI] [PubMed] [Google Scholar]
- 30.Badet L, Titus T, Metzen E, et al. The interaction between primate blood and mouse islets induces accelerated clotting with islet destruction. Xenotransplantation. 2002;9:91–6. doi: 10.1034/j.1399-3089.2002.1o040.x. [DOI] [PubMed] [Google Scholar]