Abstract
Cystic fibrosis (CF) is characterized by a neutrophil-dominated chronic inflammation of the airways with persistent infections. In order to investigate whether neutrophils contribute to an inadequacy in the pulmonary defence mechanism, the phagocytic activity of pulmonary and peripheral blood neutrophils from CF and non-CF respiratory patients were compared. Neutrophils were isolated from both the blood and bronchoalveolar lavage fluid of 21 patients with CF (12 male, 9 female; mean age 7·5 years, range 0·25–16·4 years) and 17 non-CF subjects (9 male, 8 female; mean age 5·4 years, range 0·2–13·1 years). The ex vivo phagocytic rate of normal pulmonary neutrophils to internalize zymosan particles opsonized with iC3b was faster than that of circulating neutrophils (P < 0·05), but the maximum capacity (9 particles/cell) was similar. In contrast, pulmonary neutrophils from patients with CF had a lower phagocytic capacity than circulating neutrophils either from the same patients or from normal subjects. This deficiency could not be attributed to (i) the cell surface density of CR3 (CD18/CD11b) receptors, which were not significantly different between the other groups (ii) the signalling ability of the CR3 receptors, using cytosolic free Ca2+ signalling as the receptor activity read-out or (iii) a decrease in cellular ATP concentration. As CFTR was not detectable on neutrophils from any source by either histochemistry or Western blotting, it was concluded that the reduced phagocytic capacity was not the direct result of a CFTR mutation, but was attributed to a failure of neutrophil phagocytic priming during translocation into the CF lung.
Keywords: neutrophils, phagocytosis, cystic fibrosis, Ca2+ signalling
Introduction
Cystic fibrosis (CF) is characterized by a neutrophil-dominated chronic inflammation of the airways. However, despite the presence of pulmonary neutrophils, the airways rapidly become colonized by bacteria [1], suggesting that the pulmonary neutrophils in CF may have reduced efficacy in combating infection. The mechanism of neutrophil recruitment to sites of infection is well established. TNF mediates up-regulation of ICAM-1 on endothelial cells, which is the counter ligand for β2 integrin on neutrophils and signals a sequence of neutrophil events including adherence to the endothelium, transmigration through the endothelium and chemotaxis towards the site of bacterial infection. Neutrophils thus extravasate at sites of elevated TNF, which acts on neutrophils to ‘prime’ them for subsequent activity, particularly by priming the neutrophil oxidase [2]. The extravasating neutrophils may also be primed for iC3b-mediated phagocytosis by activation of its receptor, either by an increase in affinity [3] or avidity and mobility [4]. However, the mechanism by which neutrophils are recruited to the CF airways is less clear, particularly as the accumulation of neutrophils in the CF lung may precede infection. There is evidence that neutrophil infiltration into the lung occurs before detectable lung infection in both the newborn CF child [5] and in an animal model of CF [6]. The persistent presence of neutrophils in the CF lung, which is probably in response to chronic infection and the generation of interleukin 8 [7], may exacerbate lung tissue degradation [5] and cause epithelial cell damage [8] by releasing neutrophil elastase [7]. Furthermore, the released neutrophil elastase may also degrade receptors on the pulmonary neutrophils, including the C3b receptor CR1 [9]. Although the receptor for iC3b, the main complement opsonin, CR3, is resistant to neutrophil elastase proteolytic activity, it has been suggested that this effect on CR1 may compromise pulmonary neutrophil function. In addition to the possibility that released neutrophil elastase in the lung may have a detrimental effect on neutrophil responsiveness, some studies have suggested that circulating neutrophils also have aberrant behaviour. For example, it is reported that peripheral blood neutrophils from CF patients exhibit a range of abnormal behaviours such as an altered response to N-formyl-methionyl-phenylalanine [10], increased myeloperoxidase-dependent oxidative responses [11] and decreased shedding of l-selectin [12].
The question remains, however, as to reasons behind the lack of bacterial clearance by lung neutrophils in CF. In particular, the efficacy of the main complement opsonin by which lung neutrophils ‘recognize’, bind and phagocytose bacteria, iC3b, has not been established in CF. The aim of this study therefore was to investigate the phagocytic activity, ex vivo, of pulmonary and peripheral blood neutrophils from CF patients. The phagocytic ability of neutrophils from non-CF respiratory patients undergoing investigation for persistent, or recurrent, respiratory infection was compared with that of neutrophils from patients with CF and with circulating neutrophils. We report here that neutrophils from the CF lung had a lesser capacity for phagocytosis of iC3b-opsonized particles than lung neutrophils in other situations where priming of the response was observed. This effect could not be attributed to a reduction in density or signalling ability of the iC3b receptor, CR3, or to neutrophil ATP content. These data suggest that neutrophil which infiltrate the CF lung are poorly primed for phagocytosis. The reduced phagocytic capacity of lung neutrophils in CF may be an important contributory factor towards explaining the persistent infection of CF lungs.
Subjects and methods
Subjects comprised 21 patients with CF (12 male, 9 female; mean age 7·5 years, range 0·25–16·4 years) attending the paediatric CF clinic at the University Hospital of Wales (UHW). Pulmonary lavage samples were obtained from CF patients undergoing either diagnostic flexible bronchoscopy performed under midazolam and pethidine conscious sedation, or general anaesthesia for routine surgical procedures, such as insertion of indwelling venous access device. Pulmonary neutrophils were harvested following instillation and aspiration of warmed normal saline (1 ml/kg, minimum 5 ml, maximum 20 ml). Peripheral blood was obtained by venesection at the time of procedure. There were no significant differences between the clinical parameters of patients undergoing either flexible bronchoscopy or presurgical lavage.
Genotypes for the CF population were 9 dF508/dF508, 9 dF508/other and 3 other/other. At the time of bronchoscopy 20/21 were receiving oral antibiotics, 9/21 were receiving intravenous antibiotics, 13/21 were receiving regular inhaled corticosteroids and 2/17 were receiving oral corticosteroids. Upper airway cultures or sputum in the preceding 12 months had detected Pseudomonas aeruginosa in 3, Staphylococcus aureus in 2, Haemophilus influenzae in 2, Candida albicans in 4 and atypical mycobacterium in 1. At the time of bronchoscopy, a pathogen was isolated from 6/21 CF BAL samples: 2 Pseudomonas aeruginosa (1 with concurrent Staphylococcus aureus), 1 atypical mycobacteria and 3 Candida albicans.
The non-CF group comprised 17 subjects (9 male, 8 female; mean age 5·4 years, range 0·2–13·1 years) undergoing investigation at UHW for recurrent respiratory indications. At the time of bronchoscopy 4/17 were receiving oral antibiotics, 2/17 were receiving intravenous antibiotics, 6/17 were receiving regular inhaled corticosteroids and 1/17 was receiving oral corticosteroids. A pathogen was isolated from 5/17 non-CF BAL samples: 3 Streptococcus pneumoniae and 2 Candida albicans.
The local Bro Tâf ethics committee approved the study. Parents gave written informed consent, and children gave informed assent or consent dependent on age.
Neutrophil isolation
All lavage samples were transported on ice and processed within 1 h. A typical sample (1–3 ml) contained lung detritus such as sloughed epithelial cells, mucous and DNA, white cells and, on occasion, visible bacteria. From each sample, 40 µl was cytospun for 30 s, fixed in methanol and stained with May – Grunwald/Giemsa for a visual assessment of cellular content and neutrophil morphology. The remaining volume was centrifuged (2000rpm, 2min) and the fluid aspirated and stored at −80°C. Cells were resuspended in Krebs-HEPES buffer (KHB) (120 mM NaCl, 4·8 mM KCl, 1·2 mM KH2PO4, 1·2 mM MgSO4, 1·3 mM CaC12, 25 mM HEPES, 0·1% BSA, to pH 7·4 with NaOH). Peripheral neutrophils were separated from plasma of whole heparinized blood by dextran sedimentation, hypotonic red-cell lysis and ficoll-hypaque at a density of 1·08 g/ml (179 g, 20 min). The resulting neutrophil pellet was resuspended in Krebs buffer to a concentration of 106 cells/ml.
Assessment of phagocytosis
Zymosan was opsonized with iC3b by incubation with human serum at 10 mg/ml for 30 min at 37 °C, followed by several washes in order to remove extraneous proteins. Neutrophils were incubated (0–60 mins) on glass slides with zymosan particles (l mg/ml) at 37 °C, after which nonadhered material was removed by washing and phagocytosis visualized in adherent cells after fixing (methanol) and staining (May–Grunwald/Giemsa). Phagocytosis of zymosan was assessed by light microscopy by quantifying the number of internalized zymosan particles in at least 100 neutrophils for each time point for each patient. From the distribution of zymosan particles/neutrophil, the mode value was taken. All data for phagocytosis show the mean value of the modal number of zymosan particles/neutrophil in repeat experiments. The rate of phagocytosis was defined as the initial uptake of particles up to 10 min. The capacity of the neutrophils was assessed at 60 min when zymosan uptake approached its maximum. Phagocytosis data were analysed using Student's t-test and the significant difference was set at P ≤ 0·05.
Measurement of CR3
The density of CR3 was determined using anti-CD18-FITC or anti-CD11b-phycoerthythrin (Dako, High Wycombe, UK), which was incubated with neutrophils on ice for 30 min, before washing (PBS + BSA 1%), fixing (paraformaldehyde 1% in PBS + BSA 1% + NaN3 0·01%). The binding was assessed by flow cytometry and confocal imaging (Leica CLSM; Leica Microsystems, Milton Keynes, UK). Z-plane confocal sections of adherent neutrophils were also acquired to compare CR3 expression on adherent and free neutrophil plasma membranes.
Measurement of cytosolic free Ca2+ during phagocytosis
Ca2+ signalling via CR3 was monitored in neutrophils loaded with fura2, via its acteoxymetyl ester (Molecular Probes, Oregon, USA) as previously described [13]. Ratio (340/380 nm excitation) images were acquired by an intensified IC-200 CCD camera (PTI) coupled to a Nikon Eclipse microscope and a rapid wavelength switching monochromator analysis system (Photon Technologies International, Surbiton, UK). The cytosolic free Ca2+ concentration was calculated from the mean pixel ratio value within a defined area. Phase contrast imaging was simultaneously recorded by transillumination with red light using a 690 nm band pass filter and dichroic beam splitting. iC3b-opsonized zymosan particles were presented by micromanipulation (Eppendorf, Cambridge, UK) to the neutrophil under observation, using negative pressure applied to a micropipette (diameter = 2 µm) as previously described [4,14]. On contact with the cell, the particle was released from the micropipette so that phagocytosis could proceed. Digital visualization of cytosolic free Ca2+ concentration was recorded simultaneously with phase contrast images during particle uptake [4,14].
Other measurements
CFTR detection (i) in intact adherent neutrophils was by immunofluorescence labelling and (ii) in cell extracts was by Western blotting using anti-CFTR monoclonal mouse IgM clone CF3 (804–214-R100 Alexis Corporation, San Diego, CA, USA) and polyclonal (sc-8910, Santa Cruz Biotechnology, Inc., CA, USA) antibodies. Following precipitation of soluble proteins with trichloracetic acid (10%), ATP was measured using firefly luciferase as described [15].
Results
Phagocytic activity of CF and non-CF neutrophils
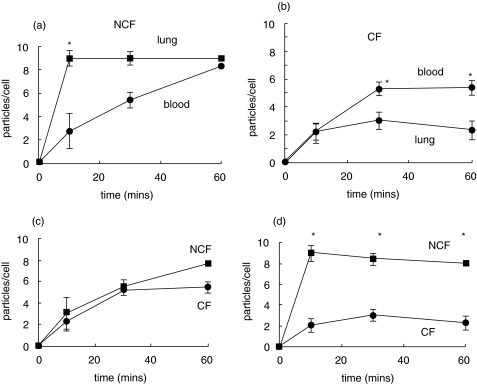
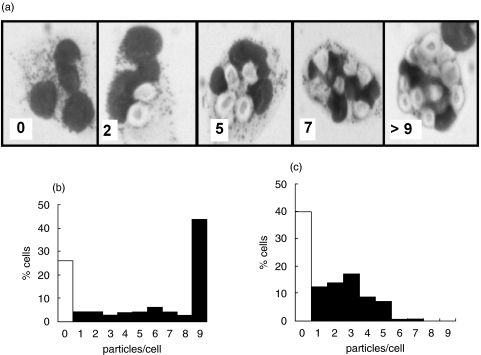
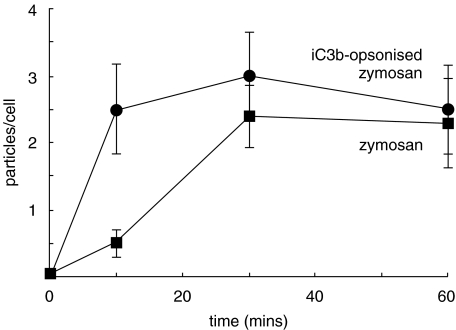
The rate of phagocytosis of iC3b-opsonized zymosan particles by pulmonary neutrophils from non-CF patients was significantly faster (P < 0·01) than peripheral neutrophils from the same patients (Fig. 1a). This was consistent with either the selection of a subpopulation of neutrophils which were able undergo rapid phagocytosis from the circulation or the ‘priming’ of neutrophils during extravasation from the circulation. By 60 min, the number of particles taken up in both groups had reached a plateau and was similar, suggesting that neutrophils from either source, though having rate different phagocytic rates, had a similar phagocytic capacity (Fig. 1a). In contrast, the initial uptake rate of iC3b-opsonized zymosan by pulmonary neutrophils from patients with CF was similar to peripheral blood neutrophils from these patients (Fig. 1b). No evidence of ‘priming’ or selection of a subpopulation of neutrophils during extravasation to the lung was found in these patients. Moreover, the maximum capacity of the pulmonary cells from CF patients was significantly decreased (P < 0·05) to only 60% of that of the blood neutrophil capacity (Fig. 1b). Interestingly, the rates of phagocytosis and the maximum capacities of the peripheral circulating neutrophils from CF and non-CF patients were similar (Fig. 1c), suggesting that there was no defect in circulating neutrophils in CF. It was the comparison between phagocytosis by pulmonary neutrophils from subjects with and without cystic fibrosis which demonstrated the most striking decrease in both the rate and capacity of C3bi-opsonized zymosan uptake by the CF neutrophil (P < 0·005) (Fig. 1d). The distribution of particles taken up by individual cells in the population of lung neutrophils for non-CF (Fig. 2b) and CF patients (Fig. 2c) was strikingly shifted towards smaller numbers of particles per cell, with a larger percentage of cells taking up zero in CF lung neutrophils and a larger number of cell taking up very high numbers of particles in non-CF lung neutrophils (Fig. 2). There were no significant differences in phagocytic capacities of neutrophils from either CF or non-CF patients without and without detectable infection or who were receiving or not receiving corticoids. This suggested that the differences in phagocytic capacity were attributable to an aspect of CF unrelated to the treatment or to the presence of detectable pathogens.
Fig. 1.
Time courses of opsonized zymosan particle phagocytosis. iC3b opsonized zymosan A particles (1 mg/ml) and neutrophils from the sources indicated were incubated on glass slides at 37 °C for 10, 30 and 60 min before washing with Krebs buffer to remove nonadhered material. The cells were then fixed and stained and the number of zymosan particles per cell counted in 100 neutrophils per slide. The time courses of zymosan uptake by neutrophils isolated from either the blood or lung fluid of patients with CF (21 patients) or the non-CF group (17 subjects) are shown. Neutrophils from non-CF (NCF) lung and circulation are compared in (a); neutrophils from CF lung and circulation are compared in (b); neutrophils from NCF and CF circulation are compared in (c) and neutrophils from NCF and CF lung are compared in (d). The mean and standard error are shown for each time point and * indicate time points at which there is a significant difference (P < 0·05).
Fig. 2.
Uptake of C3bi-opsonized zymosan particles by pulmonary neutrophils. (a) A montage of images is shown which indicates how each individual neutrophil within a population was classified as containing a specific number of zymosan particles. In each example, the number of particles per cell is indicated in the bottom left hand corner. Histograms show distributional data representative from 10 separate experiments of particles per neutrophil isolated from non-CF lung (b) and CF lung (c). In each case at least 100 neutrophils were counted. The white columns shows cells that have not taken up zymosan and the final column shows the percentage of neutrophils in which it was not possible to accurately count the number of zymosan particles within the cell but was at least 9 particles/cell.
CR3 expression and signalling by CF neutrophils
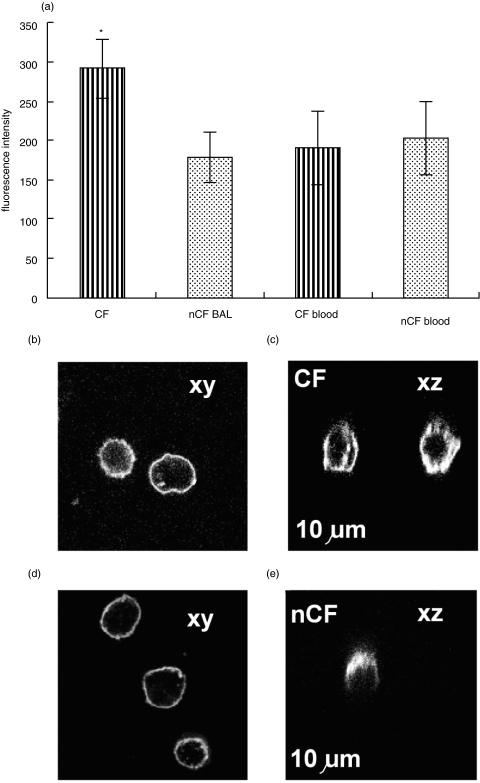
The number of iC3bi receptors (CR3) on lung neutrophils from patients with and without CF was measured, in order to establish whether a quantitative difference in receptor number was responsible for the decreased phagocytic capacity for iC3b-opsonized particles of the CF pulmonary neutrophil. There was no significant difference in the CR3 density on non-CF and circulating neutrophils on assessment by flow cytometry (Fig. 3a). However, the CR3 receptor density on CF pulmonary neutrophils was significantly increased (P < 0·05) by about 50%, and confirmed by confocal imaging (Fig. 3b,d). Furthermore, the mobility of CR3 molecules permitted them to move to sites of adhesion (Fig. 3c,e) suggesting that they may be functional [4]. Although, we failed to show the expected increase in surface CD18 in neutrophils in the lung of patients without CF, this was demonstrable in patients with CF. It was therefore clear that a lack of up-regulation of CR3 could not explain the decreased phagocytic capacity of CF lung neutrophils.
Fig. 3.
The histograms show (a) the mean of median fluorescence FACS values (arbitrary units) of CD18 surface expression on CF neutrophils (n = 5, striped columns) and non-CF neutrophils (n = 5, stippled columns) (P = 0·04). (b) CD18 expression on neutrophils from CF lung (b) and non-CF lung (c) was visualize confocally in two planes xy (i.e. standard view) and xz (i.e. perpendicular to the standard view). In the perpendicular view, a white line has been to indicate where the position of the coverslip, but is slightly below its true position so as not to obscure the level of CD18 expression at the cell-coverslip contact surface. These examples were typical of at least 5 other examples and also reflected the distribution of CD11b stained separately.
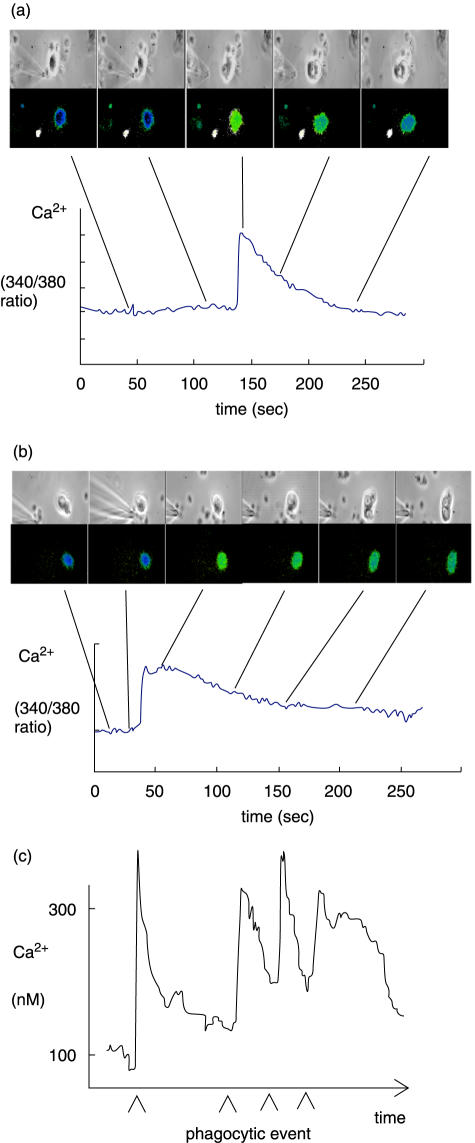
In order to further establish whether the CR3 receptors on CF lung neutrophils were functional, cytosolic free Ca2+ signalling was measured as an indicator of signalling ability of these receptors. Characteristic but variable cytosolic free Ca2+ signals are triggered after binding of iC3b-opsonized particles to circulating non-CF neutrophils via CR3 [4,14]. These signals were also triggered in circulating CF neutrophils, and in both pulmonary neutrophils from CF and non-CF (Fig. 4a,b). Although the cell-to-cell variability of the Ca2+ response was great and prevented meaningful comparisons between neutrophils from the separate groups, it was concluded that CR3 receptors on neutrophils from the CF lung were therefore capable of signalling Ca2+. Since the CF pulmonary neutrophil showed a reduced capacity for phagocytosis, the possibility that multiple phagocytic presentation might fail to trigger the Ca2+ signal was also investigated. However, CF lung neutrophils had a robust Ca2+ signalling response to successive phagocytic events with no sign of decreased Ca2+ signalling with up to 4 events (Fig. 4c).
Fig. 4.
Ca2+ signalling by CR3 on CF neutrophils. Relative changes in cytosolic free Ca2+ concentration during particle uptake are shown for neutrophils from (a) CF lung and (b) non-CF lung and (c) for successive particle uptake by a CF pulmonary neutrophil. In (a) and (b) the upper series show the delivery of a single zymosan a particle using a micropipette, the second series iof images show the cytosolic free Ca2+ pseudo-coloured image for the same view as in the upper series of images, where blues are low Ca2+ and greens are high Ca2+. The Ca2+ data is quantified in each case in the graph below the image series and the time at which the images time indicated by the lines. The data in (c) shows that an individual CF pulmonary neutrophil was able to elicit a number of phagocytic events and that each was accompanied by a typical Ca2+ signal. These data are representative of 7 separate experiments.
Another possibility considered was that although CR3 numbers and signalling were intact, these were not functionally coupled to an effect on phagocytosis. However, a comparison of the phagocytic uptake rates by CF lung neutrophils of iC3bi-opsonized and nonopsonized zymosan particles showed clearly that the presence of the opsonin accelerated phagocytosis, but did not increase the capacity (P < 0·05) (Fig. 5).
Fig. 5.
CR3 function on CF pulmonary neutrophils. The time course shows the rate of uptake by CF pulmonary neutrophils of zymosan particles either not opsonized (▪) and C3bi-opsonized zymosan particles (•) (n = 8). Opsonized zymosan were taken up at a faster rate than the unopsonized particles over the first 30 min, but had approximately the same maximum capacity. * indicates that data at that time point was significantly different (P < 0·05).
The decrease in capacity could not be attributed to a deficit in cellular ATP, which is known to limit phagocytosis [16]. ATP levels in CF pulmonary neutrophils were 4·0 ± 1·2 fmoles/cell (n = 5) and in circulating neutrophils were 2·9 ± 2·8 fmoles/cell (n = 8). These were not significantly different from pulmonary neutrophils from non-CF patients, 2·8 ± 1·8 fmoles/cell (n = 6) or circulating neutrophils, 1·9 ± 1·4 fmoles/cell (n = 5) and are similar to those reported previously [16].
Discussion
The work reported here shows that pulmonary neutrophils from patients with CF had a reduced phagocytic capacity for C3bi-opsonized zymosan. The capacity of the CF pulmonary neutrophil was only 26% that of non-CF pulmonary neutrophils. While we were not able to establish the mechanism for this decrease, it is clear that it could not be attributed a defect in CR3 functioning on CF pulmonary neutrophils. Both the expression and Ca2+ signalling function of CR3 on CF pulmonary neutrophils was demonstrated by the increased rate of zymosan uptake when the iC3b opsonin and by monitoring cytosolic free Ca2+ signalling as a output from the receptor. Instead, the defect in phagocytosis was the result of a decrease in the maximum capacity of individual neutrophils for phagocytosis.
This marked difference in phagocytic capacity between the neutrophils in the CF and non-CF lung was surprising. Since the defect was maintained ex vivo, surviving washing and incubation processes, it may be the result of either an irreversible change which occurs within the CF lung, such as a proteolytic effect, or due lack of an effect which normally occurs, such as priming during extravasation. This data is consistent with the latter possibility. However, degradation of the neutrophil C3b receptor, CR1, by neutrophil elastase [9] is intriguing. Although the exact nature of the interplay between CR1 and CR3 during phagocytosis remains unclear, it is important to note that neutrophil elastase does not degrade CR3 [9]. There is also an intriguing report of an intrinsic defect in intracellular Ca2+ homeostasis in CF neutrophils [17], which does not account for the effect reported here, as Ca2+ signalling in response to iC3b–CR3 interaction was triggered during phagocytosis. Furthermore, the reported perturbation of Ca2+ homeostasis in CF neutrophils has not been confirmed by others [18]. The unusual ionic composition of the CF airway surface liquid [19] is also unlikely to impact on neutrophil Ca2+ signalling as we have demonstrated previously that Ca2+signalling is unaffected within the osmolar range predicted for CF ASL [20].
As the molecular basis for CF is a mutation in CFTR, it was important to establish whether neutrophils express this protein. However, we were unable to detect CFTR on human neutrophils, by either immuno-labelling or Western blotting. This is in agreement with one previous finding [21], but contrary to another [22]. Even so, the absence of CFTR from mature neutrophils may not exclude a role for CFTR during maturation of these cells in the bone marrow causing a subsequently irreversible functional defect. However, the lack of significant difference in phagocytic capacity between peripheral blood neutrophils from CF and non-CF subjects suggests that, even if CFTR were expressed in these cells, suggests its role in phagocytosis is of no consequence in pulmonary, rather than circulatory, defence.
We conclude that a defect in iC3b-mediated phagocytosis in neutrophils from the CF lung may contribute to inadequate pulmonary defence in cystic fibrosis. Clearly, further work is required to identify the cause of this defect. Only then will it be possible to suggest strategies for circumventing the problem.
Acknowledgments
We are grateful to the CF Trust (UK) for the support of this work.
References
- 1.Armstrong DS, Grimwood K, Carlin JB, et al. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 1997;156:1197–204. doi: 10.1164/ajrccm.156.4.96-11058. [DOI] [PubMed] [Google Scholar]
- 2.Hallett MB, Lloyds D. Neutrophil priming; the cellular signals which say ‘amber’ but not ‘green’. Immunol Today. 1995;16:264–8. doi: 10.1016/0167-5699(95)80178-2. [DOI] [PubMed] [Google Scholar]
- 3.Li R, Rieu P, Griffith DL, Scott D, Arnaout MA. Two functional states of the CD11b A-domain: Correlations with key features of two Mn2+-complexed crystal structures. J Cell Biol. 1998;143:1523–34. doi: 10.1083/jcb.143.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewitt S, Hallett MB. Cytosolic free Ca2+ changes and calpain activation are required for β2 integrin-accelerated phagocytosis by human neutrophils. J Cell Biol. 2002;159:181–9. doi: 10.1083/jcb.200206089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan TZ, Wagener JS, Bost T, et al. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–82. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 6.Zahm JM, Gaillard D, Dupuit F, et al. Early alterations in airway mucociliary clearance and inflammation of the lamina propria in CF mice. Am J Physiol. 1997;272:C853–9. doi: 10.1152/ajpcell.1997.272.3.C853. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura H, Yoshimura K, McElvaney NG, et al. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J Clin Invest. 1992;89:1478–84. doi: 10.1172/JCI115738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venaille TJ, Ryan G, Robinson BW. Epithelial cell damage is induced by neutrophil-derived, not pseudomonas-derived, proteases in cystic fibrosis sputum. Respir Med. 1998;92:233–40. doi: 10.1016/s0954-6111(98)90101-9. [DOI] [PubMed] [Google Scholar]
- 9.Tosi MF, Zakem H, Berger M. Neutrophil elastase cleaves C3bi on opsonised pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J Clin Invest. 1990;86:300–8. doi: 10.1172/JCI114699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemp T, Schram-Doumont A, van Geffel R, et al. Alteration of the N-formyl-methionyl-leucyl-phenylalanine-induced response in cystic fibrosis neutrophils. Pediatr Res. 1986;20:520–6. doi: 10.1203/00006450-198606000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Witko-Sarsat V, Allen RC, Paulais M, et al. Disturbed myeloperoxidase-dependent activity of neutrophils in cystic fibrosis homozygotes and heterozygotes, and its correction by amiloride. J Immunol. 1996;157:2728–35. [PubMed] [Google Scholar]
- 12.Russell KJ, McRedmond J, Mukherji N, et al. Neutrophil adhesion molecule surface expression and responsiveness in cystic fibrosis. Am J Respir Crit Care Med. 1998;157:756–61. doi: 10.1164/ajrccm.157.3.9704008. [DOI] [PubMed] [Google Scholar]
- 13.Hallett MB, Davies EV, Pettit EJ. Fluorescent methods for measuring and imaging cytosolic free Ca2+ in neutrophils. Methods: Companion Meth Enzymol. 1996;9:591–606. doi: 10.1006/meth.1996.0066. [DOI] [PubMed] [Google Scholar]
- 14.Dewitt S, Laffafian I, Hallett MB. Phagosomal oxidative activity during β2 integrin (CR3)-mediated phagocytosis by neutrophils is triggered by a non-restricted Ca2+ signal: Ca2+ controls time not space. J Cell Sci. 2003;116:2857–65. doi: 10.1242/jcs.00499. [DOI] [PubMed] [Google Scholar]
- 15.Wettermark G, Stymme H, Brolin SE, et al. Substrate analysis in single cells. 1. Determination ofATP. Anal Biochem. 1975;63:293–307. doi: 10.1016/0003-2697(75)90351-6. [DOI] [PubMed] [Google Scholar]
- 16.Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982;70:550–7. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabrini G, De Togni P. Increased cytosolic calcium in cystic fibrosis neutrophils effect on stimulus secretion coupling. Life Sci. 1985;36:1561–7. doi: 10.1016/0024-3205(85)90380-7. [DOI] [PubMed] [Google Scholar]
- 18.Suter S, Lew PD, Ballaman J, et al. Intracellular calcium handling in cystic fibrosis: normal cytosolic calcium and intracellular calcium stores in neutrophils. Pediatr Res. 1985;19:346–8. doi: 10.1203/00006450-198519040-00006. [DOI] [PubMed] [Google Scholar]
- 19.Knowles MR, Robinson JM, Wood RE. Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J Clin Invest. 1997;100:2588–95. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris MR, Doull IJM, Hallett MB. Osmotically induced cytosolic free Ca2+ changes in human neutrophils. Biochim Biophys Acta. 2001;1538:20–7. doi: 10.1016/s0167-4889(00)00133-6. [DOI] [PubMed] [Google Scholar]
- 21.Crawford I, Maloney PC, Zeitlin PL, et al. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc Natl Acad Sci USA. 1991;88:9262–6. doi: 10.1073/pnas.88.20.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimura K, Nakamura H, Trapnell BC, et al. Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucl Acids Res. 1991;19:5417–23. doi: 10.1093/nar/19.19.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]