Abstract
To determine whether there is an association between atopy and recurrent vaginal candidiasis (RVC) and to evaluate the type-2 immune response in patients with RVC. Evaluation of immediate hypersensitivity skin tests to aeroallergens, measurement of total IgE and Candida albicans specific IgE and levels of IL-5 in 44 women with RVC and 26 with sporadic vaginal candidiasis (SVC). Statistical analyses were performed by Mann–Whitney test and χ2 test with Yates correction. History of atopy (68%) and positive skin test (42%) were higher (P < 0·05) in RVC than in patients with SVC. No significant difference was found in total IgE, C. albicans specific IgE and IL-5 levels. There was a strong association between atopy and RVC, but type-2 immune response to C. albicans antigen was absent or similar in the two groups of patients.
Keywords: recurrent vaginal candidiasis, immune regulation, atopy, cytokines, IgE
Introduction
Candida albicans can be isolated from the vagina of approximately 20% of the nonpregnant females of reproductive age [1,2]. While approximately 75% of women will experience a single episode of candidal vulvovaginitis in their lifetime, a significant proportion of these women (5%) will subsequently experience recurrent Candida infections [3]. The pathogenesis of recurrent vaginal candidiasis (RVC) remains controversial but evidence has been accumulated that a decrease in cell-mediated immunity occurs in these patients [4,5]. Women with RVC have a decrease in delayed type hypersensitivity reaction to C. albicans antigens, but can react to other antigens [4,6]. Also, we have previously demonstrated a lack of C. albicans-specific interferon-γ production in vitro in RVC patients [7]. It is also known that mucocutaneous candidiasis is associated with T cell impairment [8] and that C. albicans is an opportunistic infection in patients with AIDS [9].
The association between vaginal candidiasis with a type 2 immune response has been well documented in experimental models of candidiasis. While in the CBA/j mice, a type 1 immune response is generated with subsequent control of infection, BALB/c mice produce predominantly IL-4 and have recurrent infections [10]. This may imply that type 1 immune reactivity is associated with resistance to candidal disease, while type 2 immune responses are associated with pathology of RVC. However, in spite of the evidence in experimental models of RVC, that candidal disease is associated with an enhancement of type 2 response, this is not clear in humans. While we and others, evaluating patients with RVC, have failed to document IL-4 and IL-5 in supernatants of lymphocyte cultures stimulated with C. albicans antigens [6,7], a few studies have associated RVC to allergy to C. albicans[11,12]. The aim of the present study was to determine whether there was an association between atopy and RVC. Allergy was evaluated by previous history of atopy, skin prick test responses to aeroallergens and total and C. albicans specific-IgE. Moreover, levels of IL-5 were measured in supernatants of lymphocyte cultures stimulated with phytohemaglutinine (PHA) and C. albicans antigens.
Materials and methods
Participants of this study included 44 women with RVC. Age ranged from 18 to 50 years. The majority (81·8%) was using some contraceptive method. Patients were referred to the gynaecology service of the University Hospital Edgard Santos from the Federal University of Bahia for treatment of RVC defined as four episodes of candidal vulvovaginitis during the last 12 months, not correlated with menstrual cycle. Every patient had positive Candida albicans vaginal cultures prior to study entry and the tests were performed during active disease. Swab specimens were placed on Sabouraud's dextrose agar and subsequently identified at the species level with the use of the API 20C system (BioMerieux, Marcy L’Etoile, France). Patients were recruited sequentially, but were excluded from the study if the microscopical findings could not be confirmed by Candida culture or they had other infection. None had diabetes mellitus, anaemia, AIDS, autoimmune or thyroid diseases or were in use of immunosuppressive drugs or had receipted of antifungal agents in the previous four weeks. The severity of each sign and symptom was scored on a scale of 0 (absent or normal) to 3 (severe). The level of vulvovaginal discharge was not scored.
Twenty-six, nonpregnant, age-matched women from the same clinic, who had history of successfully treated nonrecurrent candidal vulvovaginitis were chosen as the control group. They were recruited simultaneously with the case group. Successful treatment was defined as absence of symptoms during a 1-year follow-up. These patients had negative Candida culture at the beginning of the study.
We used standard allergy and medical histories to obtain information about symptoms, the frequency and intensity of RVC episodes, triggering factors, past medical and familial history of allergy. Moreover, all subjects filled out the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire [13]. The women underwent physical examination and skin prick test with different allergens. Atopic status was defined as the presence of allergic respiratory disease (rhinits or asthma) in the preceeding 12 months and skin prick test positive for at least one antigen.
The study followed the guidelines of the ethics committee of the Hospital and informed consent was obtained from all patients.
Skin prick test for immediate hypersensitivity (SPT)
SPT were performed on the right forearms of all individuals using a panel with 10 relevant local allergens: Dermatophagoides pteronissynus (Der p), Blomia tropicalis (Blo t), Pool of Molds I (A. alternata, C. herbarum and C. globosum), Pool of Molds II (M. mucedo, P. pullulans), Candida albicans (Cand), dog and cat epithilia (Alk Abello; Denmark) according standardized techique [14]. Histamine (1/1·000) and saline were used as positive and negative controls, respectively. The size of the reaction was determined by measuring the greatest transverse diameter of the papulae 15 min after implantation. The skin reaction was considered positive if the diameter was > 3 mm in experimental and positive control and < 3 mm in saline negative control.
Candida albicansantigen and mitogen for in vitro studies
C. albicans isolated from a patient with RVC was cultured in Sabouraud's media. The cultures were centrifuged at 3000 g for 30 min and the pellet was washed 3 times with PBS. The pellet was re-suspended in 0·5 N sodium hydroxide and then frozen (−70 °C) and thawed at 37 °C about 20 times. After the suspension was centrifuged at 3000 g for 30 min, the supernatant was collected and the pH was adjusted to 7·3. The protein content was determined by using the method of Lowry et al. [15]. The mitogen PHA was used also. Dose–response curves were performed and the optimal concentration for C. albicans antigen was 0·05 µg/ml and for PHA was 10 µg/ml.
Cell culture and cytokine assay
Peripheral blood mononuclear cells (PBMC) were isolated from heparine-treated venous blood by Ficoll-Hypaque gradient centrifugation (LSM; Organon Teknika Corporation, Durhan, NC, USA). After being washed 3 times in 0·9% NaCl, cells were resuspended in RPMI-1640 culture medium (Gibco BRL, Grand Island, NY, USA) supplemented with 10% human AB serum, 100 IU/ml penicilin and 100 mg/ml streptomycin. Cells were adjusted to 3 × 106 cells/ml, placed in 24-well plates and stimulated with C. albicans antigen (0·05 µg/ml) or PHA (10 µg/ml). After 72 h of incubation at 37 °C, 5% CO2, supernatants were collected and stored at −70 °C. Levels of IL-5 were determined by ELISA (R & D Systems, Minneapolis, Minn, USA). Results are expressed in pg/ml.
Specific and total IgE
Total IgE was determined by ELISA as previously described [16]. The IgE concentration in sera from controls and patients with RVC was determined by an antigen-capture ELISA (Medix Biotech Inc., San Carlo, CA, USA) using an anti-human IgE monoclonal antibody and a goat anti-human IgE peroxidase conjugate. The results are expressed in International Units (IU). For determination of Candida albicans-specific IgE, polystyreme 96-well plates were coated with 40 µg/ml of C. albicans antigen in carbonate-bicarbonate buffer pH 9·6 and incubated overnight at 4 °C. After 6 washes, PBS-Tween-20 0·1% was added (100 µl/well) and incubated overnight at 4 °C. Sera were depleted of IgG prior to use by treatment with RF absorbent (Dade Bhering, Germany) and added to the plate. After washing, mouse IgG anti-human IgE antibody was added (100 µl/well) at a dilution of 1 : 100 in PBS-Tween 0·05% and incubated for 2 h at 37 °C. After a further sequence of washes, anti-mouse IgG peroxidase conjugate (1 : 3000 in PBS-Tween 0·05%) was added and the plates were incubated at room temperature for 30 min. TMB was then added (100 µl/well) and the reaction was stopped with H2SO4 (50 µl/well) within 30min.
Statistical analysis
To compare the IL-5 levels and IgE levels between RVC and SVC patients, Mann–Whitney test were used. To compare the frequencies of atopy by history and the proportion of skin prick test positivity in the two groups, chi-square tests with Yates correction were performed.
Results
The mean age ± standard deviation of cases and controls was similar: (32 ± 6 versus 31 ± 6 years). In the patients with RVC, the mean illness duration was 3 years (ranging from 1 to 7 years). Data relating to a previous personal history of allergy, family history of allergy and the frequency of positive immediate hypersensitivity skin prick test in the experimental and control patients are shown in Table 1.
Table 1. History of atopy and skin test response to aeroallergens in patients with Recurrent Vaginal Candidiasis (RVC) and Sporadic Vaginal Candidiasis (SVC).
| Variables | RVC n (%) | SVC n (%) | P-value |
|---|---|---|---|
| Personal history of atopy | 30/44 (68.1) | 4/26 (15.3) | < 0.0001 |
| Familial history of atopy | 25/44 (56.8) | 9/26 (34.6) | 0.2 |
| Positive aeroallergens skin prick test | 15/35 (42.8) | 2/21 (9.5) | 0.02 |
The majority (30/44, 68%) of women with RVC had a history of atopy versus only 15% (4 of 26) in the control group (P < 0·0001). No statistical difference was observed between the two groups in relation to family history of atopy (P= 0·2045). Eighty-three percent of the RVC patients with history of atopy reported severe symptoms, while only 14·3% of the nonatopic RVC patients had severe clinical manifestations (P= 0·0158). The majority (86·6%) of RVC women with at least one skin prick test reported severe symptoms while only 40% of RVC patients without positive SPT had such symptoms (P < 0·0001).
Tests of immediate hypersensitivity to aeroallergens were performed in 35 women with RVC and 21 controls (Table 2). The proportion of RVC patients with at least one positive skin prick test (15/35, 42·8%) was higher than that observed in the control group (2/21, 9·5%) (P < 0·02). Immediate hypersensitivity skin prick test to Candida allergen was negative in all women tested.
Table 2. Proportion of women positive for each one the allergens in the skin prick test.
| Aeroalergens | RVC patients n (%) | SVC patients n (%) |
|---|---|---|
| D. pteronyssinus | 15/35 (42.8) | 1/21 (4.7) |
| Blomia tropicalis | 8/35 (22.8) | 1/21 (4.7) |
| Fungi pool* | 1/35 (2.8) | 0/21 (0) |
| Fungi pool** | 0/35 (0) | 0/21 (0) |
| Candidina | 3/35 (8.5) | 0/21 (0) |
| Dog epithelium | 0/35 (0) | 0/21 (0) |
| Cat epithelium | 0/35 (0) | 0/21 (0) |
A. alternata, C. herbarum, C. globosum.
M. mucedo, P. pullulans.
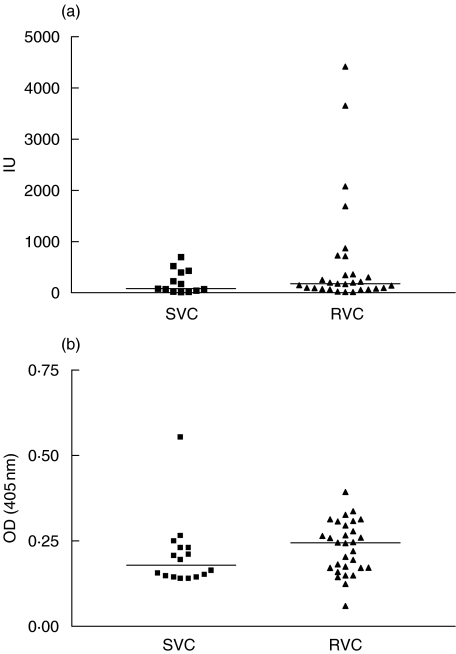
Figure 1a shows the levels of total IgE in patients with in RVC and control groups. There was a tendency for higher median IgE levels in patients with RVC (239·5 IU) ranging from 13 to 4419 IU, as compared with the control group (80 IU), ranging from 13 to 697 IU, but this difference was not statistically significant (P > 0·05). While the median of the optical density in the ELISA for Candida specific IgE (Fig. 1b) in the RVC case group was 0·223 pg/ml (range 0·0600 to 0·3930 pg/ml), in the control group it was 0·1785 pg/ml (range 0·1390–0·5540 pg/ml) (P > 0·05). Four RVC patients had very high levels of total IgE. All had history of atopy, but there was no evidence of association of IgE levels with severity of symptoms.
Fig. 1.
(a) Levels of total IgE in patients with recurrent vaginal candidiasis (RVC) and sporadic vaginal candidiasis (SVC); P = 0·3012. (b) Levels of Candida-specific IgE in patients with recurrent vaginal candidiasis (RVC) and sporadic vaginal candidiais (SVC); P = 0·0721.
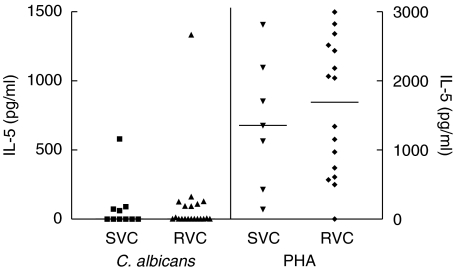
In order to discover if the RVC patients had an elevated type-2 immune response when compared with controls, the levels of IL-5 in PBMC cultures stimulated with C. albicans or PHA were determined (Fig. 2). The median of IL-5 levels in cultures stimulated with C. albicans antigen in the case group was 0 (range 0–1332 pg/ml) and in the control group was also 0 (range 0–580 pg/ml) (P= 0·7039). No statistically significant difference was found in the levels of IL-5 when the cultures were stimulated with PHA (RVC group: median 1691·5 pg/ml) (range 0–2995 pg/ml); SVC group: median 1357 pg/ml (range 143–2815 pg/ml) (P= 0·6714).
Fig. 2.
Levels of IL-5 in cultures stimulated with C. albicans and PHA in patients with recurrent vaginal candidiasis (RVC) and sporadic vaginal candidiasis (SVC); P = 0·7039.
Discussion
RVC is an important problem of young women and the understanding of the pathogenesis of this disease may help to control and prevent the recurrence of vaginal candidiasis. We have previously shown that the majority of women with RVC had impaired type-1 T cell response with inability to produce appropriate levels of IFN-γ when stimulated with C. albicans antigenin vitro[7,17]. Here we show that there is a strong association between atopy and RVC, however, the IL-5 production in response to Candida antigen was not observed. It remains to be established whether the synthesis of other type 1 and 2 cytokines were altered under other conditions.
The dichotomy of CD4 T cells in type 1 and type 2 immune response has helped the understanding of the immunopathogenesis of several infections diseases. In visceral leishmaniasis [18], chronic schistosomiasis [19] and leprosy [20] there is an antigen specific decreasing in IFN-γ production, while type 2 cytokines are increased. In this study we did not show higher IL-5 levels or higher C. albicans specific IgE antibody levels in patients with RVC when compared with women with SVC. A few reports have documented high titres of Candida specific IgE as well as eosinophils in vaginal washes suggesting that a compartmentalized immune response may occur [21]. However, the percentage of women with RVC with evidence of eosinophils or IgE in vaginal washes is much lower than the frequency of patients who report history of allergy or have evidence of atopy in this and in a previous study [22]. Although a lack of standardization of C. albicans antigen could be a bias in the results of the immune responses in these patients, the fact that both proteins and carbohydrates fractions of the yeast contain allergens [23] argue against this hypothesis. Moreover, the negative skin prick test to C. albicans antigen in all RVC patients also supports that strong type 2 immune responses to C. albicans is not an important finding in RVC. This is in agreement with our previous report that down regulation of type 1 immune responses to C. albicans antigen in patients with RVC was not associated with a strong type 2 immune response, but rather with high IL-10 levels, since neutralization of this cytokine enhanced in vitro IFN-γ production in lymphocyte cultures stimulated with C. albicans antigen [7].
Although a type 2 immune response to C. albicans antigen was not observed in patients with recurrent candidiasis, a strong association with atopy was documented. Moreover, pruritus, burning and vaginal discharge were worse in patients who had atopy, suggesting that allergy to other antigens may play a role in RVC. This observation gives support to previous studies that showed an association between RVC and rhinits and between RVC and positive immediate skin prick test to aeroallergens [23]. This is also in agreement with the observation that vaginal itching as well as allergic vulvovaginitis may be manifestations of seasonal allergy caused by pollen [24]. Vulva and vagina may be important routes for the entrance of allergens that induce local or even systemic reactions [25]. For instance: seminal fluid components, medications taken by the partner and present in his semen, spermicide, soaps, sanitary napkins, latex, food particles and house dust mite can be introduced into vagina and may induce an allergic response.
Based on this study, there is a strong association between atopy and RVC and little evidence of a strong type 2 immune response to C. albicans antigen. In such case, the allergic reaction to other antigens at the vaginal mucosa could facilitate the colonization or infection with C. albicans as a secondary event due to the break of the natural resistance to pathogens. Subsequently, this environment with a predominant type 2 immune response to other allergens or C. albicans antigen could block the development of a protective type 1 immune response. The observation that RVC is associated with both atopy and impaired type 1 immune response may have clinical implications. It is possible that enhancement of T cell response and decrease allergy may be a tool in the treatment of RVC.
Acknowledgments
We thank Givaneide Lima for her technical assistance and Elbe Silva for typing the manuscript. This study was supported by NIH Grant AI-30639. Dr Edgar M Carvalho is senior investigator of the Brazilian National Research Council (CNPq).
References
- 1.Anyon CP, Desmond FB, Esatcott DF. A study of Candida in one thousand and seven women. NZ Med J. 1971;73:9–12. [PubMed] [Google Scholar]
- 2.Giraldo P, Von Nowaskonski A, Gomes FA, Linhares I, Neves NA, Witkin SS. Vaginal colonization by Candida in asymptomatic women with and without a history of recurrent vulvovaginal candidiasis. Obstet Gynecol. 2000;95:413–6. doi: 10.1016/s0029-7844(99)00577-3. [DOI] [PubMed] [Google Scholar]
- 3.Hurly R. Recurrent candida infection. Clin Obstet Gynecol. 1981;8:209–13. [PubMed] [Google Scholar]
- 4.Corrigan EM, Clancy RL, Dunkley ML, Eyers FM, Beagley KW. Cellular immunity in recurrent vulvovaginal candidiasis. Clin Exp Immunol. 1998;111:574–8. doi: 10.1046/j.1365-2249.1998.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witkin SS. Inhibition of Candida-induced lymphocyte proliferation by antibody to Candida albicans. Obstet Gynecol. 1986;68:696–9. [PubMed] [Google Scholar]
- 6.Fidel PL, Jr, Lynch ME, Redondo-Lopez V. Systemic cell-mediated immune reactivity in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1993;168:1458–65. doi: 10.1093/infdis/168.6.1458. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho LP, Bacellar O, Neves N, de Jesus AR, Carvalho EM. Downregulation of IFN-gama production in patients with recurrent vaginal candidiasis. J Allergy Clin Immunol. 2002;109:102–5. doi: 10.1067/mai.2002.120555. [DOI] [PubMed] [Google Scholar]
- 8.Lilic D, Cant AJ, Abiunun M, Calvert JE, Spickett GP. Chronic mucocutaneous candidiasis. Clin Exp Immunol. 1996;105:205–12. doi: 10.1046/j.1365-2249.1996.d01-764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duerr A, Sierra MF, Feldman J, Clarke LM, Ehrlich I. Immune compromise and prevalence of Candida vulvovaginitis in human immunodeficiency virus-infected women. Obstet Gynecol. 1997;90:252–6. doi: 10.1016/S0029-7844(97)00253-6. [DOI] [PubMed] [Google Scholar]
- 10.Romani L, Mocci S, Bietta C, Lanfaloni L, Puccetti P, Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immuno. 1991;59:4647–54. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clancy R, Corrigan E, Dunkley M, Eyers F, Beagley K. Recurrent vulvovaginal candidiasis – allergy or immune deficiency? Int Arch Allergy Immunol. 1999;118:349–50. doi: 10.1159/000024131. [DOI] [PubMed] [Google Scholar]
- 12.Witkin SS, Jeremias J, Ledger WJ. Vaginal eosinophilis and IgE antibodies to Candida albicans in women with recurrent vaginitis. J Med Vet Mycol. 1989;27:57–8. [PubMed] [Google Scholar]
- 13.Beasley R. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis and atopic eczema: ISAAC. Lancet. 1998;351:1225–32. [PubMed] [Google Scholar]
- 14.Demoly P, Michel FB, Bousquet J. In vivo methods for study of allergy skin tests, techniques and interpretation. In: Middleton E Jr, Reed CE, Ellis EF, Adkinson NF Jr, Yunginger JW, Busse WW, editors. Allergy Principles and Practice. 5. Saint Louis: Mosby; 1998. pp. 430–7. [Google Scholar]
- 15.Lowry OH, Rousembrought NJ, Farr AL, Randal RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–72. [PubMed] [Google Scholar]
- 16.Atta AM, D'Oliveira A, Correa J, Atta MLB, Almeida R, Carvalho EM. Anti-leishmanial IgE antibodies: a marker of active disease in visceral leishmaniasis. Am J Trop Med Hyg. 1998;59:426–30. doi: 10.4269/ajtmh.1998.59.426. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho LP, Bacellar O, Neves NA, de Jesus AR. Evaluation of cellular immune response in patients with recurrent candidiasis. Rev Soc Bras Med Trop. 2003;36:571–6. doi: 10.1590/s0037-86822003000500005. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho EM, Bacellar O, Brownell C, Regis T, Coffman RL, Reed SG. Restoration of IFN-gamma production and lymphocyte proliferation in visceral leismaniaisis. J Immunol. 1994;152:5949–56. [PubMed] [Google Scholar]
- 19.Araújo MI, de Jesus AR, Bacellar O, Sabin E, Pearce E, Carvalho EM. Evidence of a T helper type 2 activation in human schistosomiasis. Eur J Immunol. 1996;26:1399–403. doi: 10.1002/eji.1830260633. [DOI] [PubMed] [Google Scholar]
- 20.Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 21.Witkin SS, Jeremias J, Ledger WJ. A localized vaginal allergic response in women with recurrent vaginitis. J Allergy Clin Immunol. 1988;81:412–6. doi: 10.1016/0091-6749(88)90909-8. [DOI] [PubMed] [Google Scholar]
- 22.Moraes PSA. Recurrent vaginal candidiasis and allergic rhinitis: a common association. Ann Allergy Asthma Immunol. 1998;81:165–9. doi: 10.1016/S1081-1206(10)62804-9. [DOI] [PubMed] [Google Scholar]
- 23.Akyiama K, Shida T, Yasueda H, et al. Alergenicity of acid protease secreted by Cândida albicans. Allergy. 1996;51:887–92. doi: 10.1111/j.1398-9995.1996.tb04489.x. [DOI] [PubMed] [Google Scholar]
- 24.Bermann B. Seasonal allergic vulvovaginitis caused by pollen. Ann Allergy. 1964;22:594–7. [PubMed] [Google Scholar]
- 25.Kudelko N. Allergy in chronic monilial vaginitis. Ann Allergy. 1971;29:266–7. [PubMed] [Google Scholar]