Abstract
In order to define the significance of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) upon progression from sepsis or severe sepsis to septic shock a prospective study was designed with 90 enrolled patients with septic syndrome due to ventilator-associated pneumonia. Blood was sampled on seven consecutive days upon initiation of symptoms and concentrations of tumour necrosis factor-alpha (TNFα), interleukin-6 (IL-6), IL-8 and sTREM-1 were estimated in serum by an enzymeimmunoassay. No differences in concentrations of TNFα, IL-6 and IL-8 were found between patients with sepsis, severe sepsis and septic shock on the first day of presentation of symptoms. Patients presenting with septic shock had concentrations of sTREM-1 significantly higher than both patients with sepsis and severe sepsis on the first day; no difference was found between patients with sepsis and severe sepsis. A positive correlation was detected between sTREM-1 and the white blood cell count. Serum levels of sTREM-1 were significantly lower in patients where VAP resolved compared to those where VAP did not resolve; similar findings were noted between patients who eventually survived and those who died. IL-6 followed the kinetics of sTREM-1 in correlation to patients's prognosis; levels of TNFα and IL-8 were unrelated to prognosis. It is concluded that sTREM-1 is particularly increased upon evolution from sepsis or severe sepsis to septic shock. Its sustained increase is an indication of poor outcome. The underlined pathophysiological role of sTREM-1 for the transition from sepsis or severe sepsis to septic shock might constitute a novel target for immunomodulatory therapy.
Keywords: sepsis, septic shock, sTREM-1, survival
Introduction
Triggering receptor expressed on myeloid cells-1 (TREM-1) is a novel membrane molecule that is largely expressed on the surface of neutrophils and mature monocytes when they are triggered by bacteria and fungi [1,2]. Its expression is increased in patients with sepsis. Though its ligand is currently unknown, its soluble form, named sTREM-1, is also detected in the blood of patients with endotoxemia and sepsis [3,4].
The exact role of sTREM-1 in the pathogenesis of the septic syndrome is yet undefined. The present study was focused on the estimation of serum levels of sTREM-1 in patients with sepsis, severe sepsis and septic shock and on the correlation of these levels to final outcome so as to disclose information about the contribution of sTREM-1 in the evolution of the septic cascade. All enrolled patients were presented with the same underlying infection, i.e. ventilator-associated pneumonia as the cause of sepsis. The uniformity for the underlying cause of sepsis was created by the necessity to minimize the discrepancy occurring when a great variety of antigenic stimuli trigger the innate immune system of the enrolled patients.
Patients and methods
Study design
A total of 90 patients were enrolled in a prospective study taking place through June 2004 to January 2005. Patients were hospitalized in the Department of Intensive Care of the ‘Evangelismos’ General Hospital and in the 2nd Department of Intensive Care of the ‘ATTIKON’ University Hospital of Athens. The study was approved by the Ethics Committee of both hospitals. All enrolled patients were intubated for at least 48 h prior enrolment and they were aged > 18 years. Written informed consent was given by their relatives. Exclusion criteria were:
neutropenia defined as < 500 × 106/l neutrophils;
HIV infection;
oral intake of corticosteroids at a dose equal to or higher than 1 mg/kg equivalent prednisone for a period greater than one month.
Inclusion criteria were the concomitant presence of ventilator-associated pneumonia (VAP) and sepsis, severe sepsis or septic shock. Diagnosis of VAP was established in any patient presenting with the following signs:
core temperature > 38 °C or < 36 °C;
new or persistent consolidation in lung X-ray;
Diagnosis of sepsis [10] was based on the presence of at least two of the following:
core temperature > 38 °C or < 36 °C;
pCO2 < 32 mmHg;
pulse rate > 90/min;
white blood cells > 12 × 109/l or < 4 × 109/l or > 10% of bands.
Severe sepsis [10] was determined as the acute dysfunction of at least one organ, i.e. the acute presentation of at least one of the following:
Acute Respiratory Distress Syndrome (ARDS), as any value of pO2/FiO2 below 200 with the presence of diffuse shadows in lung X-ray;
Acute renal failure, as the production of < 0·5 ml/kg body weight/h of urine for at least two hours provided that the negative fluid balance of the patient was corrected;
Metabolic acidosis as any pH < 7·30 or any base deficit greater than 5 mEq/l and serum lactate at least more than 2× normal value;
Acute coagulopathy as any platelet count < 100 × 109/l or INR > 1·5.
Septic shock was considered as any value of systolic pressure below 90 mmHg requiring the administration of vasopressors [10].
Upon enrolment in the study, quantitative TBS cultures were performed; TBS were collected after insertion of a sterile catheter in the intubation tube or the tracheostomy connected to a negative pressure device. Enrolled patients were followed-up on a daily basis for a total of 28 days; evaluation comprised lung X-rays, estimation of the pO2/FiO2 ratio and of the APACHE II and SOFA scores. Resolution of VAP was considered when X-ray findings were improved accompanied by an increase of the pO2/FiO2 ratio.
For the estimation of pro-inflammatory cytokines and sTREM-1 in patients's sera, 5 ml of blood were sampled after venipuncure of a peripheral vein under sterile conditions. Blood was drawn daily for seven consecutive days; it was collected into sterile tubes. After centrifugation, serum was kept at −70°C until assayed.
In order to provide control values of sTREM-1 for unintubated patients, serum was collected on the first day of diagnosis from 16 more patients; 12 presenting with severe sepsis and four with septic shock. These patients were hospitalized over the period mentioned earlier in the 4th Department of Internal Medicine of the ATTIKON University Hospital. Severe sepsis and septic shock were diagnosed by the criteria mentioned above. These critically ill patients suffered from infections other than VAP; 11 from acute pyelonephritis and five from acute cholangiitis.
Laboratory techniques
Quantitative TBS cultures were performed exactly after collection; 0·5 ml of TBS was added into a sterile tube with 2 ml of Mueller-Hinton broth and diluted five consecutive times 1 : 10. Volumes of 0·1 ml of each dilution were plated onto McConkey, blood and Saboureaux agar (Becton Dickinson). Dishes were incubated for five days at 37 °C or 42 °C for Saboureaux plates and their count was estimated after multiplying with the appropriate dilution factor. Cultures yielding a pathogen at a count ≥ 1 × 106 cfu/ml were considered positive [11]. Flasks with blood were incubated for seven days. Identification of pathogens was performed by the API20E and the API20E systems (bioMérieux, Paris, France).
Concentrations of TNFα, IL-6 and IL-8 in sera were estimated in duplicate by an enzymeimmunoassay (Diaclone, Paris, France). Lowest limits of detection were 0·5 pg/ml for TNFα, 6·25 pg/ml for IL-6, and 62·5 pg/ml for IL-8.
Estimation of sTREM-1 was performed by a hand-made enzymeimmunoassay. Capture antibody of sTREM-1 (R & D InC, Minneapolis, USA) was diluted to 4000 ng/ml and distributed in a 96-well plate at a volume of 0·1 ml per well. After overnight incubation at 25 °C, wells were thoroughly washed with a 0·05% solution of Tween in PBS (Merck) (pH: 7·2–7·4). Then 0·1 ml of standard concentrations of sTREM-1 (15·1–4000 pg/ml, R & D Inc) or serum was added in wells. After incubation for two hours, wells were washed three times, and 0·1 ml of a 400 ng/ml dilution of sTREM-1 detection antibody (R & D Inc) was added per well. The plate was then incubated for two hours, and attached antibodies were signalled by steptaverdin. Concentrations of sTREM-1 in each well were estimated by the optical density detected at 450 nm after addition of one 1 : 1 solution of H2O2: tetramethylbenzidine as a substrate (R & D Inc). All determinations were performed in duplicate; the interday variation of the assay was 5·23%. For the validation of the assay, sTREM-1 was measured in the serum of 10 healthy volunteers. In all, estimated concentrations were lower than 15·1 pg/ml.
Statistical analysis
Results were expressed as medians ± 95% confidence intervals (CI) or their range. Comparisons were performed by the Mann–Whitney U-test after correction with Bonferroni. Statistical correlations were performed after assessment of the nonparametric coefficient of Spearman (rs). Any value of P below 0·05 was considered statistically significant.
Results
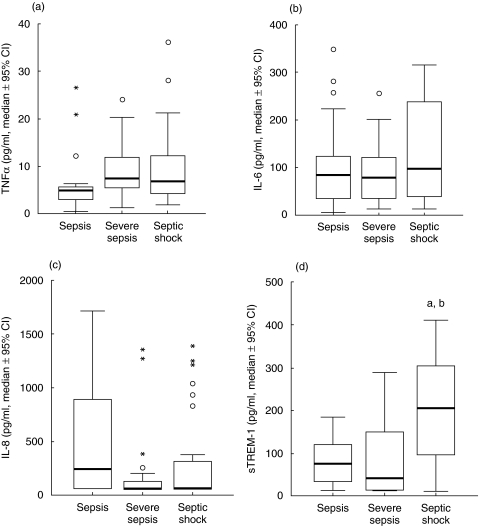
Clinical characteristics of patients enrolled in the study are shown in Table 1. Concentrations of TNFα, IL-6, IL-8 and sTREM-1 of the first day of presentation of sepsis, severe sepsis and septic shock are shown in Fig. 1. No differences were found for TNFα, IL-6 and IL-8 between sepsis, severe sepsis and septic shock. No correlations were found either between TNFα or IL-8 and APACHE II scores of the first day nor between TNFα or IL-8 and SOFA scores of the first day. IL-6 of the first day was positively correlated to both APACHE II (rs: +0·381, P < 0·0001) and SOFA (rs: +0·393, P < 0·0001) scores.
Table 1.
Clinical characteristics of patients with ventilator-associated pneumonia (VAP) enrolled in the study categorized according to the ACCP/SCCM classification
| Sepsis | Severe sepsis | Septic shock | |
|---|---|---|---|
| Number of patients | 27 | 27 | 36 |
| Age (years, mean ± SD) | 52·6 ± 20·8 | 67·7 ± 13·6 | 58·0 ± 18·1 |
| Male/female | 20/7 | 18/9 | 27/9 |
| APACHE II score (mean ± SD) | 15·63 ± 6·06 | 17·88 ± 4·19* | 19·40 ± 7·03† |
| SOFA score (mean ± SD) | 5·78 ± 2·82 | 7·21 ± 2·83* | 9·54 ± 4·10† |
| White blood cells (mean ± SD, ×106/l) | 12·132 ± 5·060 | 13·850 ± 7·026 | 14·206 ± 8·683 |
| Underlying conditions (n (%)) | |||
| Multiple injuries | 8 (29·62) | 1 (3·70) | 9 (25·00) |
| Brain haemorrhage | 6 (22·22) | 7 (25·92) | 3 (8·33) |
| Respiratory failure | 6 (22·22) | 5 (18·51) | 6 (16·66) |
| Lower respiratory tact infection | 2 (7·41) | 3 (11·11) | 5 (13·88) |
| Acute abdomen | 1 (3·70) | 2 (7·41) | 4 (11·11) |
| Celiac aortic aneurysm replacement | – | 2 (7·41) | 3 (8·33) |
| Others | 4 (14·81) | 7 (25·92) | 6 (16·66) |
| Predisposing factors (n (%)) | |||
| Diabetes mellitus 2 | 4 (14·81) | 4 (14·81) | 6 (16·66) |
| Coronary heart disease | 3 (11·11) | 8 (29·62) | 4 (11·11) |
| Hypertension | 2 (7·41) | 6 (22·22) | 4 (11·11) |
| Others | 6 (22·22) | – | 8 (22·22) |
| Pathogens (n (%)) | |||
| Acinetobacter baumannii | 10 (37·03) | 11 (40·74) | 16 (44·44) |
| Pseudomonas aeruginosa | 5 (18·51) | 7 (25·92) | 4 (11·11) |
| Others | 2 (7·41) | 3 (11·11) | 2 (5·55) |
| Bacteraemia (n (%)) | 3 (11·11) | 3 (11·11) | 5 (13·88) |
| Resolution of VAP (n (%)) | 19 (70·4) | 17 (62·9) | 13 (36·1) |
| Case-fatality (n (%)) | 6 (22·2) | 9 (33·3) | 18 (50·0) |
P = 0·017 compared to patients with sepsis;
P < 0·0001 compared to patients with sepsis and 0·011 compared to patients with severe sepsis.
Fig. 1.
Concentrations of (a) tumour necrosis factor-alpha (TNFα), (b) interleukin-6 (IL-6), (c) IL-8 and (d) sTREM-1 of the first day of presentation of sepsis, severe sepsis and septic shock in 27, 27 and 36 patients, respectively, in the field of ventilator-associated pneumonia. Circles denote outliers and asterisks denote extremes. aP = 0·003, comparison between sepsis and septic shock; bP = 0·002, comparison between severe sepsis and septic shock.
Concentrations of sTREM-1 in patients with VAP and septic shock were significantly higher than patients with sepsis and severe sepsis. No significant correlation was found between sTREM-1 and APACHE II or SOFA scores. A positive correlation was detected between sTREM-1 and the white blood cell count (rs: +0·264, P = 0·015).
Median sTREM-1 of the 12 patients with severe sepsis without VAP applied as controls was 15·1 pg/ml (range: 15·1–441·0 pg/ml). Median sTREM-1 of the four patients with septic shock without VAP applied as controls was 128·9 pg/ml (range: 15·1–887·3 pg/ml).
Concentrations of sTREM-1 TNFα, IL-6 and IL-8 on daily follow-up in relation to resolution of VAP and to final patients’ outcome are shown in Tables 2 and 3. Serum levels of sTREM-1 and IL-6 were significantly lower in patients where VAP resolved compared to those where VAP did not resolve; similar findings were noted between patients who eventually survived and those who died. Similar differences were not found for TNFα and IL-8.
Table 2. Daily estimation of serum levels of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), tumour necrosis factor-alpha (TNFα), interleukin-6 (IL-6) and IL-8 of 90 patients with septic syndrome in the field of ventilator-associated pneumonia (VAP) in relation to the resolution or not of VAP.
| Resolution of VAP (n = 49) | VAP unresolved (n = 41) | P-value | |
|---|---|---|---|
| sTREM-1 (pg/ml, median-range) | |||
| Day 1 | 79·8 (15·1–997·5) | 131·8 (15·1–513·8) | 0·049 |
| Day 2 | 75·3 (15·1–1054·5) | 172·8 (15·1–912·2) | 0·025 |
| Day 3 | 77·6 (15·1–1145·8) | 125·1 (15·1–902·4) | 0·040 |
| Day 4 | 75·6 (15·1–975·6) | 143·7 (23·4–642·2) | 0·029 |
| Day 5 | 94·1 (15·1–763·2) | 182·9 (15·1–1269·5) | 0·029 |
| Day 6 | 91·5 (15·1–887·8) | 163·3 (15·1–1630·5) | 0·013 |
| Day 7 | 87·9 (15·1–1041·2) | 200·7 (15·1–978·1) | 0·016 |
| TNFα (pg/ml, median-range) | |||
| Day 1 | 5·61 (0·70–36·17) | 6·36 (0·45–42·97) | NS* |
| Day 2 | 5·29 (0·42–37·05) | 6·09 (1·52–84·45) | NS |
| Day 3 | 5·19 (0·42–490·39) | 8·82 (1·32–178·57) | NS |
| Day 4 | 5·09 (0·39–25·94) | 6·06 (1·15–609·03) | NS |
| Day 5 | 5·08 (0·84–31·89) | 7·61 (3·20–381·08) | 0·014 |
| Day 6 | 5·36 (1·54–720·90) | 10·16 (1·35–786·07) | 0·014 |
| Day 7 | 5·46 (1·60–27·66) | 10·25 (1·63–1801·62) | 0·026 |
| IL-6 (pg/ml, median-range) | |||
| Day 1 | 89·25 (12·76–256·63) | 147·00 (15·60–347·05) | 0·022 |
| Day 2 | 85·23 (< 6·25–244·60) | 90·30 (12·86–312·72) | 0·018 |
| Day 3 | 83·29 (< 6·25–248·51) | 143·42 (17·89–314·92) | 0·001 |
| Day 4 | 74·66 (< 6·25–240·19) | 165·71 (12·63–325·35) | 0·001 |
| Day 5 | 54·72 (< 6·25–264·97) | 128·18 (< 6·25–336·46) | 0·007 |
| Day 6 | 53·76 (< 62·5–219·23) | 162·75 (13·23–364·97) | < 0·0001 |
| Day 7 | 40·49 (< 6·25–198·83) | 114·97 (20·82–326·49) | < 0·0001 |
| IL-8 (pg/ml, median-range) | |||
| Day 1 | 72·6 (< 62·5–1439·4) | 74·6 (< 62·5–1716·9) | NS |
| Day 2 | < 62·5 (< 62·5–1540·0) | < 62·5 (< 62·5–1617·6) | NS |
| Day 3 | 142·5 (< 62·5–1623·4) | < 62·5 (< 62·5–16, 137·8) | NS |
| Day 4 | 134·3 (< 62·5–1767·1) | < 62·5 (< 62·5–1886·8) | NS |
| Day 5 | 133·5 (< 62·5–1560·1) | < 62·5 (< 62·5–1683·7) | NS |
| Day 6 | 86·1 (< 62·5–1540·0) | < 62·5 (< 62·5–2594·8) | NS |
| Day 7 | 108·0 (< 62·5–1686·6) | 255·5 (< 62·5–3013·2) | 0·049 |
NS: nonsignificant
Table 3.
Daily estimation of serum levels of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), tumour necrosis factor-alpha (TNFα), interleukin-6 (IL-6) and IL-8 of 90 patients with septic syndrome in the filed of ventilator-associated pneumonia in relation to their final outcome.
| Survivors (n = 57) | Non-survivors (n = 33) | P-value | |
|---|---|---|---|
| sTREM-1 (pg/ml, median-range) | |||
| Day 1 | 79·5 (15·1–997·6) | 164·3 (15·1–513·8) | 0·031 |
| Day 2 | 83·0 (15·1–1054·5) | 165·9 (15·1–912·2) | 0·019 |
| Day 3 | 80·4 (15·1–1145·8) | 171·2 (15·1–902·4) | 0·035 |
| Day 4 | 75·6 (15·1–975·6) | 168·7 (23·4–642·2) | 0·002 |
| Day 5 | 102·2 (15·1–763·2) | 182·9 (15·1–1269·5) | NS |
| Day 6 | 91·5 (15·1–887·8) | 163·3 (15·1–1630·5) | 0·027 |
| Day 7 | 90·0 (15·1–1041·2) | 158·9 (15·1–978·1) | NS |
| TNFα (pg/ml, median-range) | |||
| Day 1 | 5·61 (0·70–42·97) | 6·36 (0·45–20·98) | NS* |
| Day 2 | 5·29 (0·42–84·45) | 8·76 (2·95–23·29) | NS |
| Day 3 | 5·19 (0·42–178·57) | 10·46 (1·49–490·39) | NS |
| Day 4 | 5·27 (0·39–491·24) | 6·36 (0·67–609·03) | NS |
| Day 5 | 5·32 (0·84–381·08) | 7·21 (1·49–135·96) | 0·044 |
| Day 6 | 5·68 (1·35–786·07) | 10·16 (2·68–75·23) | 0·040 |
| Day 7 | 5·62 (1·60–729·89) | 9·15 (4·83–1801·62) | 0·041 |
| IL-6 (pg/ml, median-range) | |||
| Day 1 | 89·25 (12·76–256·55) | 222·58 (15·60–347·05) | 0·044 |
| Day 2 | 85·23 (< 6·25–246·01) | 90·30 (15·21–312·72) | NS |
| Day 3 | 85·24 (< 6·25–248·51) | 170·18 (24·21–314·29) | < 0·0001 |
| Day 4 | 79·32 (< 6·25–240·19) | 165·71 (13·47–325·35) | 0·005 |
| Day 5 | 59·72 (< 6·25–264·97) | 180·57 (< 6·25–336·46) | 0·003 |
| Day 6 | 58·05 (< 62·5–319·04) | 156·57 (13·23–364·97) | < 0·0001 |
| Day 7 | 53·80 (< 6·25–248·89) | 206·79 (20·82–326·49) | < 0·0001 |
| IL-8 (pg/ml, median-range) | |||
| Day 1 | 97·3 (< 62·5–1439·4) | 63·66 (< 62·5–1716·9) | NS |
| Day 2 | 152·9 (< 62·5–1540·0) | < 62·5 (< 62·5–1617·6) | NS |
| Day 3 | 148·9 (< 62·5–1637·8) | < 62·5 (< 62·5–1614·7) | NS |
| Day 4 | 81·9 (< 62·5–1676·1) | < 62·5 (< 62·5–1886·8) | NS |
| Day 5 | 133·5 (< 62·5–1683·7) | < 62·5 (< 62·5–1560·1) | NS |
| Day 6 | 100.8 (< 62·5–1485·4) | < 62·5 (< 62·5–2594·8) | NS |
| Day 7 | 159·2 (< 62·5–1686·6) | 148·8 (< 62·5–3013·2) | NS |
NS, nonsignificant
Dissussion
TREM-1 is a novel receptor on cell membranes of neutrophils and monocytes that when triggered contributes to the intensification of the inflammatory process [12]. Its soluble form, named sTREM-1, has been detected in cell culture supernatants after stimulation of neutrophils and monocytes with endotoxins as well as in sera of patients with sepsis [3,12]. The exact role of sTREM-1 in the pathogenesis of sepsis is yet undefined. The present study was focused on the differences of the levels of sTREM-1 upon evolution of patients through sepsis to severe sepsis and septic shock in an attempt to define its probable pathophysiological hallmark.
The entire population enrolled in the present study became septic because of the same underlying infection, i.e. ventilator-associated pneumonia. This is a striking difference compared to all other clinical trials on sepsis and it was based on the need to elaborate an entire study population conferring an antigenic stimulus to the immune system that did not differ considerably within patients. This was evidenced by the predominance of Gram-negative pathogens creating a monomicrobial septic syndrome (Table 1). sTREM-1 was particularly elevated in the field of septic shock contrary to sepsis and severe sepsis, a finding described for the first time in the literature (Fig. 1). Measured pro-inflammatory cytokines, i.e. TNFα, IL-6 and IL-8 did not differ considerably within patients. The kinetics of sTREM-1 in the field of septic shock compared to sepsis and severe sepsis differ considerably compared to the kinetics of pro-inflammatory cytokines leading to the hypothesis that sTREM-1 might constitute a mediator implicated in the transition form sepsis and severe sepsis to septic shock.
Since all enrolled patients were subject to the same intervention, i.e. intubation and mechanical ventilation, it might be hypothesized that elevations of sTREM-1 in serum might be influenced from the applied therapeutic strategy of ventilation. Estimation of sTREM-1 in few numbers of nonventilated septic individuals with underlying infections other than pneumonias, evidenced an increase of sTREM-1 in septic shock unrelated to the occurrence of ventilation. The lack of connection between the process of ventilation is in agreement with the study of Gibot et al. [13] showing a similar increase of sTREM-1 in the bronchoalveolar lavage of either patients with community-acquired or ventilator-associated pneumonias.
Available data on the significance of TREM-1 for the evolution of the septic cascade are limited. That novel receptor is mainly expressed on neutrohils and monocytes after bacterial and fungal stimuli, so as to be considered one important counterpart of the innate immune response in sepsis [14]. Since neutrophils are highly outnumbered compared to monocytes, TREM-1 is an expression of the contribution of neutrophils in systemic inflammation [15]. The existence of a positive correlation between sTREM-1 and white blood cell counts might lead to the hypothesis that triggering of TREM-1 in neutrophil cell membranes plays a pivotal role in the pathogenesis of septic shock.
Animal studies with TREM-1 have demonstrated its significance for the intensification of the inflammatory response and for progression to death [2,12]. It was clearly shown in the present study that sTREM-1 was progressively increased upon worsening of symptoms; failing of resolution of VAP and subsequent progression to death was accompanied by higher levels of sTREM-1 (Tables 2 and 3). Based on findings of other authors showing increased levels of sTREM-1 in the bronchoalveolar lavage of patients with VAP [13], the results presented which indicated sustained serum levels of sTREM-1 in the absence of the resolution of VAP, might have been expected. These latter results differ from recent findings [16] showing higher levels of sTREM-1 in the sera of survivors compared to nonsurvivors on the first day of admission, however, they are in agreement that sTREM-1 in nonsurvivors is higher than in survivors upon evolution of the septic syndrome. The discrepancies might be attributed either to the application of a study population in the present study with the same underlying infection and/or to the enrolment of patients with sepsis, severe sepsis and septic shock in the present study compared to the study of Gibot et al. [16] which enrolled only patients with sepsis and septic shock. Among the other estimated cytokines in the present study, only IL-6 followed kinetics similar to those of sTREM-1 in relation to resolution of VAP and to final outcome (Tables 2 and 3).
The results presented reveal a significant increase of sTREM-1 upon evolution from sepsis or severe sepsis to septic shock. That sustained increase was an indication of poor outcome. The underlined pathophysiological role of sTREM-1 for the transition from sepsis or severe sepsis to septic shock might constitute a novel target for immunomodulatory therapy.
References
- 1.Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis. 2003;187(Suppl. 2):S397–S401. doi: 10.1086/374754. [DOI] [PubMed] [Google Scholar]
- 2.Gibot S, Kolopp-Sarda MN, Béné MC, Bollaert PE, Lozniewski A, Mory F, Levy B, Faure GC. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004;200:1419–26. doi: 10.1084/jem.20040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibot S, Kolopp-Sarda MN, Béné MC, Cravoisy A, Levy B, Faure GC, Bollaert PE. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med. 2004;141:9–15. doi: 10.7326/0003-4819-141-1-200407060-00009. [DOI] [PubMed] [Google Scholar]
- 4.Knapp S, Gibot S, de Vos A, Versteeg HH, Colonna M, van der Poll T. Cutting edge: expression patterns of surface and soluble receptor expressed on myeloid cells-1 in human endotoxemia. J Immunol. 2004;173:7131–4. doi: 10.4049/jimmunol.173.12.7131. [DOI] [PubMed] [Google Scholar]
- 5.Michel F, Franceschini B, Berger P, Arnal JM, Gainnier M, Sainty JM, Papazian L. Early antibiotic treatment for BAL-confirmed ventilator-associated pneumonia. A role for routine endotracheal aspirate cultures. Chest. 2005;127:589–97. doi: 10.1378/chest.127.2.589. [DOI] [PubMed] [Google Scholar]
- 6.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 7.Rello J, Paiva JA, Baraibar J, et al. International conference for the development of consensus on the diagnosis and treatment of ventilator-associated pneumonia. Chest. 2001;120:955–70. doi: 10.1378/chest.120.3.955. [DOI] [PubMed] [Google Scholar]
- 8.Baughman RP. Diagnosis of ventilator-associated pneumonia. Curr Opin Crit Care. 2003;9:397–402. doi: 10.1097/00075198-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL. Ventilator-associated pneumonia. J Hosp Infect. 2004;57:272–80. doi: 10.1016/j.jhin.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Levy M, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 11.Camargo LFA, De Marco FV, Barbas CSV, et al. Ventilator associated pneumonia: comparison between quantitative and qualitative cultures of tracheal aspirates. Crit Care. 2004;8:R422–30. doi: 10.1186/cc2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouchon A, Facchetti F, Welgand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–7. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 13.Gibot S, Cravoisy A, Levy B, Béné MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451–8. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 14.Bouchon A, Dietrich J, Colonna M. Cutting edge. inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–5. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 15.Radsak MP, Salih HR, Rammensee HG, Schild H. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses. differential regulation of activation and survival. J Immunol. 2004;172:4956–63. doi: 10.4049/jimmunol.172.8.4956. [DOI] [PubMed] [Google Scholar]
- 16.Gibot S, Cravoisy A, Kolopp-Sarda MN, Béné MC, Faure G, Bollaert PE. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit Care Med. 2005;33:792–6. doi: 10.1097/01.ccm.0000159089.16462.4a. [DOI] [PubMed] [Google Scholar]