Abstract
Chronic obstructive pulmonary disease (COPD) is characterized by a dysregulated recruitment of circulating leucocytes into the lung which is associated with the onset and progress of the disease. P-selectin glycoprotein ligand-1 (PSGL-1) is expressed on leucocytes and plays an essential role in primary leucocyte-endothelial cell adhesive contacts. The present study investigated if PSGL-1 is up-regulated on leucocytes of COPD patients. Peripheral blood samples were collected from COPD patients as well as controls (smoking, nonsmoking volunteers) and subjected to analysis of PSGL-1 expression on leucocytes, i.e. neutrophils, eosinophils, monocytes and lymphocytes by flow cytometry. No significant difference was observed between healthy nonsmoking and healthy smoking control subjects. In contrast, PSGL-1 expression was found to be significantly increased on the surface of all four leucocyte populations in COPD patients compared to both control groups. The finding that PSGL-1 surface expression is up-regulated on leucocytes of COPD patients as compared to leucocytes of controls suggests PSGL-1 as a potential target for anti-inflammatory treatment.
Keywords: COPD, leucocytes, cell adhesion molecules, inflammation
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory disorder of peripheral airways (bronchioles) and lung parenchyma which ranks twelfth among the leading diseases worldwide and fourth as cause of death in the Western world today [1–3]. At the cellular level COPD is characterized by an increased number of activated macrophages and CD8-positive T-cells in the inflamed lung parenchyma which can be paralleled by an increased number of neutrophils [4,5]. In addition, markedly increased numbers of macrophages and neutrophils can be observed in broncho-alveolar lavage fluid and induced sputum of patients with COPD [6,7]. The increase of neutrophils, monocytes and T-cells in lung parenchyma and broncho-alveolar space is achieved due to enhanced recruitment of leucocytes which is mediated by a sequence of adhesion and activation events that ends with extravasation of the leucocyte into the surrounding tissue. The first step in this cascade is the initial rolling or ‘tethering’ of leucocytes to the microvascular endothelium, which is generally considered to be the primary event in the inflammatory response. This is mediated by the selectin family of cell adhesion molecules that is comprised of three structurally related carbohydrate binding proteins, vascular E- and P-selectin and leucocyte L-selectin [8].
In COPD increased leucocyte infiltration from the circulation into the inflamed lung parenchyma and broncho-alveolar space is mediated by enhanced endothelial adherence and leucocyte migration. Whereas for the latter up-regulation of intercellular adhesion molecule-1 (ICAM-1) on bronchial epithelium has been demonstrated, enhanced adhesion is mediated by the increased expression of selectins including E-selectin on endothelial cells [9–11]. Moreover, correlation of increased plasma levels of circulating E- and P-selectin (sE-selectin and sP-selectin) with impaired blood gas tension and lung function suggested selectins as marker of disease activity [10,12]. In regard to P-selectin, clinical observations were supported by different lines of evidences in experimental animal models showing an important role for P- and E-selectin in cellular recruitment into the lung in pathological conditions like chronic inflammation [13–15]. In this respect recognition of selectin-mediated leucocyte adhesion as a critical early event in initiating and maintaining lung inflammation has made these molecules an attractive target for therapeutic intervention [16].
One of the major ligands for E- and P-selectin is the P-selectin glycoprotein ligand-1 (PSGL-1) which is expressed as homodimeric sialomucin on all leucocytes. It preferentially binds to P-selectin and with lower affinity to E-selectin. In this context, glycosylation of PSGL-1 by the tetrasaccharide sialyl Lewis X (sLex) is responsible for basal binding to E- and P-selectin whereas tyrosine sulphation is responsible for enhanced further binding which is restricted to P-selectin only [17–20]. As recently suggested, binding of PSGL-1 to P-selectin plays an important role in homing of lymphocytes to the lung. Unlike lymphocyte homing to the gut which is mainly mediated by integrin α4β7 binding to mucosal addressin cellular adhesion molecule (MAdCAM-1) or lymphocyte homing to the skin which is mainly mediated by cutaneous lymphocyte antigen (CLA) binding to E-selectin, attachment of lymphocytes to bronchial endothelium is mediated, at least in part, by binding of PSGL-1 to P-selectin [21]. In this context it was demonstrated that PSGL-1 is up-regulated on granulocytes from allergic asthmatic patients and their binding to selectins is enhanced on P- but not on E-selectin [22].
Based on the suggested prominent role for PSGl-1 in the adhesion of leucocytes to endothelial cells in the lung, we investigated in the present study the expression of PSGL-1 on leucocytes of COPD patients to determine whether this is altered in chronic obstructive lung disease. As controls circulating leucocytes of healthy nonsmoking volunteers were used as well as those of healthy smoking volunteers to investigate the effect of acute cigarette smoking on PSGL-1 levels.
Materials and methods
Study populations
After giving informed consent, 13 healthy nonsmokers, 16 patients with COPD and 14 healthy smokers were selected for the study after Ethical Commitee approval (Table 1). All patients were nonsmokers or had quitted smoking at least one year before the study. The COPD patients were consecutively recruited in our outpatient respiratory department. Diagnosis of COPD was made according to the guidelines of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) [23]. The patients reported a history of productive cough and progredient dyspnoea for at least two years (2–25 years). Spirometric investigation determined irreversible airway obstruction. None of the patients was atopic or showed signs of asthma. Exacerbation was diagnosed if at least two of the following three criteria were met: increased dyspnea, increased amount of sputum and sputum purulence [24]. Patients with a history of tuberculosis or chronic inflammatory diseases beside COPD were not included into the study. All patients were using β2-agonists, seven used also inhaled glucocorticoids (< 1000 µg/d Budesonid); patients using oral steroids were excluded from the study. No patient had undergone systemic anti-inflammatory therapy for at least one week prior to the study.
Table 1. Characteristics of study populations.
| Nonsmoker | COPD | Smoker | |
|---|---|---|---|
| Sex (m/f) | 2/11 | 7/9 | 9/5 |
| Age (years) | 42·5 ± 10·6 | 65·6 ± 7·6* | 44·4 ± 7·6 |
| Non-smoker | 13 | 3 | na |
| Ex-smoker | – | 13 | na |
| Pack years | na | 26·8 ± 21·3 | 20·8 ± 14·0 |
| Stable disease | na | 10 | na |
| Exacerbation | na | 6 | na |
| FEV1,% predicted | nd | 61·3 ± 21·7 | nd |
| FEV1/FVC,% | nd | 64·6 ± 15·9 | nd |
M, male; f, female, FEV1, forced expiratory volume in one second,; FVC, forced vital capacity; na, not applicable; nd, not determined. Data are given as mean ± sd.
P < 0·0001 versus both control groups
Healthy nonsmoking subjects and smokers without pulmonary symptoms were recruited from the clinic personnel who had no history of inflammatory or infectious diseases during the last four weeks before the study.
White blood cell (WBC) count and analysis of peripheral blood
From each individual, EDTA-blood was collected for WBC, differential cell analysis and flow cytometry. C-reactive protein (CRP) was assayed as a measure of systemic inflammation from heparinized blood. Blood analyses were made by automated blood analysers in the central laboratory.
Analysis of expression of PSGL-1 on peripheral white blood cells
Analysis of expression of PSGL-1 on peripheral leucocytes was performed by flow cytometry as previously described [22]. Briefly, whole blood was treated with PE-conjugated anti-PSGL-1 (KPL-1), FITC-conjugated anti-CD16 (3G8), or directly conjugated isotype control antibodies (MOPC-21, all from BD Biosciences, Heidelberg, Germany). Erythrocyte lysis and WBC fixation was performed with UtiLyse (DakoCytomation, Hamburg, Germany) according to the manufacturers instructions. Data acquisition was with a BD Biosiences FACScalibur flow cytometer. Monocytes, lymphocytes, neutrophils and eosinophils were identified by forward- and side-scatter properties. Eosinophils were further differentiated from neutrophils based on anti-CD16 staining and the data used to verify gating by light scatter. Data were analysed using FCS express software (De Novo Software, Thornhill, Ontario, Canada). Mean fluorescence intensity (MFI) for IgG1 was subtracted from MFI for PSGL-1 to derive net MFI.
Statistical analysis
Descriptive statistics were used to summarize the expression data. Differences between the three groups were calculated with Kruskal–Wallis anova on ranks with Dunn's posthoc analysis. Where groups were significantly different the nonparametric Mann–Whitney U-test was used to derive accurate P-values. Correlations were evaluated by Pearson product moment correlation. P < 0·05 was considered to be significant. All calculations have been carried out using SigmaStat and a data analysis system based on the R-package for statistical computing [25,26].
Results
Subject characteristics
As controls, 13 healthy nonsmokers and 14 smokers were included in the study as well as 16 patients suffering from COPD. Overall characteristics of the groups are given in Table 1. All patients were admitted to the outpatient respiratory department and randomly recruited for this study. They had chronic obstructive lunge disease in mild to very severe stage (I–IV) according to the GOLD criteria. Four of 16 patients had mild (stage I), six moderate (stage II), three severe (stage III), and three very severe COPD (stage IV) (Table 2). Six patients were found to have an acute exacerbation at the time of the visit that required intensification of medical treatment. However, there was no evidence of pneumonia, common cold or inflammatory disease beside COPD. Independent of the clinical status, stable disease or exacerbation, in 8 patients elevated CRP levels (7·6–35·8 mg/l, normal range < 5 mg/l) were found as well as in two of the smokers (6·1 and 7·7 mg/l). Despite these raised CRP levels no other clinical symptoms or signs of bacterial inflammation were found. None of the controls had elevated CRP values.
Table 2. Individual patient characteristics.
| No. | Age (years) | Sex | FEV1 (% predicted) | COPD stage | Years of bronchitis | PY/occupational exposure | Atopy | Eosinophils × 106/l |
|---|---|---|---|---|---|---|---|---|
| 1 | 64 | m | 74 | III | 10 | 0/Dust | No | 130 |
| 2 | 57 | m | 74 | II | 30 | 45 | No | 290 |
| 3 | 53 | f | 85 | I | 2 | 20 | No | 300 |
| 4 | 76 | f | 29 | IV | 25 | 40 | No | 260 |
| 5 | 77 | m | 50 | II | 20 | 20 | No | 70 |
| 6 | 58 | f | 48 | III | 7 | 20 | No | † |
| 7 | 58 | f | 80 | I | 2 | 0/Dust | No | 290 |
| 8 | 67 | f | 70 | II | 3 | 20 | No | 150 |
| 9 | 62 | m | 80 | I | 4 | 25 | No | 160 |
| 10 | 59 | f | 89 | I | 5 | 15 | No | 120 |
| 11 | 64 | f | 77 | II | 3 | 0/unknown | No | 140 |
| 12 | 75 | m | 23 | IV | 10 | 80 | No | 150 |
| 13 | 73 | f | 68 | II | 11 | 15 | No | 200 |
| 14 | 73 | m | 67 | II | 18 | 38 | No | 270 |
| 15 | 65 | f | 46 | III | 15 | 50 | No | 170 |
| 16 | 68 | m | 26 | IV | 10 | 40 | No | 50 |
FEV1, forced expiratory volume in one second; PY, pack years; m, male; f, female.
No leucocyte differential for this patient.
Differential leucocyte counts were analysed to exclude systemic dysbalance of the immune system. Compared to nonsmoking and smoking controls COPD patients showed a significantly higher neutrophil count (P = 0·002 and P = 0·004, respectively) but lower lymphocyte count (both P < 0·001) (Table 3). In COPD patients there was a correlation of lymphocyte numbers with age (r = −0·681, P = 0·005), COPD stage (r = −0·694, P = 0·004) and FEV1 (r = 0·685, P = 0·005). No such correlation between lymphocytes and age existed in the control groups. Eosinophil numbers in smokers were significantly (P = 0·009) elevated in comparison to nonsmokers and higher than in COPD patients but without reaching statistical significance (Table 3). None of the COPD patients had elevated eosinophil counts further indicating the absence of asthma (Table 2). In COPD patients the leucocyte counts did not correlate with elevation of CRP values (data not shown).
Table 3. WBC counts and leucocyte differentials from study populations.
| Nonsmoker n = 13 | COPD n = 16‡ | Smoker n = 14 | |
|---|---|---|---|
| WBC (× 106/ml) | 7·7 (5·7; 8·4) | 7·8 (7·2; 9·5) | 8·0 (7·1; 10·2) |
| Basophils (%) | 0·4 (0·3; 0·5) | 0·4 (0·3; 0·7) | 0·4 (0·3; 0·5) |
| Eosinophils (%) | 2·0 (0·9; 2·6) | 2·2 (1·5; 2·9) | 3·1 (2·4; 3·4)* |
| Neutrophils (%) | 55·8 (48·7; 46·4) | 65·4 (60·2; 69·1)† | 55·1 (49·2; 57·6) |
| Lymphocytes (%) | 35·9 (33·7; 40·7) | 25·9 (21·7; 27·7)† | 35·1 (30·7; 41·9) |
| Monocytes (%) | 7·1 (6·4; 8·0) | 7·5 (6·8; 9·0) | 6·6 (5·7; 7·0) |
Leucocyte differentials for 15 patients. WBC, white blood cells. Data are given as median (25th; 75th percentile).
P < 0·01 versus nonsmoker;
P < 0·01 versus nonsmoker and smoker.
PSGL-1 expression on leucocytes
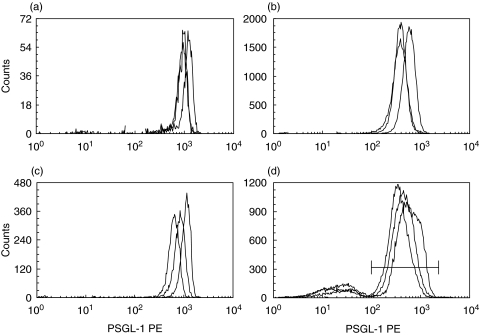
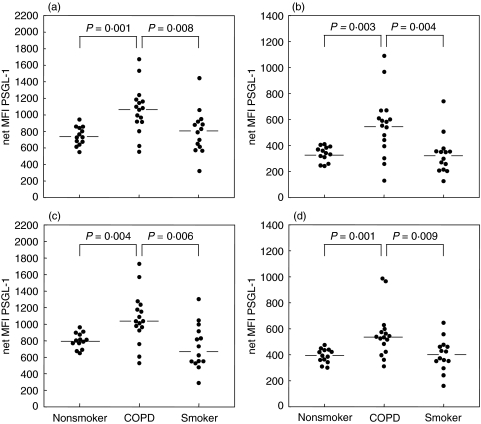
PSGL-1 expression was analysed by flow cytometry on peripheral blood leucocytes from patients with COPD and compared with that of the control groups. No differences in the shape, width or number of peaks in the histograms were present between the three groups (Fig. 1). Surface densities of PSGL-1 on circulating leucocytes showed only a narrow distribution in the nonsmoking control group with eosinophils and monocytes expressing the highest levels. In COPD patients and in smokers PSGL-1 expression showed a greater variability on all leucocyte populations while maintaining the relative differences between the populations. Mean expression levels in COPD patients were significantly increased on all four leucocyte subpopulations in comparison to healthy nonsmokers and smokers (eosinophils 1070 (1119) versus 729 (393) and 808 (1123), P < 0·001 and P = 0·008 (median (range)versus controls and smokers, respectively); neutrophils 568 (960) versus 341 (168) and 324 (615), P = 0·003 and P = 0·004; monocytes 1035 (1200) versus 801 (313) and 677 (1014), P = 0·004 and P = 0·006; and lymphocytes 535 (676) versus 392 (175) and 398 (485), P = 0·001 and P = 0·009) (Fig. 2).
Fig. 1.
Flow cytometry of WBC. Representative histograms for PE-fluorescence (PSGL-1) for nonsmoker (grey line), smoker (thin black line) and COPD (solid black line) are shown for (a) eosinophils (b) neutrophils (c) monocytes and (d) lymphocytes. For lymphocytes only the right peak within the marker region (horizontal line) was included in the analysis.
Fig. 2.
PSGL-1 expression on leucocytes. PSGL-1 levels on peripheral blood leucocytes from healthy nonsmokers, COPD patients and smokers were measured by flow cytometry and analysed separately for (a) eosinophils (b) neutrophils (c) monocytes and (d) lymphocytes. Horizontal bars denote the medians.
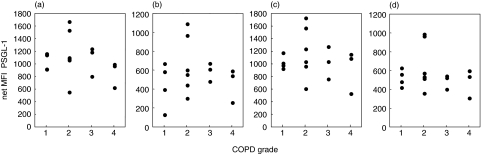
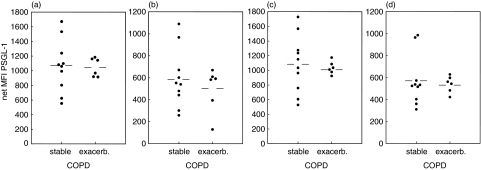
As analysis of leucocyte differentials revealed a correlation between lymphocyte counts and clinical parameter in COPD patients, we investigated whether similar relationships existed with PSGL-1 expression. Unlike the situation with the leucocyte counts the fluorescence data did not indicate a correlation between PSGL-1 expression and age, stage of the disease, FEV1 or smoking history (pack years) in COPD patients (Fig. 3 and data not shown). Furthermore, there was no difference in PSGL-1 expression between patients with normal or elevated CRP levels. Since there were also patients with exacerbation included into the study we analysed PSGL-1 expression of patients with and without exacerbation separately. No differences were observed between these groups (Fig. 4), however, due to the small number of exacerbated patients this could not be rigorously proven. In addition, neither age nor absolute leucocyte count appears to have an influence on PSGL-1 expression in any of the study groups (data not shown).
Fig. 3.
PSGL-1 expression and severity of disease. PSGl-1 levels on peripheral blood leucocytes from COPD patients are plotted against COPD stages for (a) eosinophils (b) neutrophils (c) monocytes and (d) lymphocytes.
Fig. 4.
PSGL-1 expression on leucocytes of COPD subgroups. PSGL-1 levels on peripheral blood leucocytes from stable and exacerbated COPD patients are plotted separately for (a) eosinophils (b) neutrophils (c) monocytes, and (d) lymphocytes. Horizontal bars denote the means.
Discussion
Our study demonstrates that PSGL-1 expression is significantly increased on the cell surface of neutrophils, eosinophils, monocytes and lymphocytes from COPD patients apparently irrespective of a clinically stable or exacerbated status. This observation complements previous reports demonstrating increased expression of adhesion molecules like E-selectin, P-selectin or Mac-1 in patients with COPD [9,11,12]. E-selectin was shown to be significantly higher expressed on the surface of vessels in the submucosa of COPD patients compared with asymptomatic smokers and healthy subjects [9]. Similarly, for Mac-1 it has been demonstrated that its expression is up-regulated on circulating neutrophils in patients with stable COPD [11,27]. However, there was a significant difference between stable and exacerbated COPD patients with expression levels of Mac-1 during exacerbation being the same as in healthy controls [11]. This is in contrast to our findings that the clinical status of exacerbation apperars not to influence the level of PSGL-1 expression which is elevated in both conditions. It suggests that expression of integrin ligands like Mac-1 is differently regulated than the expression of E- and P-selectin ligands on the cell surface of circulating leucocytes. This conclusion is supported by reports about the expression of other adhesion molecules in COPD. Here it was shown that L-selectin expression on neutrophils does not show any difference between stable disease and exacerbation in COPD [11].
Interestingly, we observed that there was no significant difference in cell surface expression of PSGl-1 between healthy nonsmoking and smoking control subjects. One possible explanation could be the difference in age between COPD and control groups, as age-related changes in frequencies of leucocyte populations and lymphocyte surface markers are known. However, no age-dependent modulations of PSGL-1 expression have been reported to date and our analysis showes no correlation between age and PSGL-1 surface densities on any leucocyte population in any of the three study groups. Therefore, this is unlikely to be the reason, indicating that elevated PSGl-1 levels are associated with the disease but not with its main risk factor, tobacco smoking. This observation is in line with reports about the expression of Mac-1 on the cellular surface of neutrophils showing a significantly higher expression of Mac-1 in COPD as compared to healthy nonsmoking and smoking control subjects, whereas there was no significant difference between both control groups [27]. In addition, our study suggests that increased PSGL-1 levels are not dependent on the severity of the disease, although the limited number of patients does not allow a firm statistical evaluation. While it is at present unclear whether this reflects a causal relationship with COPD or only a consequence of the disease permanently elevated PSGL-1 might play a role in maintaining the chronic inflammation due to facilitated recruitment.
Together with the findings of up-regulated E-selectin and Mac-1 expression as well as increased serum concentrations of sE- and sP-selectin in COPD patients, the result of the present study of up-regulated PSGL-1 expression suggest that adhesion molecule analysis might be an attractive approach for molecular diagnostics. For instance, previous studies demonstrated a substantial correlation between the expression profile of adhesion molecules and functional cellular and clinical parameters. Thus, it has been demonstrated that up-regulated Mac-1 expression is associated with enhanced recruitment of neutrophils and increased respiratory burst and that significantly higher plasma levels of sP-selectin, an activation marker for platelets, correlated with the impairment of blood gas tension [12,27]. In our study we investigated if the measured levels of PSGL-1 expression on leucocytes correlate with the stage of the disease, FEV1, smoking history or CRP level as a general systemic parameter for inflammation. Based on the limited number of patients included in this prospective analysis none of these parameters appears to correlate with the expression level of PSGL-1. Further more advanced studies should explore the correlation between PSGL-1 expression and functional cellular parameters in more detail.
Taken together, the results of this pilot study demonstrate a significantly higher PSGL-1 expression on the surface on neutrophils, eosinophils, monocytes, and lymphocytes of patients with COPD as compared to healthy nonsmoking and asymptomatic smoking controls. The underlying molecular mechanism of this observation and its relationship to functional parameter should be explored by more advanced and extensive studies. The fact that PSGL-1 as well as other adhesion molecules like Mac-1 or E-selectin are dysregulated only in inflammatory diseases like COPD suggests them as attractive tools for molecular diagnostics as well as targets for therapeutic interference.
Acknowledgments
This study was partly funded by a grant from the Bundesministerium für Bildung und Forschung (01 ZZ 0101; A.S., U.L) and by grants of the Ministerium für Wirtschaft, State of Brandenburg and the European Union (M.M., G.W).
References
- 1.Hurd S. The impact of COPD on lung health worldwide: epidemiology and incidence. Chest. 2000;117(Suppl.):1S–4S. doi: 10.1378/chest.117.2_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–80. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 3.Madison JM, Irwin RS. Chronic obstructive pulmonary disease. Lancet. 1998;352:467–73. doi: 10.1016/S0140-6736(97)11081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkelstein R, Fraser RS, Ghezzo H, Cosio MG. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med. 1995;152:1666–72. doi: 10.1164/ajrccm.152.5.7582312. [DOI] [PubMed] [Google Scholar]
- 5.O'Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155:852–7. doi: 10.1164/ajrccm.155.3.9117016. [DOI] [PubMed] [Google Scholar]
- 6.Pesci A, Balbi B, Majori M, Cacciani G, Bertacco S, Alciato P, Donner CF. Inflammatory cells and mediators in bronchial lavage of patients with chronic obstructive pulmonary disease. Eur Respir J. 1998;12:380–6. doi: 10.1183/09031936.98.12020380. [DOI] [PubMed] [Google Scholar]
- 7.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–4. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 8.Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–8. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 9.Di Stefano A, Maestrelli P, Roggeri A, et al. Upregulation of adhesion molecules in the bronchial mucosa of subjects with chronic obstructive bronchitis. Am J Respir Crit Care Med. 1994;149:803–10. doi: 10.1164/ajrccm.149.3.7509705. [DOI] [PubMed] [Google Scholar]
- 10.Riise GC, Larsson S, Lofdahl CG, Andersson BA. Circulating cell adhesion molecules in bronchial lavage and serum in COPD patients with chronic bronchitis. Eur Respir J. 1994;7:1673–7. doi: 10.1183/09031936.94.07091673. [DOI] [PubMed] [Google Scholar]
- 11.Noguera A, Busquets X, Sauleda J, Villaverd JM, MacNee W, Agusti AG. Expression of adhesion molecules and G proteins in circulating neutrophils in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:1664–8. doi: 10.1164/ajrccm.158.5.9712092. [DOI] [PubMed] [Google Scholar]
- 12.Ferroni P, Basili S, Martini F, Vieri M, Labbadia G, Cordova C, Alessandri C, Gazzaniga PP. Soluble P-selectin as a marker of platelet hyperactivity in patients with chronic obstructive pulmonary disease. J Invest Med. 2000;48:21–7. [PubMed] [Google Scholar]
- 13.Broide DH, Sullivan S, Gifford T, Sriramarao P. Inhibition of pulmonary eosinophilia in P-selectin- and ICAM-1-deficient mice. Am J Respir Cell Mol Biol. 1998;18:218–25. doi: 10.1165/ajrcmb.18.2.2829. [DOI] [PubMed] [Google Scholar]
- 14.Curtis JL, Sonstein J, Craig RA, Todt JC, Knibbs RN, Polak T, Bullard DC, Stoolman LM. Subset-specific reductions in lung lymphocyte accumulation following intratracheal antigen challenge in endothelial selectin-deficient mice. J Immunol. 2002;169:2570–9. doi: 10.4049/jimmunol.169.5.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Sanctis GT, Wolyniec WW, Green FH, et al. Reduction of allergic airway responses in P-selectin-deficient mice. J Appl Physiol. 1997;83:681–7. doi: 10.1152/jappl.1997.83.3.681. [DOI] [PubMed] [Google Scholar]
- 16.Romano SJ, Slee DH. Targeting selectins for the treatment of respiratory diseases. Curr Opin Invest Drugs. 2001;2:907–13. [PubMed] [Google Scholar]
- 17.Rodgers SD, Camphausen RT, Hammer DA. Tyrosine sulfation enhances but is not required for PSGL-1 rolling adhesion on P-selectin. Biophys J. 2001;81:2001–9. doi: 10.1016/S0006-3495(01)75850-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkins PP, Moore KL, McEver RP, Cummings RD. Tyrosine sulfation of P-selectin glycoprotein ligand-1 is required for high affinity binding to P-selectin. J Biol Chem. 1995;270:22677–80. doi: 10.1074/jbc.270.39.22677. [DOI] [PubMed] [Google Scholar]
- 19.Sako D, Comess KM, Barone KM, Camphausen RT, Cumming DA, Shaw GD. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell. 1995;83:323–31. doi: 10.1016/0092-8674(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 20.Pouyani T, Seed B. PSGL-1 recognition of P-selectin is controlled by a tyrosine sulfation consensus at the PSGL-1 amino terminus. Cell. 1995;83:333–43. doi: 10.1016/0092-8674(95)90174-4. [DOI] [PubMed] [Google Scholar]
- 21.Ainslie MP, McNulty CA, Huynh T, Symon FA, Wardlaw AJ. Characterisation of adhesion receptors mediating lymphocyte adhesion to bronchial endothelium provides evidence for a distinct lung homing pathway. Thorax. 2002;57:1054–9. doi: 10.1136/thorax.57.12.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dang B, Wiehler S, Patel KD. Increased PSGL-1 expression on granulocytes from allergic-asthmatic subjects results in enhanced leukocyte recruitment under flow conditions. J Leukoc Biol. 2002;72:702–10. [PubMed] [Google Scholar]
- 23.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management & Prevention of Chronic Obstructive Pulmonary Disease, NHLBI/WHO Workshop report. [06/2004];2003 (NIH publication no. 2071) [ HTTP://WWW.document]. URL http://www.goldcopd.com). [Google Scholar]
- 24.Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GJ, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team, R Foundation for Statistical Computing Vienna. R: a language and environment for statistical computing. [07/2004];2004 [ HTTP://WWW.document]. URL http://www.R-project.org. [Google Scholar]
- 26.Aydt EM, Vigano M, Meyer M, Böttcher K. Data management and data mining in biotech companies — an integrated appoach. In: Ford M, Livingsone D, Dearden J, Van de Waterbeemd H, editors. Designing Drug and Crop Protectants: Processes, Problems and Solutions. Malden, MA: Blackwell Publishing; 2003. pp. 408–13. [Google Scholar]
- 27.Noguera A, Batle S, Miralles C, Iglesias J, Busquets X, MacNee W, Agusti AG. Enhanced neutrophil response in chronic obstructive pulmonary disease. Thorax. 2001;56:432–7. doi: 10.1136/thorax.56.6.432. [DOI] [PMC free article] [PubMed] [Google Scholar]