Abstract
Antigen uptake and presentation capacities enable DC to prime and activate T cells. Recently, several studies demonstrated a diminished DC function in hepatitis C virus (HCV) infected patients showing impaired abilities to stimulate allogenic T cells and to produce IFN-γ in HCV infected patients. Moreover, DC of patients who have resolved HCV infection behave like DC from healthy donors responding to maturation stimuli, decrease antigen uptake, up-regulate expression of appropriate surface marker, and are potent stimulators of allogenic T cells. A number of studies have demonstrated in tumour models and models of infectious diseases strong induction of immune responses after DC vaccination. Because DC are essential for T-cell activation and since viral clearance in HCV infected patients is associated with a vigorous T-cell response, we propose a new type of HCV vaccine based on ex vivo stimulated and matured DC loaded with HCV specific antigens. This vaccine circumvents the impaired DC maturation and the down regulated DC function of HCV infected patients in vivo by giving the necessary maturation stimuli and the HCV antigens in a different setting and location ex vivo. Strong humoral and cellular immune responses were detected after HCV core DC vaccination. Furthermore, DC vaccination shows partial protection in a therapeutic and prophylactic model of HCV infection. In conclusion, mice immunized with HCV core pulsed DC generated a specific antiviral response in a mouse HCV challenge model. Our results indicate that HCV core pulsed DC may serve as a new modality for immunotherapy of HCV especially in chronically infected patients.
Keywords: hepatitis C virus, dendritic cell, immunization, BALB/ c, novel treatment
Introduction
Hepatitis C virus infection (HCV) is the major causative agent of transfusion-associated and community-acquired non-A,non-B hepatitis [1]. An estimated 170 million individuals are infected worldwide and thus HCV infection represents a viral pandemic [2]. Infection with HCV is associated with high morbidity and mortality and has therefore become a major interest in public health [3]. Of those persons exposed, 75–85% develop chronic infection and hepatitis, and about one third progress to cirrhosis and eventual liver failure [4]. Moreover, HCV infected individuals are at increased risk for hepatocellular carcinoma (HCC) within 10–20 years following infection [5]. Interferon and ribavirin therapy, beneficial in about half of the patient population [6–9], is expensive and associated with significant side-effects making the development of a therapeutic and/or prophylactic vaccine of paramount clinical importance [10,11]. However, the feasibility of developing an effective HCV vaccine has been questioned, mainly because prophylactic immunity against HCV has not been reproducibly induced in chimpanzees by either vaccination or previous HCV infection, and reinfection in humans has been reported. Nonetheless, in chimpanzees previously infected or vaccinated with a plasmid vaccine, viral persistence and severity of subsequent HCV infections are strikingly diminished [12,13]. Recent data from injection drug users suggest that immunity against HCV viral persistence can be acquired and therefore further improved vaccines should be developed and tested [14].
Each design for a vaccine candidate (live, attenuated virus; inactivated virus; recombinant protein; plasmid DNA; live, recombinant vectors) has its own limitations in the vaccinee, e.g. induction of only borderline immunity after DNA-immunization or safety issues with viral vectors [15,16]. A number of different vaccine approaches have been investigated against HCV infection in the past like DNA vaccination [17–19], vaccination with recombinant proteins or peptides [11], vaccines based on virus-like particles [20] and viral or other pathogen vectors containing genetic information of HCV proteins [21].
DC vaccination holds its promises by playing a pivotal and central role in the initiation of immune responses and is considered in a large number of gene therapeutic studies against cancer and infectious diseases [22–24]. DC process captured or intracellularly produced antigens into peptides, migrate via afferent lymphatics to lymph nodes, and present MHC-peptide complexes to naive T cells. DC also control the type of immune responses by different cytokines, i.e. IFN-γ (Th1), IL-4 (Th2) or IL-10 to stimulate regulatory T cells. Such control can be influenced by the subset of DC and the type and duration of maturation signals they receive [23,25–27]. DC are found in the peripheral blood and the bone marrow and their number and activation state depend on growth factors such as GM-CSF or Flt3-L. Monocyte derived CD11+ DC induce T cells to produce Th1 cytokines in vitro, whereas the CD11c- plasmacytoid T cell-derived DC elicit the production of Th2 cytokines [28,29]. In this study we investigated the properties of a DC vaccine pulsed with recombinant HCV protein and peptides and analysed humoral and cellular immune responses. Moreover, we evaluated prophylactic and/or therapeutic efficacy of DC vaccination by using a syngenic tumour model expressing HCV core protein in an immunocompetent host. Since a number of studies recently described a significant down-regulation of DC function in HCV infected patients [30–33], a therapeutic DC vaccine may hold its promises by ex vivo maturation and stimulation of DC, because these DC in the chronically HCV infected patient are under the negative regulation of the virus itself.
Materials and methods
Animals
BALB/c (H-2b) mice were purchased from Charles River Laboratories and maintained under standard pathogen-free conditions in the animal facility (Zentrales Tierlabor, University of Heidelberg) and used at the age of 6–20 week for in vivo studies. Mice received standard care according to our institutional and national guidelines.
Antigens and cell lines
Recombinant HCV core protein covering amino acid (aa) 1–115 was purchased from Mikrogen (Munich, Germany). Peptides were proposed by computer simulation (University of Wisconsin Genetics Computer Group (UWGCG), peptide structure program) and prepared by EMC microcollections (Tübingen, Germany) with the following sequences: YQVRNSSGLYHVTNDCPNSS (1–20), PGCVPCVREGNAS RCWVAVT (33–53), REGNASRCWVAVTPTVATRDGKL (40–62) and PRRHWTTQDCNCSIYPG (104–120). Establishment and characterization of the stable transfected cell line expressing HCV core protein (SP2-19) and the control cell line SP2-0 have been described previously [34]. Cells were grown in DMEM, 10% FCS, l-glutamine, penicillin 5000 U/ml/streptomycin 5000 µg/ml and G418 1 µg/ml for positive selection. For HCV core protein expression Western blot analysis was performed as published previously [18,34].
Generation of dendritic cells
Bone marrow-derived DC were generated from female BALB/c mice (6–12 weeks old) by using a modified version of the method described by Inaba et al. [35] Briefly, femurs and tibias were removed from mice, and bone marrow cells were flushed from the femurs and tibias and cultured in RPMI-1640 (Gibco BRL) supplemented with 10% fetal bovine serum, penicillin 5000 U/ml, streptomycin 5000 µg/ml, 20 ng of granulocyte-macrophage colony-stimulating factor (GM-CSF)/ml (R & D) at 1 × 106 cells/ml in a 24 well plate. On day 3 of culturing, the nonadherent cells were removed and fresh medium containing GM-CSF was added. On day 5 of culturing, DC were isolated by transferring the nonadherent and loosely adherent cells to new culture plates (leaving behind the adherent macrophages), incubating the plates at 37 °C for at least 2 h, and then repeating the procedure to remove any contaminating macrophages. The DC were further enriched by density gradient centrifugation with 14·5% metrizamide solution (Sigma) in culture medium. DC purity was determined by cell size, dendritic morphology was assessed by phase-contrast microscopy, and viability was assessed by trypan blue exclusion. The purity of the DC fraction was higher than 90% as determined by FACS analysis of surface molecules expression (CD11c, CD11b, B7-2).
Pulsing DC and adoptive immunization with antigen-pulsed DC
At day 5 DC were panned twice and purified by density gradient centrifugation. DC (2 × 107 DC in RPMI-10) were incubated in the presence of 5 µg/ml recombinant HCV core protein and 2·5 µM recombinant HCV core peptides for 2 h at 37 °C with gentle mixing every 15 min. Afterwards cells were centrifuged, washed twice with PBS and injected in a volume of 100 µl in both footpads (50 µl/each). At the first immunization event a total of 1 × 106 DC, at the second booster immunization a total of 5 × 105 cells were injected into the footpad (6–10 mice per treatment group). Unpulsed DC were cultured and adoptively transferred in the same manner into control mice.
Two different immunization regiments were evaluated in this study: in the prophylactic model (P) DC were given on day 0 and 21; 5 days after the last immunization event mice were challenged with SP2-19 into the right flank. In the therapeutic model (T) mice were challenged at day 0; 5 and 26 days later mice were immunized with a therapeutic DC vaccine pulsed with recombinant HCV core protein and HCV peptides. To measure humoral immune responses and T cell proliferation mice were immunized in the prophylactic modus without tumour challenge.
Measurement of humoral immune responses
Anti-HCV core antibody levels in the serum of each immunized animal were determined by ELISA individually. In brief, microtitre plates (Nunc Maxisorp) were coated with 0·5 µg/well recombinant HCV core protein (Mikrogen, Munich, Germany), incubated overnight at 4 °C and blocked with foetal bovine serum (FBS) for 2 h at 20 °C. A 1 : 50 dilution of mouse serum was added to the plates, incubated an additional 1 h at 20°C, and washed 4 times with phosphate buffered saline (PBS) containing 0·05% Tween-20. A peroxidase-conjugated AffinePure Goat anti Mouse IgG (Dianova, Hamburg, Germany) was applied to the plates at a 1 : 2000 dilution, incubated for 1 h, washed, and substrate was added for colour development (Abbott).
T-cell proliferation assay
Mice were anaesthetized with diethylether for harvesting of spleen cells. Erythrocytes were removed by incubation in 0·83% NH4Cl/0·17 M Tris (pH 7·4) for 5 min at 25 °C. Spleen cells were washed 2 times and cultured in triplicate in 96 well round bottom plates at 5 × 105 cells/well in 200 µl DMEM (Cellgro Mediatech, Washington, DC) containing 10% FBS and 2-mercaptoethanol (50 µM). Cells were stimulated for 3 d with recombinant HCV core protein (aa 1–120) (Mikrogen, Munich, Germany) or recombinant HCV peptides at various concentrations (0, 0·01, 0·1, 1 µg/ml). Effector cells (negative controls) were stimulated with recombinant HBsAg proteins (Engerix™) at the same concentrations for control of antigen specificity. Following the addition of radioactive [3H]-thymidine (1 µCi/well), cells were incubated an additional 18 h, and [3H]-thymidine incorporation into DNA was measured after harvesting.
ELISPOT assay
To assess the number of IFN-γ secreting cells at the individual cell level, single cell suspensions from spleens harvested from immunized mice were analysed in an IFN-γ ELISPOT assay. Cells were directly assessed in this assay without prior in vitro expansion in the presence or the absence (neg. control) of 0,1 or 1 µg/ml recombinant HCV core protein at 37 °C (5%CO2) in IFN-γ bound microtitre plates to measure IFN-γ cytokine secretion as means of CD8 ± T-cell function (AID, Germany). After washing with PBS/Tween cells were incubated with a secondary antibody suspended in DMEM supplemented with 10% FCS and 2-mercaptoethanol. After rewashing, spots representing individual cytokine-producing cells were visualized by developing with substrate according to manufacturers guidelines and counted.
Assessment of prophylactic and therapeutic vaccination efficacy in vivo
One week after the last immunization event (prophylactic model) or five days prior to the first immunization with pulsed DC (therapeutic model) 2 × 106 syngenic SP2-0-derived cells stably expressing HCV core (designated SP2-19) were washed, resuspended in 200 µl PBS, and inoculated s.c. into the right flank. SP2-0 cells (not expressing a HCV antigen) were used as a control in selected animals to assess antigen specificity in this model. Tumor formation, tumour size, mouse weight and mouse survival were assessed at distinct intervals post inoculation. The DC inoculation was repeated once after 14 days and tumour size was measured every 3–5 days over 4 weeks and mouse survival until day 120 (to evaluate long-term survival). To assess CD4+ or CD8+ T cell specificity selected animals were treated with anti-CD4 or anti-CD8 antibodies as described previously [18,34]. A total of 1 mg per mouse per injection of anti-CD8 (clone YTS 169) or anti-CD4 (clone YTS 191·1) was injected on days 5, 3, and 1 before tumour challenge and every 5 days thereafter.
Results
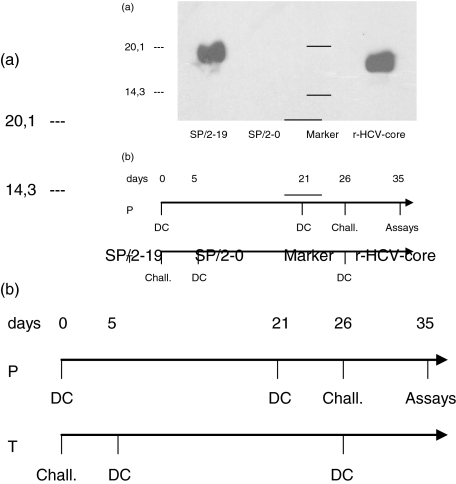
In order to investigate prophylactic and therapeutic efficacy of a DC vaccine we used a syngenic mouse myeloma cell line stably expressing HCV core. Stable expression was shown by Western blot analysis revealing a 21 kD protein in SP2-19 cells. Recombinant HCV core protein is visualized as a slightly smaller protein in the same blot (Fig. 1a). Two different immunization regiments were evaluated in this study as shown in Fig. 1b: in the prophylactic model DC were given on day 0; 21 days later a booster injection was performed. Five days after this immunization event mouse were challenged with an HCV-core expressing syngenic mouse myeloma cell line into the right flank. In the therapeutic model mice were challenged at day 0 with the HCV core expressing tumour cell line; 5 and 26 days later mice were immunized with a DC vaccine pulsed with recombinant HCV core protein and HCV peptides. In addition, to analyse humoral and cellular immune responses by in vitro assays (ELISA, T-cell-proliferation assay) mice were immunized twice with a three week interval and sacrificed 14 days after the last booster injection.
Fig. 1.
(a) To assess prophylactic and therapeutic efficacy of DC vaccination a syngenic mouse myeloma cell line expressing HCV core (SP2-19) was used for challenging experiments. Stable expression is shown by Western blot analysis revealing a 21 kD protein in SP2-19 cells (lane 1); lane 2, control, nonexpressing SP2-0 cell lysate; Lane 3, marker lane; Lane 4, E. coli expressed recombinant HCV core protein reveals a slightly smaller protein band. (b) Time schedule for vaccination in a prophylactic (P) or therapeutic (T) model.
Humoral immune response
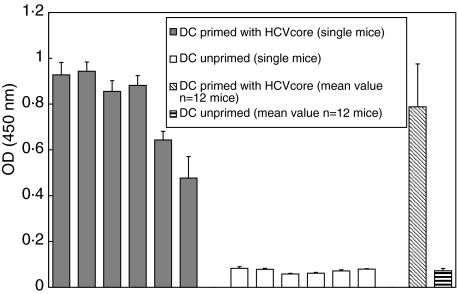
A strong HCV core antibody response was detected in each individual mouse after two immunizations with pulsed DC (Fig. 2), demonstrating the central role of DC in immune activation and the strength of our vaccination approach [18,34]. Notably, no antigen specific immune responses were detected in mice immunized with unpulsed DC. This antibody response may have importance for using a DC vaccine for individuals at high risk for an HCV infection (e.g. i.v. drug users, health care professionals).
Fig. 2.
HCV-core ELISA after immunization with HCV core recombinant protein and HCV-peptide pulsed DC. DC were cultured for five days in the presence of GM-CSF and pulsed for two hours ex vivo. Strong humoral immune responses were detectable by ELISA two weeks after the last immunization event.
T-cell proliferative response
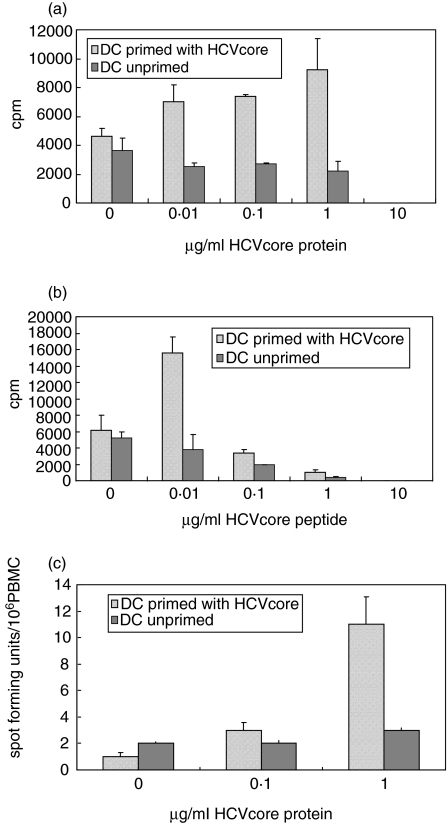
To investigate cell-mediated immune responses against HCV core protein, spleen cells were harvested and restimulated either with recombinant core protein or peptides. T-cells were stimulated with varied concentrations of recombinant HCV core protein or HCV core peptides (0; 0,01; 0,1; 1 µg/ml) for 3 days, and then underwent a 18-h radioactive thymidine incorporation assay. Significant T-cell proliferation levels were obtained at 1 µg/ml recombinant protein concentrations as compared to mice immunized with unprimed and unstimulated DC (Fig. 3a,b). A strong T-cell proliferative response was seen in T cells stimulated with HCV core peptides already at a concentration as low as 0,01 µg/ml. At higher concentrations (10 µg/ml) recombinant HCV core protein and > 0·1 µg/ml core peptides toxic effects to T cells were observed. This effect is most likely due to denaturing buffer conditions of solubilized proteins (containing 0·05% SDS) or peptides. The T-cell proliferative response was antigen-specific since stimulation with a nonrelevant protein (HBsAg) revealed only background proliferative activity (data not shown).
Fig. 3.
Proliferation of CD4+ T cells after 3 days stimulation in the presence of (a) 1 µg/ml recombinant HCV core and (b) 0.01 µg/ml recombinant HCV core peptide mix (n = 10 mice/group). (c) Assessment of mIFN-γ secreting cells in an ELISPOT assay as means of T-cell effector function. Spleen cells were stimulated with recombinant HCV-core protein at 0,1 and 1 µg/ml, and the number of spot forming units/106 PBMC were counted after 24 h incubation period (n = 8 mice/group).
CD8+ T cell function: ELISPOT assay
We used IFN-γ bound microtitre plates to measure IFN-γ cytokine secretion as means of CD8 ± T cell function in an ELISPOT on an individual cell level. In contrast to the chromium release assay T cells were not in vitro expanded or restimulated. Spleen cells were harvested and cultured overnight in the presence or the absence (neg. control) of 0.1 or 1 µg/ml recombinant HCV core protein. The number of spot forming units/106 PBMC were counted after 24 h incubation period. A significant increase of HCV core specific CD8 ± T cells was measured without in vitro without restimulation at 1 µg/ml, demonstrating the efficacy of a DC-vaccine to induce cellular and cytotoxic immune responses (Fig. 3c).
In vivo cytotoxic T-cell response
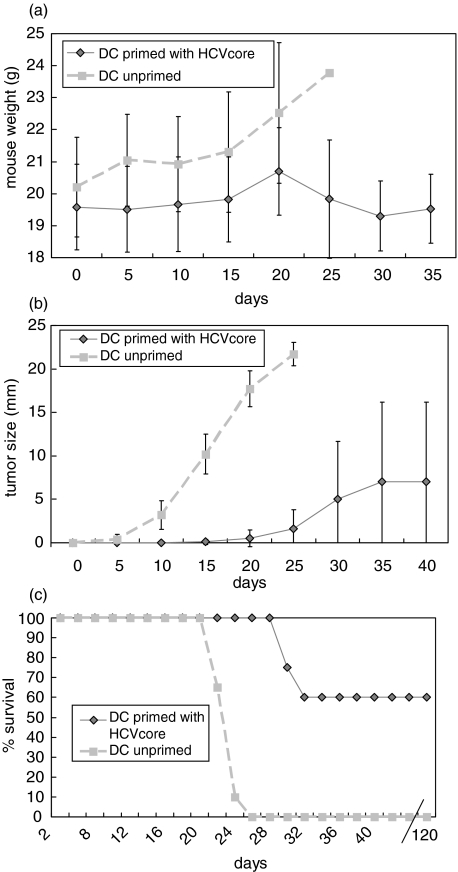
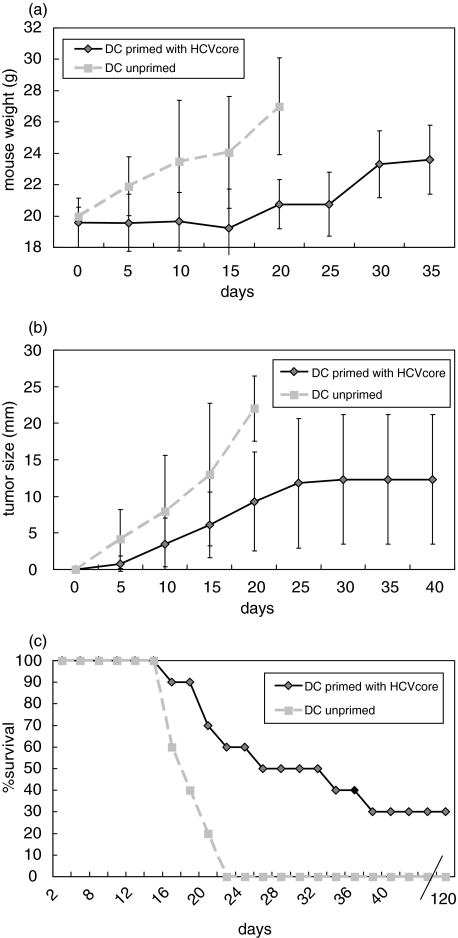
In vivo antiviral activity was assessed by a tumour model in a prophylactic (Fig. 4) and in a therapeutic setting (Fig. 5). Each group contained 10 animals and experiments were done twice. Naïve mice immunized with HCV-core pulsed DC are partially protected against a challenge with syngenic mouse myeloma cells expressing HCV core. Mouse weight after vaccination is stable throughout the observation period, whereas control mice increased their weight due to tumour formation and ascites. Some mice immunized with HCV core pulsed DC developed tumours, but they were smaller then those of mice immunized with unpulsed DC. Finally, in the control group (mice immunized with unpulsed DC) all mice died by day 27. Sixty percent of mice immunized with HCV-core pulsed DC were long-term survivors over a period of four month with no sign of any tumour growth. Similar results were obtained in the treatment group were mice were first inoculated with HCV core expressing tumours and thereafter vaccinated with HCV core pulsed or unpulsed (control group) DC. However, the number of long-term survivors was lower than in the prophylactic model; only 30% showed no tumour burden and survived until day 120. Antigen specificity was demonstrated by tumour formation in all mice immunized with a SP2-0 cell line (no expression of HCV core protein), where all mice died by day 26 (n = 5) (data not shown). Moreover, mice immunized with HCV pulsed DC and challenged with SP2-19 cells and treated at the same time with an anti-CD8 antibody (n = 3) developed all tumours and died (data not shown) confirming the effector function of CD8+ cytotoxic T lymphocytes after DC vaccination.
Fig. 4.
Mice (n = 10) immunized with HCV core pulsed DC are long-term protected (> 120 days) following challenge with an HCV core expressing syngenic mouse myeloma cell line in about 60% of mice. This response was CD8+ T cell mediated since mice (n = 4) treated with anti-CD8 antibodies were all dead by day 25. In the control group (unpulsed DC) all mice were dead by day 27 and tumour weight as well as mouse weight due to tumour formation and aszites was significantly higher until day 25. (a) mouse weight; (b) tumour size; (c) % survival.
Fig. 5.
In a therapeutic vaccination approach mice were inoculated with 2 × 106 cells of a HCV core expressing syngenic mouse myeloma cell line into the right flank (n = 10). Five and 26 days later mice were treated with HCV core pulsed or unpulsed DC. About 30% of mice long-term survived this treatment regiment with no signs of tumour formation until day 120. In contrast all mice died by day 23 in the group treated with unpulsed DC. Again, as for the prophylactic model tumour size and mouse weight differed strikingly in both treatment groups. (a) mouse weight; (b) tumour size; (c) % survival.
Discussion
Chronic HCV infection may progress to chronic hepatitis, cirrhosis, hepatic failure and HCC. Attempts to elucidate the polyclonal and multispecific host response as they relate to liver cell injury are emerging and the generation of CD4+ and CD8+ CTL activity in chronic hepatitis appears to be associated with viral replication and sometimes viral clearance. Thus, broad-based and vigorous CD4+ and CD8+ immune responses appear to promote viral clearance and may generate protective immunity [36–38]. However, although B and T lymphocytes respond to antigens with high specificity, they are by themselves not capable of making the complex decisions of immune activation. Induction of an effective immune response requires the participation of professional host antigen presenting cells (APC), the most potent of which are DC. They play a key role in the initiation of immune responses to foreign antigens. Their antigen uptake and presentation capacities enable them to prime and activate T cells [23,25]. The functions of DC differs according to their localization and level of maturation. In general these cells are strong stimulators of the allogenic mixed leucocyte reaction, induce antigen specific humoral and cellular immune responses, produce and induce a variety of cytokines of both Th1 and Th2 types, ensure the survival and activity of the antigen-specific lymphocytes including CTL and induce immunogenic tolerance [25,27,29]. HCV has been shown like other viruses (e.g. herpes simplex virus, measles virus) to affect and to impair DC function. DC derived from HCV patients and from patients with HCC in a HCV cirrhotic liver showed impaired abilities to stimulate allogenic T cells and to produce IFN-γ[30–32]. Monocyte derived DC from patients with chronic infection fail to respond to maturation stimuli. Instead they maintain their immature phenotype, reflected by their pattern of cell surface markers and by their continued capacity to uptake antigen. Moreover, DC from patients who have resolved HCV infection behave like DC from healthy donors: in response to maturation stimuli, they decrease antigen uptake, up-regulate expression of appropriate surface marker, and are potent stimulators of allogenic T cells [33]. The communication between DC and T cells in this area of host–pathogen interaction seems to be a dialogue rather than a monologue in which the mature DC respond to the T cell as well. The fact that anergic T cells can inhibit the allostimulatory capacity of DC and down-regulate MHC-class II, CD80 and CD86 surface expression, may negatively influence DC function in chronically HCV infected patients. Most of CD8+ T cells were negative for activation markers resembling an anergic phenotype which may lead in consequence to the impaired maturation of DC [33,39]. Furthermore, in the context of an allostimulatory defect of monocyte-derived DC, they may also constitute an extrahepatic reservoir for HCV [32].
DC have been successfully used as cellular adjuvants in mice to elicit protective T cell immunity against pathogens and tumours [22,25,27]. Because DC are essential for T-cell activation and since viral clearance in HCV infected patients is associated with a vigorous T-cell response [36], we propose a new type of HCV vaccine based on ex vivo matured, stimulated and ex vivo HCV specific antigen pulsed DC. This vaccine used in a therapeutic setting circumvents the diminished and down-regulated DC function of HCV infected patients in vivo by giving the necessary maturation stimuli ex vivo. This is important to keep in mind, since DC maturation and DC function are modulated by the microenvironment, which is very different in HCV infected patients in vivo then in the cell culture dish ex vivo. Especially as a therapeutic immunization approach this vaccine holds important new aspects since other vaccines are faced in HCV infected patients with the described down-regulated DC function.
Mice immunized with a HCV core based DC vaccine showed humoral and more importantly cellular immune responses by ELISA, T-cell proliferation assay and by ELISPOT, demonstrating CD8+ antigen-specific T cell activity. Interestingly, levels of HCV core antibodies were about 10 fold higher than compared to earlier results after DNA immunization against HCV core protein or the nonstructural proteins, demonstrating the central role of DC in immune activation and the strength of our vaccination approach [18,34]. Naïve mice and mice inoculated with a HCV core expressing cell line were partially protected against tumour formation and indeed 60% after prophylactic and 30% after therapeutic immunization were long-term survivors and protected through the induction of a strong B but more importantly T cell response. In the control group mice were vaccinated with unpulsed DC and all mice died at about 25 days. This confirms that immune responses were antigen specific and not anti-tumour immune responses due to DC vaccination against tumour cells. Moreover, all mice treated with an anti-CD8 antibody behaved like the control group, demonstrating the in vivo CD8+ T cell activity through DC vaccination. It is also known from our previous studies using this challenge model that tumour rejection in this animal model is mediated by CD8 T cells [18,34].
Despite the development of significant B cell responses with high antibody levels in our model, the clinical role of neutralizing antibodies in a prophylactic or therapeutic immunization approach in HCV infected patients remains to be determined. To our knowledge no clinical data exist to date that neutralizing antibodies in HCV infection are able to control viral disease. Furthermore, in our model we do not know if antibodies generated through DC vaccination have neutralizing capacity.
Recently, Shimizu et al. [40] demonstrated the utility of a HBV dendritic cell vaccine in HBV transgenic mice. This study suggests that antigen presentation by cytokine-activated DCs can break tolerance and trigger an antiviral CTL response in HBV transgenic mice. They showed that this strategy is more efficient than DNA immunization in this setting. Indeed, we confirm these results in our HCV model were our laboratory previously induced cellular and humoral immune responses in naïve BALB/c mice to prophylactically assess HCV DNA vaccination [34,41]. Antibody levels and T cell activation were dramatically lower after DNA immunization then described here in our HCV DC vaccination study.
This is to our knowledge the first systematic analysis of in vitro and in vivo immune responses after DC vaccination against an HCV antigen. Only one report exists where in a side arm of studying a novel vaccine system with the anthrax toxin-mediated antigen delivery system, DC primed with this vector system were evaluated in a HCV model, however, DC vaccination itself was not the aim of the study [42]. Immunization strategies in chronic HCV infection may have one central aim in the future: targeting directly or indirectly DC to induce vigorous immune responses which are able to eliminate the virus. The use of HCV primed DC for vaccination in chronic infected patients in a therapeutic setting or in high risk groups as a prophylactic vaccine seems to be a new promising modality for immunotherapy of HCV.
Acknowledgments
We are most grateful to Christoph Eisenbach and Michael Stephan for critical reading of the manuscript. Supported in part by a grant from the ‘Forschungsförderungsprogramm der Medizinischen Fakultät der Universität Heidelberg’ and by grant En 338/4–1 from the Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany.
References
- 1.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–62. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 2.Thomas DL. Hepatitis C epidemiology. Curr Top Microbiol Immunol. 2000;242:25–41. doi: 10.1007/978-3-642-59605-6_2. [DOI] [PubMed] [Google Scholar]
- 3.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 4.Theodore D, Fried MW. Natural history and disease manifestations of hepatitis C infection. Curr Top Microbiol Immunol. 2000;242:43–54. doi: 10.1007/978-3-642-59605-6_3. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 6.Manns MP, McHutchinson JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C. a randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 7.Zeuzem S, Feinman SV, Rasenack J, et al. Peginterferon α-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343:1666–72. doi: 10.1056/NEJM200012073432301. [DOI] [PubMed] [Google Scholar]
- 8.McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–92. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 9.Poynard T, McHutchinson J, Manns M, et al. Impact of pegylated interferon α-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–13. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 10.Lechmann M, Liang TJ. Vaccine development for hepatitis C. Semin Liver Dis. 2000;20:211–26. doi: 10.1055/s-2000-9947. [DOI] [PubMed] [Google Scholar]
- 11.Houghton M. Strategies and prospects for vaccination against the hepatitis C viruses. Curr Top Microbiol Immunol. 2000;242:327–39. doi: 10.1007/978-3-642-59605-6_15. [DOI] [PubMed] [Google Scholar]
- 12.Forns X, Payette PJ, Ma X, et al. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology. 2000;32:618–25. doi: 10.1053/jhep.2000.9877. [DOI] [PubMed] [Google Scholar]
- 13.Bassett SE, Guerra B, Brasky K, et al. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology. 2001;33:1479–87. doi: 10.1053/jhep.2001.24371. [DOI] [PubMed] [Google Scholar]
- 14.Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–83. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 15.Letvin NL. Strategies for an HIV vaccine. J Clin Invest. 2002;110:15–27. doi: 10.1172/JCI15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho DD, Huang Y. The HIV-1 Vaccine Race. Cell. 2002;110:135–8. doi: 10.1016/s0092-8674(02)00832-2. [DOI] [PubMed] [Google Scholar]
- 17.Encke J, zu Putlitz J, Wands JR. DNA Vaccines. Intervirol. 1999;42:117–24. doi: 10.1159/000024971. [DOI] [PubMed] [Google Scholar]
- 18.Encke J, zu Putlitz J, Geissler M, Wands JR. Genetic immunization generates cellular and humoral immune responses against the nonstructural proteins of the hepatitis C virus in a murine model. J Immunol. 1998;161:4917–23. [PubMed] [Google Scholar]
- 19.Satoi J, Murata K, Lechmann M, et al. Genetic immunization of wild-type and hepatitis C virus transgenic mice reveals a hierarchy of cellular immune response and tolerance induction against hepatitis C virus structural proteins. J Virol. 2001;75:12121–7. doi: 10.1128/JVI.75.24.12121-12127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechmann M, Murata K, Satoi J, Vergalla J, Baumert TF, Liang TJ. Hepatitis C virus-like particles induce virus-specific humoral and cellular immune responses in mice. Hepatology. 2001;34:417–23. doi: 10.1053/jhep.2001.26523. [DOI] [PubMed] [Google Scholar]
- 21.Wedemeyer H, Gagneten S, Davis A, Bartenschlager R, Feinstone S, Rehermann B. Oral immunization with HCV-NS3-transformed Salmonella: induction of HCV- specific CTL in a transgenic mouse model. Gastroenterology. 2001;121:1158–66. doi: 10.1053/gast.2001.29311. [DOI] [PubMed] [Google Scholar]
- 22.Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002;109:1519–26. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 24.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 25.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 26.Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106:271–4. doi: 10.1016/s0092-8674(01)00448-2. [DOI] [PubMed] [Google Scholar]
- 27.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253–6. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 28.Pulendran B, Bancherau J, Burkeholder S, et al. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J Immunol. 2000;165:566–72. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 29.Pulendran B, Banchereau J, Maraskovsky E, Maliszewski C. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 2001;22:41–7. doi: 10.1016/s1471-4906(00)01794-4. [DOI] [PubMed] [Google Scholar]
- 30.Kakumu S, Ito S, Ishikawa T, et al. Decreased function of peripheral blood dendritic cells in patients with hepatocellular carcinoma with hepatitis B and C virus infection. J Gastroenterol Hepatol. 2002;15:431–6. doi: 10.1046/j.1440-1746.2000.02161.x. [DOI] [PubMed] [Google Scholar]
- 31.Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–91. [PubMed] [Google Scholar]
- 32.Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo C, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–24. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 33.Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171–6. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 34.Geissler M, Gesien A, Tokushige K, Wands JR. Enhancement of cellular and humoral immune responses to hepatitis C virus core protein using DNA-based vaccines augmented with cytokine-expressing plasmids. J Immunol. 1997;158:1231–7. [PubMed] [Google Scholar]
- 35.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rehermann B, Chisari FV. Cell mediated immune response to the hepatitis C virus. Curr Top Microbiol Immunol. 2000;242:299–325. doi: 10.1007/978-3-642-59605-6_14. [DOI] [PubMed] [Google Scholar]
- 37.Chang KM, Thimme R, Melpolder JJ, et al. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–76. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 38.Chisari FV. Cytotoxic T cells and viral hepatitis. J Clin Invest. 1997;99:1472–7. doi: 10.1172/JCI119308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He XS, Rehermann B, Lopez-Labrador FX, et al. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci USA. 1999;96:5692–7. doi: 10.1073/pnas.96.10.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimizu Y, Guidotti LG, Fowler P, Chisari FV. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J Immunol. 1998;161:4520–9. [PubMed] [Google Scholar]
- 41.Tokushige K, Wakita T, Pachuk C, et al. Expression and immune response to hepatitis C virus core DNA-based vaccine constructs. Hepatology. 1996;24:14–20. doi: 10.1002/hep.510240104. [DOI] [PubMed] [Google Scholar]
- 42.Moriya O, Matsui M, Osorio M, et al. Induction of hepatitis C virus-specific cytotoxic T lymphocytes in mice by immunization with dendritic cells treated with an anthrax toxin fusion protein. Vaccine. 2001;20:789–96. doi: 10.1016/s0264-410x(01)00407-8. [DOI] [PubMed] [Google Scholar]