Abstract
Systemic immunization of BALB/c mice with human cartilage proteoglycan (PG) aggrecan induces progressive polyarthritis. The G1 domain of the PG aggrecan molecule contains most of the T cell epitopes, including three immunodominant (‘arthritogenic’) and at least six subdominant T cell epitopes. The three dominant T cell epitopes (P49, P70 and P155) were deleted individually or in combination by site directed mutagenesis, and the recombinant human G1 (rhG1) domain (wild type and mutated) proteins were used for immunization. Close to 100% of BALB/c mice immunized with the wild-type (nonmutated) rhG1 domain developed severe arthritis, which was 75% in the absence of P70 (5/4E 8) epitope, and very low (< 10% incidence) when all three dominant T cell epitopes were deleted. The onset was delayed and the severity of arthritis reduced in animals when dominant T cell epitopes were missing from the immunizing rhG1 domain. The lack of T cell response to the deleted epitope(s) was specific, but the overall immune response against the wild-type rhG1 domain of human PG was not significantly affected. This study helped us to understand the dynamics and immune-regulatory mechanisms of arthritis, and supported the hypothesis that the development of autoimmune arthritis requires a concerted T cell response to multiple epitopes, rather than the immune response to a single arthritogenic structure.
Keywords: aggrecan, animal model, arthritis, rheumatoid arthritis, T cell epitopes
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that affects approximately 1% of the human population. Although the aetiology of this disease is not known, it is strongly believed that T-cell-driven mechanisms against joint/cartilage-derived macromolecules determine the organ-specificity of the disease. Among these macromolecules, type II collagen, proteoglycan (PG) aggrecan, link protein, and glycoprotein-39 are the most abundant components in cartilage, which became most likely candidates as autoantigens in RA. Indeed, T cell responses to these matrix molecules have been detected in patients with RA [1–8], and cartilage macromolecules induce arthritis, when used for immunization of genetically susceptible rodents [9–14].
Systemic immunization of BALB/c mice with cartilage PG aggrecan induces progressive polyarthritis [11,15]. This PG-induced arthritis (PGIA) model bears many similarities to human RA and might be a relevant model for, at least a subset of RA. The clinical features shown for PGIA, along with histological studies, radiographic analyses and scintigraphic bone scans of diarthrodial joints exhibited many similarities to RA [11,15–17]. PGIA can be transferred only with T cells from arthritic BALB/c mice to syngeneic recipients [18,19], a PG-specific T cell hybridoma can induce arthritis [20], and the depletion of CD4+ T cells protects susceptible animals from arthritis [15,21]. In contrast, while PG-specific antibodies and/or PG-presenting B cells [22] could accelerate the onset and severity of adoptively transferred disease [18,19], neither the PG-specific antibodies nor B cells (without T cells) can transfer arthritis. Moreover, recent genome-wide screening studies showed that most of the genomic loci for arthritis incidence were associated with T cell responses, and only a limited number of quantitative traits linked to antibody/B cell function in PGIA [15,23,24]. In conclusion, we believe that PGIA is a T-cell-dependent antibody-mediated autoimmune disease [15,22,25].
The aggrecan molecule contains several T cell epitopes essential for the induction of arthritis, and eventually all the dominant/subdominant T cell epitopes are present in the G1 domain [15,26,27]. Recently, an extensive study has been completed to map a total of 143 predicted T cell epitopes along the core protein of the aggrecan molecule in genetically susceptible BALB/c mice [27], and in RA-predisposing DR4/DQ8 allele-specific transgenic BALB/c mice [28]. The G1 domain of PG aggrecan carries at least three dominant arthritogenic epitopes (P49, P70 or 5/4E8, and P155) [20,27,29] and a few subdominant and/or cryptic structures depending upon the strain-specific haplotype (major histocompatibility complex [MHC] in mice) [27,30]. The level of T cell responses against these three dominant/arthritogenic peptide sequences highly correlated with the severity of arthritis in BALB/c mice [22,27,29]. These three dominant (arthritis-associated) epitopes are present in the A and B loops of the G1 domain of aggrecan (Fig. 1). The aim of this study was to investigate the immune pathological function of these three dominant epitopes when disrupted (deleted) individually or in combination using site directed mutagenesis, and to determine which dominant/arthritogenic epitope might be critical for arthritis induction.
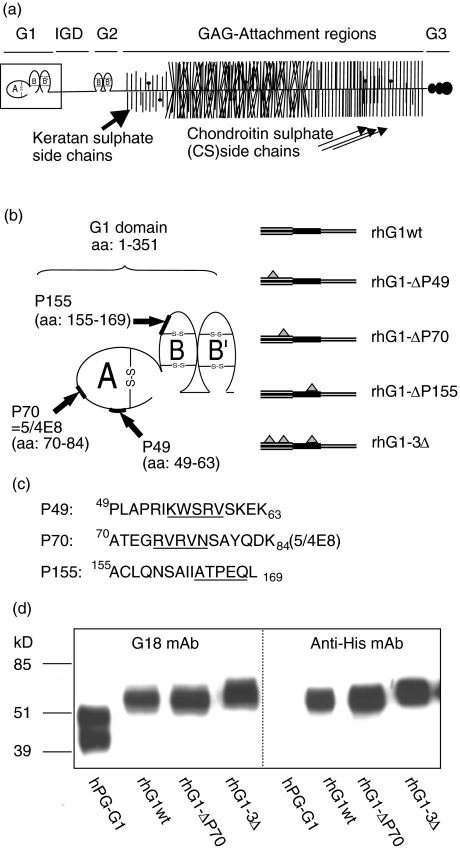
Fig. 1.
Schematic structure of cartilage PG aggrecan, localizations of dominant/arthritogenic epitopes in the G1 domain, and Western blots of wild-type (hPG-G1) and recombinant human G1 (rhG1) domain proteins. (a) A schematic presentation of the aggrecan molecule with its different domains. These are the G1, G2, and G3 domains, the interglobular domain (IGD), and the glucosaminoglycan (GAG) altered region. (b) The G1 domain (framed in (a)) consists of 3 loops: A, which binds to the A loop of link protein and BB′ loops which bind to hyaluronan. The positions of the three dominant/arthritogenic epitopes (P49, P70 (also named 5/4E8), and P155) in the G1 domain of aggrecan are indicated by arrows (b) and the corresponding amino acid sequences are shown (c). The deleted 5 amino acids within these epitopes (created by site directed mutagenesis) are underlined. Three clones, each having one (single) epitope deletion, were created, and another clone which had all three deletions (rhG1–3Δ). (d) The Western blot shows a stromelysin-generated and affinity-purified G1 domain (hPG-G1) isolated from native human cartilage PG aggrecan (lane 1; positive control) as described [22], and the three rhG1 domain proteins used in this study. Monoclonal antibody G18, which recognizes a linear peptide sequence in the A loop of human G1 domain [22], and anti-His mouse monoclonal antibody (Invitrogen) which recognizes the poly His tag at the C-terminus of the rhG1 were used for immunodetection shown in (d). Approximately the same amounts of proteins (0·8 µg) were loaded on a 12% sodium-duodecyl/acrylamide gel, transferred onto nitrocellulose membrane and stained with antibodies as described [17].
Materials and methods
Animals, human material, and chemicals
The use of animals for immunization and arthritis induction was approved by the Institutional Animal Care and Use Committee of Rush University Medical Center. The use of human cartilage PG aggrecan (obtained from joint replacement surgeries) was approved by the Institutional Review Board. All chemicals (unless indicated) were purchased from Sigma Chemical Company (St. Louis, MO, USA) or Fisher Scientific (Chicago, IL, USA).
Baculovirus expression system
As the non-glycosylated G1 domain is completely insoluble [22], and the G1 domain without glucosaminoglycan-attachment regions (Fig. 1a) is not secreted by avian or mammalian cells [31–34], we have used a baculovirus expression system [14]. This method resulted in relatively large amounts of secreted rhG1 domains, both wild-type and mutated. The baculovirus and the recombinant bacmids were prepared according to the Bac-to-Bac Baculovirus Expression System (Invitrogen, Carlsbad, CA, USA). The cDNA coding for the human G1 domain of aggrecan was generated by reverse transcription of RNA isolated from human chondrocytes and amplified by polymerase chain reaction (PCR). A clone in pSecTagII vector (Invitrogen) was prepared earlier in our laboratory [22] that contained the first 360 amino acids in the aggrecan molecule (G1 domain and a short sequence of the interglobular domain up to the stromelysin cleavage site [VDIPEN360]). This G1-coding domain cDNA (Fig. 1b–1c) was recloned into the donor plasmid pFastBac1 (Invitrogen) along with secretion and poly His tags from the G1 domain containing pSecTagII vector. T7 and BGH universal primers were used for this purpose. The T7 (forward) primer had the XbaI linker, and the BGH (reverse) primer had the KpnI linker. The PCR product and the donor plasmid were digested with the two restriction enzymes, ligated, and DH5α competent cells (Invitrogen) were transfected with this new pFastBac1-G1 plasmid.
Minipreps were prepared and relevant clones selected by PCR, which were then sequenced. Recombinant pFastBac1-G1 donor plasmids with or without mutated G1 domain were transformed into DH10Bac competent cells (Invitrogen) and plated on Luria-Bertini agar plates containing 7 µg/ml gentamicin, 10 µg/ml tetracyclin, 50 µg/ml kanamycin, 100 µg/ml BlueGal, and 40 µg/ml β-D-isopropyl-thiogalactopyranoside. The recombinant bacmid was transfected into SF9 cells (Invitrogen) using Cellfectin reagent (Invitrogen). The supernatant containing the virus particles was collected after 72 h for further amplification and optimization of protein expression.
Virus amplification was performed in SF9 cells cultured in SF-900 II SFM medium (Invitrogen) at 0·1 multiplicity of infection (MOI), and the titre of the produced virus was measured using the BD BacPAK Rapid Titer kit (BD Bioscience, Palo Alto, CA, USA). For recombinant protein expression, ‘High Five’ cells (Invitrogen) were used. These cells were cultured in Express Five SFM (Invitrogen), and infected with the recombinant baculovirus at 5·0 MOI at a cell density of 2 million cells/ml. The supernatant, which contained the recombinant protein, was collected by centrifugation 3 days after infection. We achieved a yield of about 0·1–0·5 mg of purified rhG1 domain protein per litre of insect cell cultures using the combination of anion exchange Q-Sepharose chromatography and Ni-NTA purification system described below (Fig. 1d).
Site directed mutagenesis
Site directed mutagenesis kit (Stratagene, La Jolla, CA, USA) was used for the deletion of amino acid residues within the arthritogenic epitopes of the G1 domain of aggrecan (Fig. 1b). Briefly, this PCR-dependent method uses two complementary primers that contain the mutation of interest (deletion, insertion, or substitution) to perform a PCR on the used cDNA template (vector plus insert). High fidelity DNA polymerase was used to perform PCR on the pFastBac1-G1 template. The PCR product was used to transform ultracompetent XL10-Gold E. coli cells (Stratagene) after digestion with the restriction enzyme Dpn I, which digests only methylated DNA of the template derived from a bacterial source. Thus, this digestion simultaneously eliminated nonmutated colonies. Selected clones were screened for the mutation by sequencing the region of interest.
Purification of recombinant proteins using anion exchange chromatography (Q-Sepharose) and metal affinity column (Ni-NTA)
First, Q-Sepharose (Amersham Bioscience, Piscataway, NJ, USA) anion-exchange chromatography was used for prepurification of recombinant proteins. Collected media were cleared from cells by centrifugation at 5800 × g, the pH adjusted to pH 10·0, and then run through a Q-Sepharose column that was equilibrated and washed with phosphate-buffered saline (25 mM, pH 10·0). The bound proteins were eluted with a 0·5 M NaCl in 25 mM phosphate buffer (pH 6·0). The eluted material from the Q-Sepharose column was collected and dialysed against the washing buffer (0·5 M NaCl solution in 25 mM phosphate buffer, pH 6·0) prior to loading on a Ni-NTA column (Qiagen, Valencia, CA, USA). Unbound proteins were removed from the Ni-NTA column by extensive washing with 40 mM imidazole in the washing buffer, and then the recombinant proteins eluted with 300 mM imidazole. The purities of rhG1 proteins were tested in sodium-dodecyl sulphate acrylamide gel electrophoresis in reducing condition and Western blotting using different antibodies (Fig. 1d).
Immunization protocols and assessment of arthritis
Female BALB/c mice were purchased from Charles River Breeding Laboratories (Kingston colony, NY, USA). Mice were immunized intraperitonially with 50 µg of the different versions of the recombinant protein (wild-type or mutated G1 domain of aggrecan) on days 0, 21, 42, 63 and 84. These injections were given with 2 mg of DDA (dimethyldioctadecyl ammonium bromide) adjuvant in 100 µl PBS [35]. This adjuvant is a bacterium- and oil-free bifunctional (hydrophilic/lypophylic) quaterner ammoinium basis [35]. Human PG aggrecan-immunized age-matched BALB/c mice were used as positive controls [15,17,22]. Mice were scored for arthritis (paws swelling) three times a week [17], and killed approximately 2 weeks after the 5th antigen injection (on days 96–98).
Measurement of antigen specific T cell responses, antibodies, and cytokines
Sera and spleens were collected from immunized mice killed at the end of the experiment. Antigen-specific T cell responses were measured in quadruplicate samples of spleen cells (3 × 105 cells/well) in the presence of 10–50 µg/ml of synthetic peptides representing individual epitopes or 25–50 µg/ml antigenic proteins. Antigen-specific interleukin (IL)-2 production was measured in 2-day-old supernatants by CTLL-2 bioassay, and T cell proliferation was assessed on day 5 by H3-thymidine incorporation [17,22,27]. The antigen specific T cell response was expressed as stimulation index, which was calculated as the ratio of the count per minute (cpm) of H3-thymidine incorporated in antigen-stimulated cultures relative to the cpm measured in nonstimulated cultures. PG-specific antibodies were measured by enzyme linked immunosorbent assay (ELISA) as described previously [17,27,28,35]. Tumour necrosis factor-α (TNF-α), IL-1β, IL-4, IL-6, IL-10 and interferon-γ (IFN-γ) were measured in sera collected at the end of experiments, and in antigen (hPG, rhG1 or its mutated versions) or peptide-stimulated 3-day old supernatants of spleen cells as described [17,27,28,35]. The capture ELISA method (BD Bioscience, San Diego, CA or R & D Systems, Minneapolis, MN, USA) was used as described previously [17,19].
Results
The immuno-functionality of the rhG1 wild-type (rhG1wt) protein (without mutation) was tested in vitro by using a T cell hybridoma (5/4E8) which is specific to the P70 epitope (70ATEGRVRVNSAYQDK; core peptide is underlined) [22]. The antigen-specific IL-2 production shown on Fig. 2 was determined by CTLL-2 bioassay using different synthetic peptides, rhG1wt protein, human PG aggrecan, and the mutated rhG1-ΔP70 protein, which has a 5 amino acid-long deletion within the P70-84 (5/4E8) epitope (Fig. 1c). The rhG1wt domain, the human PG aggrecan, and the synthetic peptide (70ATEGRVRVNSAYQDK) corresponding to the P70 (5/4E8) epitope, showed marked stimulation of the 5/4E8 T cell hybridoma. The rhG1-ΔP70 failed to stimulate hybridoma cells, likewise the rhG1–3Δ which also lacks the P70-84 [5/4E 8] epitope (Fig. 2). These in vitro stimulation experiments indicated that rhG1wt domain (Fig. 2), and the hG1-ΔP49 and rhG1-ΔP155 recombinant proteins (data not shown) contained the P70 (5/4E8) epitope, these recombinant proteins were presented by antigen presenting cells, and recognized by a peptide-specific hybridoma.
Fig. 2.
In vitro functional tests of rhG1 domain proteins using T cell hybridoma 5/4E8, which is specific for P70-84 peptide sequence within the G1 domain [22,39]. The peptide sequence and the 5-amino-acid deletion of the P70-84 (5/4E8) epitope are shown in Fig. 1c. CTLL-2 bioassay was used for the detection of antigen/peptide-specific IL-2 production. Antigenic peptides/proteins were presented by irradiated A20 mouse (BALB/c) B lymphoma cells as described [22]. Human P70-84 synthetic peptide was used at 10, 5 and 1 µg/ml, the P70-84 mouse analogous peptide at 100, 50 and 25 µg/ml, and the rhG1 domain proteins at 4, 0·8 and 0·16 µg/ml. The human PG aggrecan (hPG) was used at 40, 16 and 1·6 µg/ml concentrations. (Antigen/peptide concentrations are indicated: black columns represent the highest, and empty columns the lowest concentrations in each group). Note, 5/4E8 T cell hybridoma was stimulated in a dose-dependent manner with antigens (peptides), except the rhG1-ΔP70 and rhG1–3Δ recombinant proteins both having the same 5-amino-acid deletion within the P70 (5/4E8) core peptide (Fig. 1c) were negative. *P70 human/mouse (5/4E8) peptide: 70ATEGR/QVRVNSAYQ/IDK84.
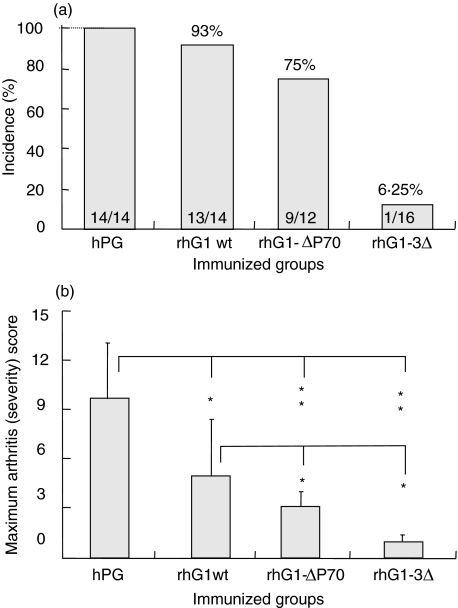
To test how the deletions of one or all three arthritogenic epitopes in the rhG1 domain protein affect the incidence, onset, and/or severity of arthritis, recombinant proteins (rhG1wt and those having deletions within one or all three dominant epitopes; Fig. 1b) were used to immunize BALB/c mice. The results of the most characteristic experiments with these three recombinant proteins (rhG1wt, rhG1-ΔP70, and rhG1–3Δ) are summarized in this paper. In three independent experiments, 14 age-matched female BALB/c mice were immunized with rhG1wt, 12 with rhG1-ΔP70, 16 with rhG1–3Δ (Fig. 3a). Mice immunized with mutated rhG1 proteins uniformly responded to the other rhG1 domain protein, but never to the mutated hG1 used for immunization or the corresponding synthetic peptide (data not shown). High incidence with relatively severe arthritis developed in the rhG1wt-immunized group. Mice immunized with rhG1-ΔP70 protein (or with rhG1-ΔP49 or rhG1-ΔP155, five-nine BALB/c mice with each; data not shown) developed high incidence of arthritis (75–80% in each case) with less severe inflammation in arthritic animals compared to rhG1wt-immunized group (Fig. 3). In contrast, BALB/c mice immunized with rhG1 lacking three arthritic epitopes (rhG1–3Δ) had very low incidence of the disease, and only one animal developed a very mild arthritis (Fig. 3). These results indicated that the development of PGIA is likely dependent on the immune responses to several dominant T cell epitopes, and that probably a single epitope may not play a definitive role. Rather PGIA develops as a response to multiple T cell epitopes.
Fig. 3.
Incidence and severity of arthritis in rhG1-immunized mice compared to hPG-immunized mice. Incidence was significantly lower only in the rhG1–3Δ protein-immunized mice (a), whereas the severity of arthritis (b) was significantly lower in BALB/c mice immunized with deleted (rhG1–3Δ or rhG1-Δ70) T cell epitopes. The number of arthritic/immunized animals are shown inside the columns, and the levels of significant differences on panel B: *P < 0·05, **P < 0·01. As PGIA is a progressive autoimmune disease with acute exacerbations and remissions leading to the complete deterioration of the articular cartilage, and ankylosis of peripheral joints (Fig. 4), the maximum arthritis score (ever reached during the experimental period) is used to show the maximum cumulative score of each group.
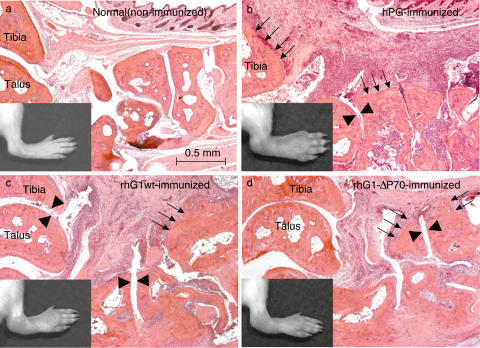
Histology sections were prepared from ankle joints of mice immunized with rhG1 domain (wild-type or mutated) proteins, and compared with sections prepared from ankle joints of normal (non-arthritic) and human PG aggrecan (hPG) immunized mice (Fig. 4). Inflammation and synovial hyperplasia along with leucocyte infiltration were comparable in arthritic joints and reflected the severity of the inflamed paw. Pannus formation led to cartilage degradation and bone erosion (Fig. 4).
Fig. 4.
Histology of ankle joints of non-immunized (a), human PG aggrecan (hPG), and rhG1wt and rhG1-ΔP70 protein-immunized (c,d) BALB/c mice. All sections were prepared in a sagittal plane and show the structure of ankle joints. (b–d) are inflamed arthritic ankles from mice immunized with either hPG (b) or rhG1 domain proteins (c,d) approximately five weeks after the onset of arthritis. Synovial hyperplasia accompanied by infiltrating cells and pannus formation are evident. Arrowheads show cartilage degradation, and thin arrows point to the site of bone erosion. The overall histopathology of joint inflammation and tissue destruction in rhG1 protein-immunized mice (c,d) is similar, but less extensive than seen in mice immunized with hPG (b). Sections are stained with haematoxylin and eosin.
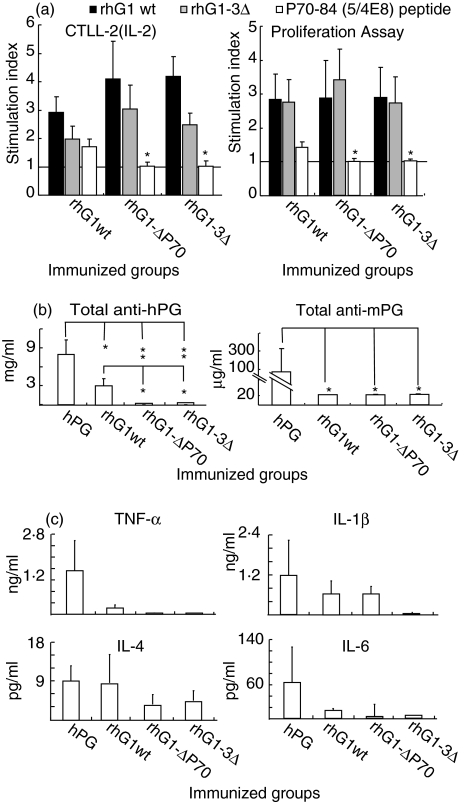
Antigen (rhG1)-specific T cell response (IL-2 production and T cell proliferation) was almost uniformly detected in all (wild-type or mutated) rhG1 protein-immunized mice (Fig. 5a) independently of the presence or absence of arthritis (Fig. 3a). This was the case when antigen (rhG1 domain or its mutated versions)-induced cytokine productions (TNF-α, IFN-γ, IL-4 and IL-10) were measured in 3-day old supernatants of spleen cell cultures. While the hPG usually induced highest, and the rhG1–3Δ the lowest, cytokine production, the secreted cytokines did not show correlation with arthritis, and the differences, like in sera (Fig. 5a), never reached significant levels (data not shown). This might be the result of the combined affect of multiple T cell epitopes present in the G1 domain.
Fig. 5.
Antigen-specific T cell responses and serum antibody and cytokine levels in mice immunized with human PG aggrecan (hPG) or recombinant hG1 domain proteins (either with wild-type or those having a single (rhG1-ΔP70) or three (rhG1-3Δ) deleted T-cell epitopes). Animals and groups represent those shown in Fig. 3. (a) Antigen-specific IL-2 production and T cell proliferation of splenocytes of mice immunized with rhG1wt, rhG1ΔP70, or rhG1–3Δ, were measured in vitro using 50 µg/ml synthetic peptide or 10 µg/ml rhG1 protein as indicated. The results are shown as stimulation indices. Anti-human (hPG) and antimouse (mPG) antibodies (b), and pro- and anti-inflammatory cytokines TNF-α, IL-1β, IL-4 and IL-6 (c) were measured by ELISA in sera of immunized BALB/c mice at the end of experiment (on days 96–98).
Antibody response to PG aggrecan was significantly higher in mice immunized with hPG than in mice immunized with rhG1 (either mutated or wild-type) proteins. Sera from mice immunized with rhG1wt protein showed significantly higher titres to hPG aggrecan than those mice immunized with either rhG1Δ-P70 or rhG1–3Δ (Fig. 5b). Nevertheless, all three groups immunized with rhG1 proteins showed less antibodies against native hPG aggrecan when compared to mice immunized with hPG aggrecan (Fig. 5b). This difference was due to the high B-cell response to glucosaminoglycan epitopes (Fig. 1a) present in the PG aggrecan [22] but lacking in recombinant proteins. Mice immunized with hPG aggrecan (positive control group) showed significantly higher (auto) antibody titre to mouse PG than rhG1-immunized animals (Fig. 5b). These observations, together, indicate that the G1 domain has a relatively low number of B-cell epitopes, i.e. G1 domain carries mostly T cell epitopes.
Concentrations of pro- and anti-inflammatory cytokines TNF-α, IL-1β, IL-6 and IL-4 were also measured in the sera of immunized mice, and in spleen cell cultures stimulated with rhG1 domain or its mutated variants. Even though we can detect a trend in the values of these cytokines corresponding to the level of inflammation seen in immunized mice, we could not find significant differences in cytokine levels when different groups of immunized mice were compared (Fig. 5c).
Discussion
The association between immunity against cartilage components and the development of arthritis has prompted many investigators to question whether the immunity is causally related to the development of the disease or is a secondary consequence of cartilage destruction and subsequent exposure of autoepitopes to the immune system. There is evidence in animals (and possibly in humans) that immunity to cartilage PG aggrecan may play a role in the development or pathogenesis of some forms of inflammatory arthritis and spondylitis [1–8,14,36–38]. The presence of autoantigenic epitopes of aggrecan molecules in articular cartilage, together with a genetic predisposition, may determine the organ-specificity of inflammation in susceptible individuals.
In a recent study, arthritogenic epitopes of human PG aggrecan were mapped [27], and several dominant epitopes were found, including the three dominant/arthritogenic sequences discussed in this paper. All three dominant epitopes were located in the G1 domain of aggrecan. A T cell hybridoma (5/4E8) specific to one of these epitopes was generated to determine MHC and TCR binding sites of the corresponding peptide [39]. Also, in an earlier study that established a connection between PGIA and RA, PGIA was induced in BALB/c mice deficient in H2-d (mouse MHC), but carrying the human leucocyte antigen (HLA) molecules DR4 or DQ8 [28]. The results of these experiments indicated that the human HLA was indeed able to present the human aggrecan epitopes to mouse TCR, and contributed to arthritis induction. When immune responses to predicted T cell epitopes (synthetic peptides) of the aggrecan molecule were tested, the three dominant/arthritogenic epitopes of the G1 domain, positive in BALB/c mice, were also positive in DR4 and/or DQ8 transgenic mice [28]. Of a total of 31 positive peptides tested in four different HLA transgenic mice (HLA-DR2, -DR3, -DR4, -DQ8), nine epitopes were the same as those identified earlier in BALB/c mice [27].
The aim of this study was to investigate the role of the most important arthritogenic T cell epitopes in the hPG aggrecan, that proved to be arthritogenic in BALB/c mice [27], employing targeted mutagenesis in sequences encoding these epitopes. Reduction in incidence, accompanied by less severe arthritis, occurred only when all three dominant T cell epitopes were deleted from the rhG1 domain. In this experimental group only one out of 16 mice showed mild arthritis, whereas 75% of rhG1-ΔP70-immunized mice (single epitope deletion) developed arthritis, and 13 became arthritic when 14 mice were immunized with rhG1wt protein. As shown on Fig. 3, the deletion of a single epitope (e.g. P70-84) could not abrogate the arthritogenic character of the human G1 domain, even though this P70-84 (5/4E8) T cell epitope seemed to be the most dominant and arthritogenic sequence within the G1 domain of the human PG aggrecan molecule [22,27]. These results supported the hypothesis that PGIA requires the presence of several dominant/arthritogenic epitopes in the aggrecan molecule. Thus, it is very likely that the development of arthritis (at least in PGIA) requires a coordinated (auto)immune response to multiple T cell epitopes within the G1 domain of aggrecan.
When we compared the cytokine levels in sera of immunized mice, and in vitro cytokine production to rhG1-stimulation, we could not detect differences between the different groups, even though a trend was seen toward a correlation between the levels of proinflammatory cytokines and the degree of inflammation/arthritis in these animals. This observation supports another hypothesis that certain regulatory events (e.g. T- and B-cell responses, cytokine production) are needed to initiate the disease, and the more epitopes that are recognized by the immune system, the more severe the inflammation and the disease. Data from this study, and from several others conducted in animals [11,15,16,22,28] or using lymphocytes from RA patients [2,4,6–8], show that antigenic molecules derived from immunoprivileged cartilage tissue and lack of self tolerance may play a role in the aetiology of RA and RA-like models. The question whether the immune response towards these molecules occurs as a result of the inflammatory damage to cartilage (which may expose new antigenic determinants as foreign structures to the immune system), or is a part of the pathological process leading to that damage, remains to be answered.
Several other studies used T cells from patients with RA or ankylosing spondylitis and showed cellular immune responses to a variety of T cell epitopes present in the aggrecan G1 domain [4–8,38]. Eventually, all positive T cell epitopes (peptides) identified in human patients with RA or ankylosing spondylitis, were the same or overlapping with those found to be positive in wild-type [22,27] or DR4/DQ8 transgenic BALB/c mice [28]. Studies using lymphocytes from either RA patients [7] or HLA transgenic mice [28,38] have demonstrated the relevance of the G1 domain of aggrecan to the pathomechanism of RA [4,6–8]. Together, the association of RA with certain HLA class II molecules (especially DR4 and DQ8/DQ6) supports the hypothesis that these MHC class II molecules can effectively present tissue (joint/cartilage) antigens to T cells in patients who also have a defect in immunoregulation, thus predisposing these individuals to RA.
RA is a complex autoimmune disease, and animal models for this disease serve simplified experimental settings for the investigation of disease mechanisms. These animal studies are employed to determine whether such cartilage antigens (PG, type II collagen, link protein or glucose-6-phosphate isomerase) could be causative agents in RA. In the case of PGIA, an arthritis-susceptible mouse strain (BALB/c) was used to study an RA-like disease [15] by one of the candidate autoantigens of human cartilage [11,16]. If the critical role of either a single or multiple dominant epitopes of these macromolecules becomes evident in human patients with RA, such knowledge could be used to introduce new innovative treatments that may involve, for example, inducing tolerance in patients against these epitopes. Recently, we found that nasally applied cartilage PG [40], synthetic peptides with altered peptide ligand of the dominant/arthritogenic epitopes of aggrecan [27], could indeed effectively induce tolerance in mice against PGIA, whereas some other altered peptides accelerated the development of arthritis (unpublished observation).
Acknowledgments
The authors wish to thank members of the Departments of Biochemistry, Immunology/Microbiology, and Orthopedic Surgery for their help and participation in this project. We would also like to thank Drs Alison Finnegan and Katalin Mikecz for valuable discussions of the manuscript, and Sonja Velins for assistance in preparation of the manuscript. This research was funded in part by the NIH (AR40310, AR45652 and AR47657).
References
- 1.Herman JH, Wiltse DW, Dennis MV. Immunopathologic significance of cartilage antigenic components in rheumatoid arthritis. Arthritis Rheum. 1973;16:287–97. doi: 10.1002/art.1780160302. [DOI] [PubMed] [Google Scholar]
- 2.Glant T, Csongor J, Szücs T. Immunopathologic role of proteoglycan antigens in rheumatoid joint diseases. Scand J Immunol. 1980;11:247–52. doi: 10.1111/j.1365-3083.1980.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 3.Golds EE, Stephen IBM, Esdaile JM, Strawczynski H, Poole AR. Lymphocyte transformation to connective tissue antigens in adult and juvenile rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, systemic lupus erythematosus and a non-arthritic control population. Cell Immunol. 1983;82:196–209. doi: 10.1016/0008-8749(83)90153-3. [DOI] [PubMed] [Google Scholar]
- 4.Guerassimov A, Zhang YP, Banerjee S, et al. Cellular immunity to the G1 domain of cartilage proteoglycan aggrecan is enhanced in patients with rheumatoid arthritis but only after removal of keratan sulfate. Arthritis Rheum. 1998;41:1019–25. doi: 10.1002/1529-0131(199806)41:6<1019::AID-ART8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 5.Guerassimov A, Zhang YP, Banerjee S, Cartman A, Webber C, Esdaile J, Fitzcharles MA, Poole AR. Autoimmunity to cartilage link protein in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 1998;25:1480–4. [PubMed] [Google Scholar]
- 6.Li NL, Zhang DQ, Zhou KY, Cartman A, Leroux JY, Poole AR, Zhang YP. Isolation and characteristics of autoreactive T cells specific to aggrecan G1 domain from rheumatoid arthritis patients. Cell Res. 2000;10:39–49. doi: 10.1038/sj.cr.7290034. [DOI] [PubMed] [Google Scholar]
- 7.Zou J, Zhang Y, Thiel A, et al. Predominant cellular immune response to the cartilage autoantigenic G1 aggrecan in ankylosing spondylitis and rheumatoid arthritis. Rheumatology. 2003;42:846–55. doi: 10.1093/rheumatology/keg230. [DOI] [PubMed] [Google Scholar]
- 8.Zou J, Appel H, Rudwaleit M, Thiel A, Sieper J. Analysis of the CD8+ T cell response to the G1 domain of aggrecan in ankylosing spondylitis. Ann Rheum Dis. 2004;64:722–9. doi: 10.1136/ard.2004.024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen: an experimental model of arthritis. J Exp Med. 1977;146:857–68. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunization against heterologous type II collagen induces arthritis in mice. Nature. 1980;282:666–8. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 11.Glant TT, Mikecz K, Arzoumanian A, Poole AR. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 1987;30:201–12. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]
- 12.Verheijden GFM, Rijnders AWM, Bos E, et al. Human cartilage glycoprotein-39 as a candidate autoantigen in rheumatoid arthritis. Arthritis Rheum. 1997;40:1115–25. doi: 10.1002/art.1780400616. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Guerassimov A, Leroux J-Y, et al. Induction of arthritis in BALB/c mice by cartilage link protein. Involvement of distinct regions recognized by T- and B lymphocytes. Am J Pathol. 1998;153:1283–91. doi: 10.1016/S0002-9440(10)65673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi S, Ciurli C, Cartman A, Pidoux I, Poole AR, Zhang Y. Experimental immunity to the G1 domain of the proteoglycan versican induces spondylitis and sacroiliitis, of a kind seen in human spondylarthropathies. Arthritis Rheum. 2003;48:2903–15. doi: 10.1002/art.11270. [DOI] [PubMed] [Google Scholar]
- 15.Glant TT, Finnegan A, Mikecz K. Proteoglycan-induced arthritis. immune regulation, cellular mechanisms and genetics. Crit Rev Immunol. 2003;23:199–250. doi: 10.1615/critrevimmunol.v23.i3.20. [DOI] [PubMed] [Google Scholar]
- 16.Mikecz K, Glant TT, Poole AR. Immunity to cartilage proteoglycans in BALB/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis Rheum. 1987;30:306–18. doi: 10.1002/art.1780300310. [DOI] [PubMed] [Google Scholar]
- 17.Glant TT, Mikecz K. Proteoglycan aggrecan-induced arthritis: a murine autoimmune model of rheumatoid arthritis. Meth Mol Med. 2004;102:313–38. doi: 10.1385/1-59259-805-6:313. [DOI] [PubMed] [Google Scholar]
- 18.Mikecz K, Glant TT, Buzás E, Poole AR. Proteoglycan-induced polyarthritis and spondylitis adoptively transferred to naive (nonimmunized) BALB/c mice. Arthritis Rheum. 1990;33:866–76. doi: 10.1002/art.1780330614. [DOI] [PubMed] [Google Scholar]
- 19.Bárdos T, Mikecz K, Finnegan A, Zhang J, Glant TT. T and B cell recovery in arthritis adoptively transferred to SCID mice: Antigen-specific activation is required for restoration of autopathogenic CD4+ Th1 cells in a syngeneic system. J Immunol. 2002;168:6013–21. doi: 10.4049/jimmunol.168.12.6013. [DOI] [PubMed] [Google Scholar]
- 20.Buzás EI, Brennan FR, Mikecz K, et al. A proteoglycan (aggrecan)-specific T cell hybridoma induces arthritis in BALB/c mice. J Immunol. 1995;155:2679–87. [PubMed] [Google Scholar]
- 21.Banerjee S, Webber C, Poole AR. The induction of arthritis in mice by the cartilage proteoglycan aggrecan: roles Cd4+ Cd8+ T Cells. Cell Immunol. 1992;144:347–57. doi: 10.1016/0008-8749(92)90250-s. [DOI] [PubMed] [Google Scholar]
- 22.Glant TT, Buzás EI, Finnegan A, Negroiu G, Cs-Szabó G, Mikecz K. Critical role of glycosaminoglycan side chains of cartilage proteoglycan (aggrecan) in antigen recognition and presentation. J Immunol. 1998;160:3812–9. [PubMed] [Google Scholar]
- 23.Adarichev VA, Valdez JC, Bárdos T, Finnegan A, Mikecz K, Glant TT. Combined autoimmune models of arthritis reveal shared and independent qualitative (binary) and quantitative trait loci. J Immunol. 2003;170:2283–92. doi: 10.4049/jimmunol.170.5.2283. [DOI] [PubMed] [Google Scholar]
- 24.Glant TT, Adarichev VA, Nesterovitch AB, et al. Disease-associated qualitative and quantitative trait loci in proteoglycan-induced arthritis and collagen-induced arthritis. Am J Med Sci. 2004;327:188–95. doi: 10.1097/00000441-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan CD, O'Neill SK, Koreny T, Czipri M, Finnegan A. Development of inflammation in proteoglycan-induced arthritis is dependent on FcgammaR regulation of the cytokine/chemokine environment. J Immunol. 2002;169:5851–9. doi: 10.4049/jimmunol.169.10.5851. [DOI] [PubMed] [Google Scholar]
- 26.Glant TT, Fülöp C, Cs-Szabó G, Buzás EI, Ragasa DR, Mikecz K. Mapping of arthritogenic/autoimmune epitopes of cartilage aggrecans in proteoglycan-induced arthritis. Scand J Rheumatol. 1995;24:43–9. doi: 10.3109/03009749509100899. [DOI] [PubMed] [Google Scholar]
- 27.Buzás E, Vegvari A, Murad YM, Hudecz F, Mikecz K, Glant TT. T-cell recognition of differentially tolerated epitopes of cartilage proteoglycan aggrecan in arthritis. Cell Immunol. 2005;234 doi: 10.1016/j.cellimm.2004.08.006. in press. [DOI] [PubMed] [Google Scholar]
- 28.Szanto S, Bárdos T, Szabo Z, David CS, Buzás E, Mikecz K, Glant TT. Induction of arthritis in HLA-DR4-humanized and HLA-DQ8-humanized mice by human cartilage proteoglycan aggrecan but only in the presence of an appropriate (non-MHC) genetic background. Arthritis Rheum. 2004;50:1984–95. doi: 10.1002/art.20285. [DOI] [PubMed] [Google Scholar]
- 29.Leroux J-Y, Guerassimov A, Cartman A, Delaunay N, Webber C, Rosenberg LC, Banerjee S, Poole AR. Immunity to the G1 globular domain of the cartilage proteoglycan aggrecan can induce inflammatory erosive polyarthritis and spondylitis in BALB/c mice but immunity to G1 is inhibited by covalently bound keratan sulfate in vitro and in vivo. J Clin Invest. 1996;97:621–32. doi: 10.1172/JCI118458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bárdos T, Buzás EI, Finnegan A, Mikecz K, Glant TT. Altered peptide ligands dictate cytokine profiles and modify the clinical appearance of arthritis. Arthritis Rheum. 2000;43:S396. [Google Scholar]
- 31.Vertel BM, Grier BL, Li H, Schwartz NB. The chondrodystrophy, nanomelia: biosynthesis and processing of the defective aggrecan precursor. Biochem J. 1994;301:211–6. doi: 10.1042/bj3010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo W, Kuwada TS, Chandrasekaran L, Zheng J, Tanzer ML. Divergent secretory behavior of the opposite ends of aggrecan. J Biol Chem. 1996;271:16447–50. doi: 10.1074/jbc.271.28.16447. [DOI] [PubMed] [Google Scholar]
- 33.Kiani C, Lee V, Cao L, Chen L, Wu Y, Zhang Y, Adams ME, Yang BB. Roles of aggrecan domains in biosynthesis, modification by glycosaminoglycans and product secretion. Biochem J. 2001;354:199–207. doi: 10.1042/0264-6021:3540199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang BL, Cao L, Kiani C, Lee V, Zhang Y, Adams ME, Yang BB. Tandem repeats are involved in G1 domain inhibition of versican expression and secretion and the G3 domain enhances glycosaminoglycan modification and product secretion via the complement-binding protein- like motif. J Biol Chem. 2000;275:21255–61. doi: 10.1074/jbc.M001443200. [DOI] [PubMed] [Google Scholar]
- 35.Hanyecz A, Berlo SE, Szanto S, Broeren CPM, Mikecz K, Glant TT. Achievement of a synergistic adjuvant effect on arthritis induction by activation of innate immunity and forcing the immune response toward the Th1 phenotype. Arthritis Rheum. 2004;50:1665–76. doi: 10.1002/art.20180. [DOI] [PubMed] [Google Scholar]
- 36.Guerassimov A, Zhang Y, Cartman A, Rosenberg LC, Esdaile J, Fitzcharles M-A, Poole AR. Immune responses to cartilage link protein and the G1 domain of proteoglycan aggrecan in patients with osteoarthritis. Arthritis Rheum. 1999;42:527–33. doi: 10.1002/1529-0131(199904)42:3<527::AID-ANR18>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Guerassimov A, Leroux J-Y, et al. Arthritis induced by proteoglycan aggrecan G1 domain in BALB/c mice. Evidence for T cell involvement and the immunosuppressive influence of keratan sulfate on recognition of T and B cell epitopes. J Clin Invest. 1998;101:1678–86. doi: 10.1172/JCI1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuon W, Kuhne M, Busch DH, et al. Identification of novel human aggrecan T cell epitopes in HLA-B27 transgenic mice associated with spondyloarthropathy. J Immunol. 2004;173:4859–66. doi: 10.4049/jimmunol.173.8.4859. [DOI] [PubMed] [Google Scholar]
- 39.Buzás EI, Hanyecz A, Murad Y, Hudecz F, Rajnavolgyi E, Mikecz K, Glant TT. Differential recognition of altered peptide ligands distinguishes two functionally discordant (arthritogenic and non-arthritogenic) autoreactive T cell hybridoma clones. J Immunol. 2003;171:3025–33. doi: 10.4049/jimmunol.171.6.3025. [DOI] [PubMed] [Google Scholar]
- 40.Bárdos T, Czipri M, Vermes C, Zhang J, Mikecz K, Glant TT. Continuous nasal administration of antigen is critical to maintain tolerance in adoptively transferred autoimmune arthritis in SCID mice. Clin Exp Immunol. 2002;129:224–31. doi: 10.1046/j.1365-2249.2002.01903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]