Abstract
The aim of this prospective study was to examine gender-related differences of cytokines in the plasma and urine of healthy individuals that might provide a clue concerning the lower rate of chronic renal diseases in females. Soluble interleukin-1 receptor antagonist (sIL-1RA), interleukin (IL)-1α, IL-1β, IL-2, sIL-2R, IL-3, IL-4, IL-6, sIL-6R, IL-10, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β2 and interferon (IFN)-γ were determined using standard enzyme-linked immunosorbent assay (ELISA). Cytokine levels were determined in simultaneously obtained plasma and urine samples of 18 male and 28 female healthy members of our laboratory staff. Urine cytokine levels were studied three times at 1-month intervals. All individuals had a negative urine nitrite test and showed no symptoms of urinary tract infection (UTI). Plasma levels of all studied cytokines were similar in males and females (P = n.s.). However, females had significantly higher urine IL-1α (P < 0·0001; P < 0·0001; P < 0·0001) and sIL-1RA (P = 0·0001; P = 0·0003; P = 0·0002) than males at three and higher IL-1β at one of the three investigations (P = 0·098; P = 0·003; P = 0·073). Urine levels of the other cytokines were similar in males and females. Higher urine levels of IL-1α, IL-1β and sIL-1RA in females may result from stimulation of cells in the urinary tract. Increased sIL-1RA might block T lymphocyte activation. The elevated cytokines may play a role in the protection of the female urinary tract from certain renal diseases, such as pyelonephritis and other inflammatory and sclerotic kidney diseases.
Keywords: gender-related differences, protection, scarring, urine sIL-1RA, urine IL-1α, urine IL-1β, urinary tract infection
Introduction
It is well known that men are more prone to develop membranous nephropathy, immunoglobulin A nephropathy or polycystic kidney disease than women [1,2], and that women experience urinary tract infection and slower progression of chronic renal diseases more often than men [1,3]. It has been hypothesized that the underlying mechanisms for this gender disparity might be related to gender-specific differences in glomerular structure, glomerular hemodynamics, diet, variations in the production and activity of local cytokines and hormones, and/or the effect of sex hormones on kidney cells [1,2].
In this prospective study we investigated a series of 13 different cytokines, soluble cytokine receptors and soluble cytokine receptor antagonists in simultaneously obtained plasma and urine samples of healthy females and males. We investigated gender-related differences of monocyte- and lymphocyte-derived mediators of immune responses that might be protective against urinary tract infection (UTI) and certain renal diseases, such as pyelonephritis and other inflammatory and sclerotic kidney diseases.
Methods and subjects
Female and male healthy individuals
Cytokine levels were determined in simultaneously obtained plasma and urine samples of 18 male and 28 female healthy members of our laboratory staff. Because gender-related differences were found only in the urine, we studied urine cytokine levels in 18 male and 28 female staff members two times after a 1-month interval, and in a subgroup of 18 males and 24 females a third time after another month. The age of male and female staff members was similar (36·9 ± 10·8 years versus 36·9 ± 7·7 years: P = n.s.) and ranged from 21 to 63 years. All subjects were free of acute or chronic disease and urinary tract infection, and none was on any medication. The women had no menstrual bleeding at the time of investigation. Anamnestic data were obtained using a questionnaire. To exclude bacterial contamination and undiagnosed infection, all urine samples were tested for nitrite using urine sticks (Medi-Test, Macherey-Nagel, Düren, Germany). All individuals had negative test results at the three determinations. Urine cultures for the detection of asymptomatic bacteriuria were not performed. The study was conducted in accordance with local ethical guidelines and all individuals gave informed consent for analysis of their plasma and urine samples.
Determination of plasma and urine cytokines, soluble cytokine receptors and soluble cytokine receptor antagonists
Plasma and urine levels of soluble interleukin-1 receptor antagonist (sIL-1RA), IL-1α, IL-1β, IL-2, sIL-2R, IL-3, IL-4, IL-6, sIL-6R, IL-10, tumour necrosis factor (TNF)-α, transforming growth factor (TGF)-β2 and interferon (IFN)-γ were determined using standard enzyme-linked immunosorbent assay (ELISA). IL-1α, IL-1β, sIL-1RA, IL-2, sIL-2R, IL-3, IL-4, IL-6, sIL-6R, IL-10, TGF-β2, and TNF-α were measured using Quantikine kits (R&D Systems, Wiesbaden, Germany), and IFN-γ using HBT kits (Holland Biotechnology BV, Firma Biermann, Bad Nauheim, Germany). Plasma was snap-frozen within 2 h after the blood was drawn. The urine samples were freshly obtained in the morning, snap-frozen within 2 h and stored at −30°C until testing.
Statistical analysis
The Mann–Whitney U-test was applied using the Statistical Package for the Social Sciences (SPSS, Chicago, USA). Adjustment for multiple testing (n = 13) was performed according to Bonferroni's method. P-values of < 0·01 were considered significant and are shown in Tables 1 and 2.
Table 1. Plasma cytokine levels of male and female healthy staff members.
| Parameter | Males (n = 18) | Females (n = 28) | P |
|---|---|---|---|
| P_IL-1α (pg/ml) | 1·7 ± 2·7 | 2·3 ± 3·0 | 0·044 |
| P_IL-1β (pg/ml) | 1·0 ± 2·8 | 0·5 ± 0·7 | 0·738 |
| P_sIL-1RA (pg/ml) | 205 ± 88 | 334 ± 480 | 0·964 |
| P_IL-2 (pg/ml) | 0·6 ± 2·7 | 1·8 ± 4·2 | 0·122 |
| P_sIL-2R (pg(ml) | 559 ± 191 | 566 ± 198 | 0·848 |
| P_IL-3 (pg/ml) | 16·8 ± 24·7 | 4·2 ± 7·1 | 0·133 |
| P_IL-4 (pg/ml) | 2·6 ± 7·5 | 0·2 ± 0·7 | 0·298 |
| P_IL-6 (pg/ml) | 1·8 ± 3·2 | 0·5 ± 0·9 | 0·136 |
| P_sIL-6R (pg/ml) | 34 536 ± 9871 | 29 298 ± 9802 | 0·068 |
| P_IL-10 (pg/ml) | 8·1 ± 17·5 | 3·9 ± 10·2 | 0·124 |
| P_TNF-α (pg/ml) | 1·4 ± 3·3 | 0·8 ± 1·5 | 0·719 |
| P_TGF-β2 (pg/ml) | 3·6 ± 15·3 | 0·8 ± 2·8 | 0·596 |
| P_IFN-γ (pg/ml) | 1194 ± 1737 | 489 ± 624 | 0·653 |
P_ = plasma level. All data are given as mean ± 1 s.d. P-values were calculated using the Mann–Whitney U-test. Adjustment for multiple testing (n = 13) was performed according to Bonferroni's method. Only P-values of < 0·01 were considered to be significant.
Table 2. Urine cytokine levels in males and females measured in 1-month intervals at three different investigations.
| 1st investigation* | 2nd investigation | 3rd investigation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Male (n = 18) | Female (n = 28) | P | Male (n = 18) | Female (n = 28) | P | Male (n = 18) | Female (n = 24) | P |
| U_IL-1α (pg/ml) | 0·7 ± 1·1 | 12·6 ± 14·3 | < 0·0001 | 0·6 ± 1·2 | 17·9 ± 18·7 | < 0·0001 | 1·9 ± 1·5 | 8·1 ± 7·5 | < 0·0001 |
| U_IL-1β (pg/ml) | 0·4 ± 0·8 | 5·2 ± 11·9 | 0·098 | 0·8 ± 1·0 | 5·1 ± 7·9 | 0·003 | 0·4 ± 0·5 | 1·8 ± 3·6 | 0·073 |
| U_sIL-1RA (pg/ml) | 1727 ± 801 | 2686 ± 573 | 0·0001 | 2010 ± 790 | 2859 ± 662 | 0·0003 | 1414 ± 805 | 2452 ± 877 | 0·0002 |
| U_IL-2 (pg/ml) | 1·8 ± 5·1 | 1·4 ± 2·9 | 0·082 | 4·8 ± 16·5 | 4·3 ± 0·10·8 | 0·543 | 2·0 ± 4·0 | 3·8 ± 9·4 | 0·432 |
| U_sIL-2R (pg/ml) | 750 ± 540 | 535 ± 520 | 0·163 | 695 ± 561 | 685 ± 623 | 0·644 | 753 ± 566 | 448 ± 407 | 0·049 |
| U_IL-3 (pg/ml) | 5·7 ± 11·3 | 3·1 ± 6·9 | 0·928 | 3·1 ± 4·2 | 2·6 ± 5·0 | 0·363 | 1·2 ± 2·2 | 1·8 ± 3·1 | 0·587 |
| U_IL-4 (pg/ml) | 0·7 ± 1·7 | 0·4 ± 0·8 | 0·904 | 0·7 ± 1·5 | 0·8 ± 4·0 | 0·280 | 0·5 ± 0·9 | 0·4 ± 1·2 | 0·266 |
| U_IL-6 (pg/ml) | 0·9 ± 1·5 | 1·3 ± 2·9 | 0·620 | 2·1 ± 3·2 | 1·5 ± 1·8 | 0·731 | 1·3 ± 2·4 | 0·4 ± 1·1 | 0·111 |
| U_sIL-6R (pg/ml) | 814 ± 500 | 1142 ± 2285 | 0·558 | 1478 ± 1867 | 743 ± 482 | 0·115 | 673 ± 502 | 700 ± 566 | 0·990 |
| U_IL-10 (pg/ml) | 2·7 ± 4·53 | 7·3 ± 10·2 | 0·136 | 4·4 ± 8·0 | 7·1 ± 13·6 | 0·246 | 1·4 ± 2·1 | 3·5 ± 6·5 | 0·366 |
| U_TNF-α (pg/ml) | 4·0 ± 7·0 | 3·4 ± 4·1 | 0·583 | 3·8 ± 2·7 | 3·6 ± 3·3 | 0·569 | 2·3 ± 2·4 | 1·9 ± 2·1 | 0·575 |
| U_TGF-β2 (pg/ml) | 1·5 ± 4·4 | 3·2 ± 7·8 | 0·904 | 0·8 ± 2·2 | 2·5 ± 5·4 | 0·115 | 2·3 ± 5·4 | 3·4 ± 6·1 | 0·473 |
| U_IFN-γ (pg/ml) | 11·8 ± 19·4 | 26·7 ± 44·5 | 0·551 | 16·1 ± 23·5 | 39·4 ± 74·8 | 0·147 | 102·5 ± 125·8 | 71·3 ± 87·5 | 0·990 |
U_ = urine level. All data are given as mean ± 1s.d.
Urine samples of the first investigation were obtained simultaneously with plasma samples (see Table 1). P-values were calculated using the Mann–Whitney U-test. Adjustment for multiple testing (n = 13) was performed according to Bonferroni's method. P-values of < 0·01 were considered to be significant and are shown in bold type.
Results
Cytokine levels in simultaneously obtained plasma and urine samples
Plasma levels of IL-1α, IL-1β, sIL-1RA, IL-2, sIL-2R, IL-3, IL-4, IL-6, sIL-6R, IL-10, TNF-α, TGF-β2 and IFN-γ were similar in males and females (P = n.s.) (Table 1). In contrast, IL-1α and sIL-1RA levels in simultaneously obtained urine samples were significantly higher in females than males (IL-1α: P < 0·0001; sIL-1RA: P = 0·0001), whereas the other cytokines were similar in females and males and apparently not affected by gender (P = n.s.) (Tables 2; first investigation).
Urine cytokine levels studied three times at 1-month intervals
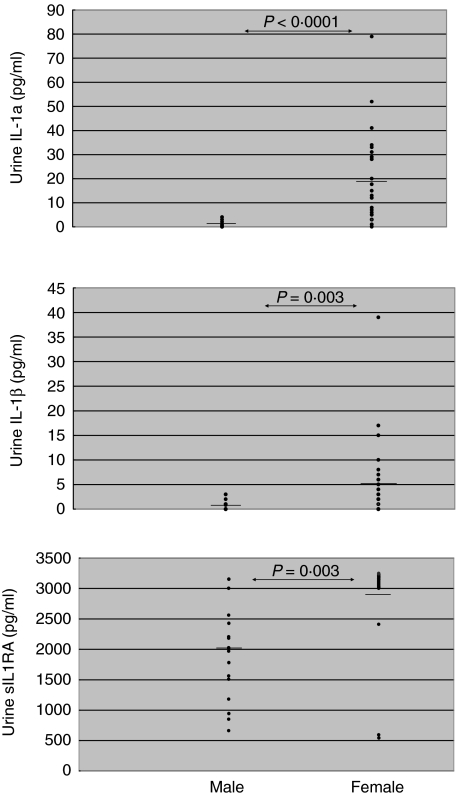
When the urine samples of the 18 male and 28 female staff members were investigated a second time after a 1-month interval, and in a subgroup of 18 males and 24 females a third time after another month, females had consistently higher urine IL-1α (P < 0·0001 at first, second and third investigations) and urine sIL-1RA (P = 0·0001 at firsst, P = 0·0003 at second and P = 0·0002 at third investigations) (Table 2) than male individuals. In addition, urine IL-1β was increased significantly in females at one and slightly increased at the other two investigations (P = 0·098 at first, P = 0·003 at second and P = 0·073 at third investigations). In contrast to cytokines of the IL-1 family, urine levels of IL-2, sIL-2R, IL-3, IL-4, IL-6, sIL-6R, IL-10, TNF-α, TGF-β2 and IFN-γ were consistently similar in females and males at all measurements (P > 0·01) (Table 2). The data suggest that only cytokines of the IL-1 family are increased in the urine of females, whereas the urine levels of other cytokines are not gender-related. Figure 1 shows the IL-1α, IL-1β and sIL-1RA urine levels of males and females.
Fig. 1.
Interleukin (IL)-1α, IL-1β and sIL-1RA levels in urine samples of 18 male and 28 female healthy individuals. Female individuals had significantly higher urine IL-1α (P < 0·0001), IL-β (P = 0·003) and soluble interleukin-1 receptor antagonist (sIL-1RA) (P = 0·0003) levels than male individuals. Means are represented by horizontal bars.
Discussion
The aim of this study was to examine gender-related differences in mono- and lymphokine levels in plasma and urine under physiological conditions in order to obtain information concerning the pathogenesis of gender-related differences in the rate of infectious diseases and certain renal diseases, such as pyelonephritis and other inflammatory and sclerotic kidney diseases.
Our data agree with the findings of Lynch et al. and Rauta et al. who reported a higher urinary excretion of IL-1RA and IL-1β in healthy females than males [4,5]. Moreover, Lynch et al. described a 5–10× higher secretion of IL-1α, IL-1β and sIL-1RA from mononuclear cells (MNC) obtained from the blood of healthy female controls during the luteal phase, and a 13–28× higher secretion of these cytokines from MNC obtained during the follicular phase compared to the secretion obtained with cells from healthy males [4]. The finding of a consistently higher spontaneous release of IL-1α, IL-1β and sIL-1RA in vitro from MNC of females stands in contrast to our results, which showed similar cytokine levels in the plasma of females and males. Dilution of in-vivo secreted cytokines with plasma to undetectable levels can be ruled out as a source of error for IL-α and sIL-1RA because only two of 18 male and two of 28 female individuals had undetectable plasma IL-1α, and none of the male and only one female individual had undetectable plasma sIL-1RA. IL-1β levels were generally low, and 11 of 18 males and 16 of 28 females had undetectable levels. That the mean concentrations of IL-1α and IL-1β in plasma samples of males were higher than those in simultaneously obtained urine samples also argues against an influence of a limited sensitivity of the detection method. Unfortunately, we did not measure urine creatinine levels in our healthy controls. However, the reproducibility of increased IL-1α, IL-1β and sIL-1RA concentrations in female urine samples and the similar levels of lymphocyte-derived cytokines in male and female urine samples indicate that intra- and interindividual differences in urine volumes as well as urine creatinine levels did not markedly distort our results. As shown in Fig. 1, gender-related differences of the three cytokines were so profound that small dilution effects caused by varying urine volumes were most probably irrelevant.
Cytokines have a low molecular weight and can therefore easily undergo glomerular filtration followed by reabsorption in the tubuli [6]. However, reabsorption should strongly decrease the urine levels and should increase the plasma levels of the corresponding cytokines. Because cytokines of the IL-1 family were increased only in urine but not in plasma samples of females we favour the hypothesis that the increased urine levels reflect an increased production of these cytokines in the female urinary tract. Continuous stimulation in the urinary tract of females, inducing the production of these cytokines, could be the reason for the high urine levels of IL-1α, IL-1β and sIL-1RA. Production of IL-1 by activated infiltrating mononuclear cells as well as activated resident cells, including glomerular endothelial cells, capsular epithelial cells, smooth muscle cells of vessel walls, fibroblasts and some tubular epithelial cells, has been reported previously [7–11]. IL-1 is a profibrogenic cytokine capable of inducing epithelial–myofibroblast transdifferentiation, and thereby renal fibrosis through a TGF-β1-dependent mechanism that can be inhibited completely by IL-1RA [12]. Increased sIL-1RA production of glomerular cells might protect the female kidney against glomerular diseases, resulting in renal fibrosis [12,13]. Female gonadal steroids at normal physiological levels can induce expression of sIL-1RA [14]. A protective role of oestrogen and female gender in non-diabetic chronic renal disease, such as polycystic kidney disease, chronic glomerulonephritis, hypertensive angionephrosclerosis, chronic tubulointerstitial nephritis, IgA nephropathy and membranous nephropathy, has been established in a meta-analysis by Neugarten et al. [15]. Oestrogen suppresses TGF-β, α1 type IV collagen gene expression and the synthesis of type I collagen, preventing renal injury [16–20]. Androgens, the natural opponent of oestrogens, inhibit Th1 cytokines such as IL-2 and IFN-γ and induce IL-10. Androgens represent natural anti-inflammatory hormones [21,22]. One might speculate that the higher oestrogen levels in females induce a consistent prophylactic anti-inflammatory response of monocytes and endothelial cells in the urinary tract, intensified by low anti-inflammatory androgen levels, and that this might block Th1 activation and the development of inflammation and scarring in the female urinary tract.
We hypothesize that IL-1α, IL-1β and sIL-1RA, produced by activated resident cells of the urinary tract as well as activated infiltrating mononuclear cells within the urinary tract, protect the kidney against acute and chronic inflammation induced by bacterial infection ascending from the urethra via the bladder to the kidney. The bactericidal milieu, including activated monocytes, prevents or at least decreases the antigenic stimulation of the female urinary tract by bacteria that otherwise would have resulted in chronic inflammation and fibrosis [23,24]. Tullus et al., studying IL-1α and sIL-1RA in the urine of children with acute pyelonephritis, suggested that persisting high urine levels of IL-1α may protect the urinary tract from inflammation and scarring [25]. Interestingly, they reported that urine sIL-1RA levels were higher in healthy controls than in children with recurrent pyelonephritis or children convalescent after acute pyelonephritis, but they did not differentiate between males and females [25]. Because sIL-1RA has been shown to function as an anti-inflammatory cytokine, it seems reasonable to suggest that higher levels of IL-1 and its receptor antagonist may play a role in the protection of female kidneys from T lymphocyte-mediated immune responses and/or certain infections and renal diseases, such as pyelonephritis. Differences in cytokine responses may be a result of gender-related differences in the response to bacteria in the urinary tract, and a consequence of the body's defence mechanism against increased urinary tract infections in females.
We did not find literature reports on gender-related differences in the cytokine production of renal cells in cell culture experiments. However, several studies have described the influence of gender on cytokine production [26–30]. Further, there are no reports on the effect of sex hormones on the production of IL-1 and sIL-1RA in tubular cells. However, we found three citations in the literature on an effect of oestrogen on TGF-β production in renal cells [18–20].
The described cytokine increases might have a prophylactic effect because they were observed in healthy individuals. All individuals in our study had a negative urine nitrite test and were asymptomatic. As shown in Fig. 1, nearly all females had higher sIL-1RA and higher IL-1α urine levels than males (minimal overlap), suggesting that increased cytokine urine levels in our healthy female controls did not originate from undiagnosed UTI. In a previous study, we showed that female transplant recipients with or without bacteriuria had significantly higher sIL-1RA urine levels than male transplant recipients with or without bacteriuria [31]. We believe that pro- and anti-inflammatory cytokines in females regulate each other at a higher level than in males, thus establishing a balance with higher IL-1α, IL-1β and sIL-1RA urine levels. sIL-1RA produced by stimulated cells in the female urinary tract suppresses T lymphocytes that would otherwise initiate a T cell-mediated immune response. The results of many studies in humans and animals establish the importance of endogenous sIL-1RA as part of the host's response against infection and in limiting organ damage [32]. New potential approaches to modify glomerular inflammation using anti-inflammatory cytokines have been highlighted recently by Kluth and Rees [33].
Acknowledgments
We would like to acknowledge the skilful technical assistance of Martina-Kutsche-Bauer and Regina Seemuth.
References
- 1.Gandolfo MT, Verzola D, Salvatore F, et al. Gender and the progression of chronic renal diseases: does apoptosis make the difference? Minerva Urol Nefrol. 2004;56:1–14. [PubMed] [Google Scholar]
- 2.Silbiger SR, Neugarten J. The role of gender in the progression of renal disease. Adv Ren Replace Ther. 2003;10:3–14. doi: 10.1053/jarr.2003.50001. [DOI] [PubMed] [Google Scholar]
- 3.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49:53–70. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 4.Lynch EA, Dinarello CA, Cannon JG. Gender differences in IL-1 alpha, IL-1 beta, and IL-1 receptor antagonist secretion from mononuclear cells and urinary excretion. J Immunol. 1994;153:300–6. [PubMed] [Google Scholar]
- 5.Rauta V, Teppo AM, Tornroth T, Honkanen E, Gronhagen-Riska C. Lower urinary-interleukin-1 receptor-antagonist excretion in IgA nephropathy than in Henoch–Schonlein nephritis. Nephrol Dial Transplant. 2003;18:1785–91. doi: 10.1093/ndt/gfg234. [DOI] [PubMed] [Google Scholar]
- 6.Kudo S, Goto H. Intrarenal handling of recombinant human interleukin-1alpha in rats: mechanism for proximal tubular protein reabsorption. J Interferon Cytokine Res. 1999;19:1161–8. doi: 10.1089/107999099313109. [DOI] [PubMed] [Google Scholar]
- 7.Tesch GH, Yang N, Yu H, et al. Intrinsic renal cells are the major source of interleukin-1 beta synthesis in normal and diseased rat kidney. Nephrol Dial Transplant. 1997;12:1109–15. doi: 10.1093/ndt/12.6.1109. [DOI] [PubMed] [Google Scholar]
- 8.Noronha IL, Kruger C, Andrassy K, Ritz E, Waldherr R. In situ production of TNF-alpha, IL-1 beta and IL-2R in ANCA-positive glomerulonephritis. Kidney Int. 1993;43:682–92. doi: 10.1038/ki.1993.98. [DOI] [PubMed] [Google Scholar]
- 9.Waldherr R, Noronha IL, Niemir Z, Kruger C, Stein H, Stumm G. Expression of cytokines and growth factors in human glomerulonephritides. Pediatr Nephrol. 1993;7:471–8. doi: 10.1007/BF00857578. [DOI] [PubMed] [Google Scholar]
- 10.Brauner A, Soderhall M, Jacobson SH, Lundahl J, Andersson U, Andersson J. Escherichia coli-induced expression of IL-1 alpha, IL-1 beta, IL-6 and IL-8 in normal human renal tubular epithelial cells. Clin Exp Immunol. 2001;124:423–8. doi: 10.1046/j.1365-2249.2001.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niemir ZI, Stein H, Dworacki G, et al. Podocytes are the major source of IL-1 alpha and IL-1 beta in human glomerulonephritides. Kidney Int. 1997;52:393–403. doi: 10.1038/ki.1997.346. [DOI] [PubMed] [Google Scholar]
- 12.Fan JM, Huang XR, Ng YY, et al. Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor-beta1-dependent mechanism in vitro. Am J Kidney Dis. 2001;37:820–31. doi: 10.1016/s0272-6386(01)80132-3. [DOI] [PubMed] [Google Scholar]
- 13.Lan HY, Nikolic-Paterson DJ, Zarama M, Vannice JL, Atkins RC. Suppression of experimental crescentic glomerulonephritis by the interleukin-1 receptor antagonist. Kidney Int. 1993;43:479–85. doi: 10.1038/ki.1993.70. [DOI] [PubMed] [Google Scholar]
- 14.Lee BY, Huynh T, Prichard LE, McGuire J, Polan ML. Gonadal steroids modulate interleukin-1 receptor antagonist mRNA expression in cultured human monocytes. Biochem Biophys Res Commun. 1995;209:279–85. doi: 10.1006/bbrc.1995.1500. [DOI] [PubMed] [Google Scholar]
- 15.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11:319–29. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 16.Negulescu O, Bognar I, Lei J, Devarajan P, Silbiger S, Neugarten J. Estradiol reverses TGF-beta1-induced mesangial cell apoptosis by a casein kinase 2-dependent mechanism. Kidney Int. 2002;62:1989–98. doi: 10.1046/j.1523-1755.2002.00679.x. [DOI] [PubMed] [Google Scholar]
- 17.Lei J, Silbiger S, Ziyadeh FN, Neugarten J. Serum-stimulated alpha 1 type IV collagen gene transcription is mediated by TGF-beta and inhibited by estradiol. Am J Physiol. 1998;274:F252–8. doi: 10.1152/ajprenal.1998.274.2.F252. [DOI] [PubMed] [Google Scholar]
- 18.Silbiger S, Lei J, Ziyadeh FN, Neugarten J. Estradiol reverses TGF-beta1-stimulated type IV collagen gene transcription in murine mesangial cells. Am J Physiol. 1998;274:F1113–8. doi: 10.1152/ajprenal.1998.274.6.F1113. [DOI] [PubMed] [Google Scholar]
- 19.Birch Nielsen C, Krag SO, Sterby R, et al. Transforming growth factor beta 1-induced glomerulopathy is prevented by 17beta-estradiol supplementation. Virchows Arch. 2004;444:561–6. doi: 10.1007/s00428-004-1006-4. [DOI] [PubMed] [Google Scholar]
- 20.Blush J, Lei J, Ju W, Silbiger S, Pullman J, Neugarten J. Estradiol reverses renal injury in Alb/TGF-beta1 transgenic mice. Kidney Int. 2004;66:2148–54. doi: 10.1111/j.1523-1755.2004.66005.x. [DOI] [PubMed] [Google Scholar]
- 21.Cutolo M, Seriolo B, Villaggio B, Pizzorni C, Craviotto C, Sulli A. Androgens and estrogens modulate the immune and inflammatory responses in rheumatoid arthritis. Ann NY Acad Sci. 2002;966:131–42. doi: 10.1111/j.1749-6632.2002.tb04210.x. [DOI] [PubMed] [Google Scholar]
- 22.Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167:2060–7. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 23.Hertting O, Khalil A, Jaremko G, et al. Enhanced chemokine response in experimental acute Escherichia coli pyelonephritis in IL-1beta-deficient mice. Clin Exp Immunol. 2003;131:225–33. doi: 10.1046/j.1365-2249.2003.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitschke M, Wiehl S, Baer PC, Kreft B. Bactericidal activity of renal tubular cells: the putative role of human beta-defensins. Exp Nephrol. 2002;10:332–7. doi: 10.1159/000065296. [DOI] [PubMed] [Google Scholar]
- 25.Tullus K, Escobar-Billing R, Fituri O, et al. Interleukin-1 alpha and interleukin-1 receptor antagonist in the urine of children with acute pyelonephritis and relation to renal scarring. Acta Paediatr. 1996;85:158–62. doi: 10.1111/j.1651-2227.1996.tb13984.x. [DOI] [PubMed] [Google Scholar]
- 26.van den Broek HH, Damoiseaux JG, De Baets MH, Hupperts RM. The influence of sex hormones on cytokines in multiple sclerosis and experimental autoimmune encephalomyelitis: a review. Mult Scler. 2005;11:349–59. doi: 10.1191/1352458505ms1174rr. [DOI] [PubMed] [Google Scholar]
- 27.Mo R, Chen J, Grolleau-Julius A, Murphy HS, Richardson BC, Yung RL. Estrogen regulates CCR gene expression and function in T lymphocytes. J Immunol. 2005;174:6023–9. doi: 10.4049/jimmunol.174.10.6023. [DOI] [PubMed] [Google Scholar]
- 28.Nalbandian G, Kovats S. Understanding sex biases in immunity. effects of estrogen on the differentiation and function of antigen-presenting cells. Immunol Res. 2005;31:91–106. doi: 10.1385/IR:31:2:091. [DOI] [PubMed] [Google Scholar]
- 29.Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I. Estrogen and cytokines productio – the possible cause of gender differences in neurological diseases. Curr Pharm. 2005;11:1017–30. doi: 10.2174/1381612053381693. [DOI] [PubMed] [Google Scholar]
- 30.Kher A, Wang M, Tsai BM, et al. Sex differences in the myocardial inflammatory response to acute injury. Shock. 2005;23:1–10. doi: 10.1097/01.shk.0000148055.12387.15. [DOI] [PubMed] [Google Scholar]
- 31.Sadeghi M, Daniel V, Naujokat C, Wiesel M, Hergesell O, Opelz G. Strong inflammatory cytokine response in male and strong anti-inflammatory response in female kidney transplant recipients with urinary tract infection. Transpl Int. 2005;18:177–85. doi: 10.1111/j.1432-2277.2005.00007.x. [DOI] [PubMed] [Google Scholar]
- 32.Arend WP. The balance between IL-1 and IL-1RA in disease. Cytokine Growth Factor Rev. 2002;13:323–40. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 33.Kluth DC, Rees AJ. New approaches to modify glomerular inflammation. J Nephrol. 1999;12:66–75. [PubMed] [Google Scholar]