Abstract
Increasing numbers of patients are choosing to interrupt highly active antiretroviral therapy (HAART). We describe the effect of patient-directed treatment interruption (PDTI) on plasma viral loads (pVL), proviral DNA (pDNA), lymphocyte subsets and immune responses in 24 chronically HIV-1 infected individuals. Patients were divided into group A with pVL > 50 copies/ml and group B with pVL < 50 copies/ml, prior to the PDTI. pVL rose significantly in group B during the first month off HAART and was associated with a significant decrease in CD4 T-cell count. At baseline there was a significant difference in HIV-1 pDNA levels between groups A and B, however, levels significantly increased in group B, but not in group A during PDTI becoming equivalent after 1 month PDTI. We have previously shown no increase in pDNA over the time of substitution in patients switching HAART regimens despite a small rebound in pVL. These observations indicate that to protect low pDNA levels PDTI should be discouraged and that changing regimen at the first sign of failure should be advised where possible. Only transient, no longer than 4 week, HIV-1-specific responses were observed during PDTI in 5/24 patients, 2 from group A and 3 from group B. The low numbers of responders and the transient nature of the anti-HIV-1 immune responses do not favour the auto-vaccination hypothesis.
Keywords: chronic HIV-1 infection, HAART, therapy interruption, pro-viral DNA
Introduction
Although highly active antiretroviral therapy (HAART) has proven to be effective at reducing HIV-1 replication and improving the clinical course of infection since its introduction in 1996 [1,2] it is now evident that due to the latently infected, resting CD4+ T-cell population, life-long HAART alone is unlikely to achieve full eradication of the virus [3]. Long-term use of antiretrovirals has been associated with increased risk of drug related resistance and side-effects including fat redistribution or lipodystrophy and insulin intolerance [4,5]. A major cause of concern is evidence from clinical trials showing that only 70% of previously untreated patients achieve complete virological suppression (HIV-1 plasma viral load (pVL) of < 50 copies/ml) [6]. Added to the failure to completely suppress pVL in 30% of patients, there are problems associated with adherence to therapy regimens, such as those highlighted in a recent study showing an increasing trend towards interruption of HAART [7]. Patients often interrupt therapy for short periods, due to personal choice, adverse side-effects, adherence problems, toxicity or drug resistance. In addition to the adverse side-effects and lack of initial efficacy, up to 50% of patients starting on HAART regimens will fail virologically within the first year [8]. Interruption of therapy is generally associated with a rapid increase in pVL [9–11]. Little is known about the immunological and virological consequences of these interruptions in HAART beyond observations made in structured (clinician defined) treatment interruption (STI) trials. Such STI has been studied extensively in both primary (PHI) and chronic (CHI) HIV-1 infection. The concept that interruption of HAART may be of benefit, allowing regeneration of HIV-1-specific responses, stems from the initial report on the ‘Berlin patient’ in 1997 [12,13]. The most comprehensive study of STI to date has been the Swiss Spanish cohort study, which studied 133 patients undergoing an STI. Patients with a median CD4 T cell count of 740 cells/µl and undetectable pVL for a median of 21 months, underwent an STI schedule of 2 weeks off, followed by 8 weeks on HAART for a total of 4 cycles, and at week 40 HAART was indefinitely suspended. Responders were defined as those with pVL < 5000 copies/ml. By week 52 17% of patients were responders and by week 96 this had fallen to 8%. Low pre-HAART pVL and lack of rebound during weeks 0–40 were predicative of continued viral suppression. IFN-γ production was also measured by ELISpot, using HLA matched HIV-1 peptides and the number of spot forming cells (SFC) observed at week 52 correlated with the degree of antigen exposure [6].
During primary HIV-1 infection a latent reservoir is established, it has been shown by the presence of resting memory CD4+ T cells containing replication-competent provirus that, this latent infection is not completely blocked by HAART [3,14]. Resting memory CD4+ T cells can persist for many months, and represent a long-term viral reservoir in patients receiving HAART. The persisting proviral DNA (pDNA) can be detected in patients in the absence of detectable HIV-1 plasma viral RNA load [15]. The presence of drug resistant provirus, in the PBMC allows the ability to produce ART resistant virions upon activation of these cells, this storage of provirus during viral replication allows the cataloguing of drug resistance within the latent viral reservoir. Recently, it has been shown in patients receiving HAART that higher levels of pDNA are associated with an increase in the proportion of effector CD4 T cells and reductions in the proportions of naïve CD4 and CD8 T cells, indicating that higher levels of pDNA interfere with immune reconstitution in patients receiving HAART [16]. We have previously shown that HAART initiated during PHI reduces the level of pDNA in PBMC to levels seen in long-term nonprogressors (LTNP), however, this is not observed if therapy is initiated in CHI [17].
We have previously also studied the effect of switching from protease inhibitor (PI) to non-nucleoside reverse transcriptase inhibitor (NNRTI) based antiretroviral regimens. Following therapy switch we observed an increase in cellular response to mitogen and an increase in viral and recall antigen responses measured by proliferation, and all patients demonstrated an increase in HIV-1-specific T-cell responses. However, despite a transitory small elevation in plasma HIV-1 RNA load, pDNA studies revealed no alteration in numbers of DNA copies of virus per total cellular DNA [18].
The principal focus of this study was to observe the immunological and virological changes in the numerous patients who do now interrupt HAART, outside of the controlled conditions of STI trials. The present study was specifically designed to establish whether temporary patient-directed discontinuation of HAART resulted in differential immunological and/or virological outcomes in patients who had either persistent measurable pVL or suppressed pVL whilst receiving HAART, with particular focus on the level of HIV-1 pDNA in the two groups.
Materials and methods
Patient cohort
Patients who had personally taken the decision to interrupt therapy were recruited via the St Stephen's Clinic, Chelsea and Westminster Hospital and HIV/GU Medicine, St George's Hospital. The patients recruited had chosen to interrupt HAART due to adverse side-effects, adherence problems, toxicity, drug resistance or for other personal reasons. Patients were divided into one of two groups after recruitment Group A comprised of individuals who had detectable viremia (pVL > 50 copies/ml) at the time of HAART withdrawal and Group B comprised of individuals who had virological suppression below the level of detection (BLD) (pVL < 50 copies/ml) at the time of HAART withdrawal. Recruitment continued until we had 12 individuals in each group. The duration of the interruption and the choice of therapy upon re-start were determined by the patient and their physician and did not follow predetermined study timings. All patients were followed up during their routine clinic visits, until they had restarted HAART, this was at approximately 1 month intervals. All patients were enrolled with informed consent and local ethics committee approval (Riverside Ethics Committee number RREC2852).
Sampling procedure
Thirty mls of blood was collected into lithium heparin vacutainers and 10 mls into EDTA vacutainers (Becton Dickinson, Oxford, UK). Samples were taken on the day of HAART discontinuation (day 0), twice during the off HAART period (visit 1: median day 30, range day 21–42; visit 2: median day 63, range day 53–80) and within the first month of re-introduction of HAART. In addition to the study assays HIV-1 pVL and lymphocyte subsets were measured at each time point.
Lymphocyte subset quantification
The Epics XL-MCL (Beckman Coulter, High Wycombe, UK) was used for four colour flow cytometric analysis. Anti-human CD3, CD4, CD8, CD45, CD56 and CD19 were used to analyse T cell subsets, NK and B cells, respectively. Briefly, specific cell staining was achieved by incubating 100 µl whole blood with 10 µl of either CYTO-STATtetraCHROME (CD45-FITC/CD4-RD1/CD8-ECD/CD3-PC5) or (CD45-FITC/CD56-RD1/CD19-ECD/CD3-PC5) (Beckman Coulter) for 15 min at room temperature. Red blood cells were then lysed with the COULTER ImmunoPrep Reagent System and the TQ-Prep Workstation (Beckman Coulter). The white cells were then analysed on the Epics XL-MCL flow cytometer using system II software in conjunction with control reagents (Beckman Coulter) which provide automated colour compensation, light scatter and colour intensities.
Plasma viral RNA assay
The Bayer HIV-1 RNA 3·0 bDNA assay (Bayer, Newbury, UK) was used to determine pVL with a lower detection limit of 50 copies/ml plasma, and an upper detection limit of 500 000 copies/ml plasma. This was used according to the manufacturer's instructions.
Proviral DNA quantification
HIV-1 proviral DNA was measured utilizing fluorometric PCR methodology as previously described [19], with an analytic sensitivity of 10 copies/µg of total cellular DNA. Briefly PBMC were lysed using proteinase K and the DNA quantified using Hoechst dye. Lysates were coamplified for 30 cycles with an internal DNA quantification standard using the Amplicor HIV-1 Monitor v1·5 test primers (SK145-SKCC1B). Amplified products were then quantified in microwell plates using the Amplicor HIV Monitor format. The number of HIV-1 DNA copies per µg of total cellular DNA was then calculated according to the manufacturers instructions.
Peptide pools, mitogens and antigens
Peptide pools, of HIV-1 clade B Nef, Gag and Tat (provided by the EU Programme EVA/MRC Centralized facility for AIDS Reagents, NIBSC, UK (Grant number QLK2-CT-1999–00609 and G9828102)) were added to appropriate wells, at a final concentration of 5 µg/ml of each individual peptide. The 22 gag p24 peptides were 20-mers with a 10 amino acid (aa) overlap covering p24 gag (aa 133–363 of HIV-1 SF2, ARP788·1–22), these were used in a pool of 22. The 21 Nef peptides were mostly 20-mers with a 10 aa overlap (the exceptions are peptide 7, a 21-mer, and peptide 21, a 15-mer) covering Nef from strain HIV-1 Bru (ARP7074·1–21), these were used in a pool of 21. The Tat peptide pool (EVA779·1–8) was used as a pool of 8 peptides, the peptides are from the HIV-1 LAI stain and were all 20mers with a 10 aa overlap. HIV-1 antigens were baculovirus derived recombinant p24 and baculovirus derived recombinant gp160, added to wells at a final concentration of 5 µg/ml (both Protein Sciences, Meridan, USA). Empty baculovirus vector was used as control for the HIV-1 recombinant antigens. Recall antigens used were tetanus toxoid, candida (both Pasteur Sanofi, Guildford, UK), PPD (Statens Serum Institute, Copenhagen, Denmark) and streptokinase (Sigma, Poole, UK) at final concentrations of 5 µg/ml, 25 µg/ml, 1 µg/ml and 5 µg/ml, respectively, along with viral antigens CMV 1/2000 and HSV 1/400 final dilution (both Bio Whittaker, Wokingham, UK). Mitogen control PHA (Sigma) was used as a positive control in all assays at a final concentration of 5 µg/ml.
Proliferation assays
PBMC (105/well) were cultured with antigen or mitogen in round bottomed microtitre plates (Greiner, Stonehouse, UK), in 10% AB plasma/RPMI (200 µl) (Sigma) for 5 days. Each well was then pulsed with 1 µCi 3H-methyl thymidine (3H-TdR; Amersham International, Amersham UK) and 16 h later cells were harvested onto glass fibre filtermats (Wallac Oy, Turku, Finland). Proliferation measured by 3H-TdR incorporation was evaluated by liquid scintillation spectroscopy using a 1205 Betaplate counter (Wallac). Control wells, for calculation of background activity, contained PBMC only, with the exception of the baculovirus-derived HIV-1 antigens which were controlled with empty baculovirus vectors. Results are expressed as stimulation indices (SI) and as the mean counts per minute (cpm) for triplicate cultures, with percentage error of the mean < 15%. SI is defined as the experimental triplicate mean divided by background mean. A positive response is defined as an SI score of ≥5 coupled with a Δcpm of >2000.
IFNγ ELISPOT assays
Detection of single cell IFNγ release by ELISPOT assays was carried out as previously described [20,21]. Briefly, 1 × 105 PBMC/well were cultured in 10% AB plasma/RPMI (200 µl; Sigma) in 96-well polyvinylidene difluoride backed plates (Millipore, Watford, UK), coated with anti-IFNγ mAb (Mabtech, Stockholm, Sweden). Cells were stimulated with appropriate peptide, antigen, mitogen or control (see above) in 200 µl 10% AB plasma/RPMI (Sigma). Plates were incubated overnight at 37 °C for 16 h or 2 days for detection of peptide-specific or antigen-specific IFNγ producing cells, respectively. Spot-forming cells (SFC) were then quantified according to the manufacturer's instructions (Mabtech). A positive result is defined as a score of > 50 SFC/106 PBMC above background and at least 2× the background.
Statistical analysis
Statistical significance of change within a group was calculated using the Wilcoxon's signed-rank test. The Mann–Whitney U-test was used to compare between the two groups. The results are presented as P-values, with a cut off for significance of P < 0·05. Statistical software (Statview 5·01; Abacus, Cary, NC, USA) was used for all calculations.
Results
Twenty-four patients who had commenced HAART during chronic HIV-1 infection, who wished to interrupt HAART for a variety of reasons, were studied before, during and after PDTI. Patients were divided into two groups. Group A comprised of 12 individuals who had detectable viremia (pVL > 50 copies/ml) at the time of HAART withdrawal. Clinical notes indicate adverse drug reactions in 3/12, resistance in 5/12 individuals and poor compliance in 1 out of the 12 individuals. Three of 12 sets of patient notes listed virological failure but with no cause indicated.
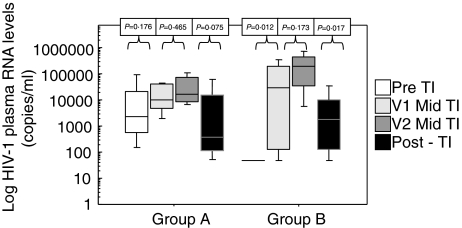
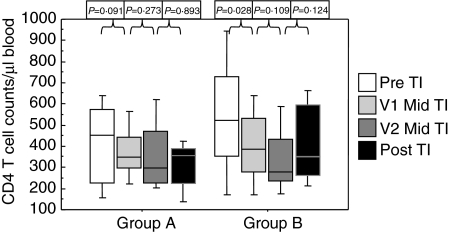
Group B comprised of 12 individuals who had virological suppression below the level of detection (BLD) (pVL < 50 copies/ml) at the time of HAART withdrawal. Twenty-one patients were male with 1 female in group A and 2 females in group B. There was no statistical difference between the ages of the patients at entry into the study, Group A mean 40 years (range 31–64 years) and group B mean 46 years (range 31–68 years). Patient characteristics at baseline are detailed in Table 1. Patients CD4 T cell counts and pVL prior to the initiation of initial HAART regimen are described in Table 2, there was no significant differences between the two groups in their pre-HAART characteristics. There was no significant difference in the length of time off HAART between the two groups (Group A median 102 days range 82–190 days; Group B median 112 days range 90–203 days). CD4 counts and pVL were statistically different between the two groups at baseline (P = 0·0342 and P = 0·0022, respectively) but CD3, CD8 and CD19 lymphocyte counts did not differ (Table 1). HIV-1 RNA pVL rose in both groups during the first month off HAART although this increase was only statistically significant in group B (P = 0·012). The trend in pVL increase continued during the 2nd off HAART month in both groups. pVL decreased within the 1st month after re-introduction of HAART although only statistically significant in group B (P = 0·017). However there was no significant difference between the two groups at the last time point (Fig. 1). The viral loads in group A were lower post-PDTI compared to pre-PDTI although this was not statistically significant. CD3, CD8 and CD19 lymphocyte counts did not change significantly following the off HAART period in either group (Table 1). There was a decrease in CD4 T cell counts in both groups once HAART was withdrawn although this decrease only reached significance in group B during the first month of HAART withdrawal (P = 0·0284). Once HAART was reintroduced CD4 T cell counts increased in both groups and there was no difference between the groups post-PDTI (Fig. 2).
Table 1. Overall patient characteristics pre- and post PDTI: plasma HIV-1 loads and lymphocyte subset counts.
| Pre – PDTI | Post – PDTI | |||
|---|---|---|---|---|
| Group | A | B | A | B |
| Plasma HIV-1 RNA copies/ml | 2342 (143–166 170) | 49* (49–49) | 1066 (49–76 366) | 1748 (49–42 809) |
| CD4 T-cells/µl blood | 450 (41–649) | 523** (118–1217) | 356 (104–435) | 353 (207–689) |
| CD8 T-cells/µl blood | 830 (350–1244) | 894 (328–3653) | 819 (340–1802) | 1346 (457–2387) |
| CD3 T-cells/µl blood | 1121 (430–1922) | 1337 (619–3161) | 1309 (465–2161) | 1452 (680–3341) |
| CD19 B-cells/µl blood | 132 (19–769) | 282 (73–434) | 281 (144–659) | 207 (28–357) |
Median and range values for plasma HIV-1 loads and lymphocyte counts for each group. Samples were taken on the day of HAART withdrawal (day 0), and within the first month of HAART re-introduction. Group A had a pre-TI HIV-1 pVL of > 50 copies/ml and group B had a pre-TI HIV-1 pVL of < 50 copies/ml.
pVL values were statistically different between the groups at baseline (P = 0·0022).
CD4 T cell count values were statistically different between the groups at baseline (P = 0·0342). There were no statistically significant differences between groups post PDTI.
Table 2. Individual patient characteristics pre-HAART: Nadir plasma HIV-1 loads and CD4+ T lymphocyte counts.
| Group A | Group B | |||
|---|---|---|---|---|
| Patient | Plasma HIV-1 RNA copies/ml | CD4 T-cells/µl blood | Plasma HIV-1 RNA copies/ml | CD4 T-cells/µl blood |
| 1 | 290 540 | 594 | 80 748 | 127 |
| 2 | 395 219 | 359 | 23 306 | 195 |
| 3 | 20 331 | 244 | 408 727 | 498 |
| 4 | 50 119 | 90 | 19 918 | 188 |
| 5 | 61 610 | 125 | 66 560 | 311 |
| 6 | 101 170 | 139 | 428 093 | 95 |
| 7 | 42 880 | 264 | 171 259 | 285 |
| 8 | 150 403 | 207 | 64 884 | 467 |
| 9 | 29 341 | 380 | 500 000 | 314 |
| 10 | 602 921 | 373 | 43 600 | 88 |
| 11 | 13 201 | 190 | 6 582 | 96 |
| 12 | 52 554 | 122 | 135 000 | 239 |
| Median | 57 082 | 226 | 73 654 | 217 |
| Range | 13 201–602 921 | 90–594 | 6 582–500 000 | 95–498 |
Individual, median and range values for plasma HIV-1 loads and CD4+ T lymphocyte counts prior to the initiation of HAART for all patients.
Fig. 1.
Log HIV-1 plasma RNA levels (copies/ml). Samples were taken on the day of HAART discontinuation (day 0), twice during the off HAART period (V1: median day 30, range day 21–42; V2: median day 63, range day 53–80) and within the first month of HAART re-introduction. Group A had a pre-TI HIV-1 plasma RNA load of > 50 copies/ml and group B had a pre-TI HIV-1 plasma RNA load of < 50 copies/ml.
Fig. 2.
CD4+ T lymphocyte count/µl peripheral blood. Samples were taken on the day of HAART discontinuation (day 0), twice during the off HAART period (V1: median day 30, range day 21–42; V2: median day 63, range day 53–80) and post TI within the first month of HAART re-introduction. Group A had a pre-TI pVL of > 50 copies/ml and group B had a pre-TI pVL of < 50 copies/ml.
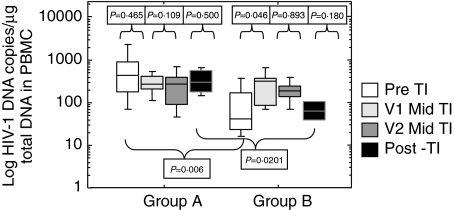
HIV-1 pDNA levels significantly increased in group B during the first month to visit 1 off HAART (P = 0·0464), however, no significant change was seen in group A (P = 0·4652). There was no significant difference in either group between the pre and post PDTI levels of pDNA although the pDNA significantly increased during the PDTI compared to baseline in group B (V1 P = 0·0464; V2 P = 0·0284). There was a significant difference in the level of HIV-1 pDNA between groups A and B at baseline (P = 0·0060) prior to therapy interruption, and at the post PDTI time point (P = 0·0201) (Fig. 3).
Fig. 3.
Log HIV-1 DNA copies/µg total DNA measured in peripheral blood. Samples were taken on the day of HAART discontinuation (day 0), twice during the off HAART period (V1: median day 30, range day 21–42; V2: median day 63, range day 53–80) and within the first month of HAART re-introduction. Group A had a pre-TI HIV-1 plasma RNA viral load of > 50 copies/ml and group B had a pre-TI HIV-1 plasma RNA viral load of < 50 copies/ml. HIV-1 pDNA, measured as described in materials and methods, with an analytical sensitivity of 10 copies HIV-1 DNA/µg of total cellular DNA.
Mitogen PHA was used as a positive control in all proliferation and ELIspot assays, all assays were included in the analysis as the mitogen control met the criteria for positivity in all assays.
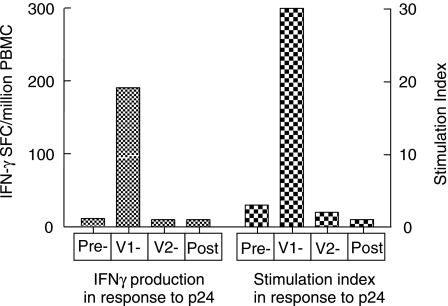
No significant changes in proliferative or IFN-γ response to HIV-1, other viral and recall antigens were seen during the PDTI in either group nor any differences between the groups (Table 3). When analysed on a single patient basis 5 gained IFN-γ producing responses associated with proliferation to HIV-1 antigens. None of the patients had a response at baseline to the HIV-1 antigens, however, responses were observed in 2 patients from group A and 3 patients from group B at visit 1, but all were transient and lost by visit 2 post therapy interruption. A representative patient is depicted in Fig. 4. Using regression analysis no correlation was seen between pVL, lymphocyte subset counts or pDNA load and response to HIV-1 antigens.
Table 3. Proportion of patients responding with IFN-γ production and proliferation.
| Group A | Group B | |||||||
|---|---|---|---|---|---|---|---|---|
| Antigen | Baseline (n = 12) | Visit 1 (n = 12) | Visit 2 (n = 12) | Post PDTI (n = 12) | Baseline (n = 12) | Visit 1 (n = 12) | Visit 2 (n = 12) | Post PDTI (n = 12) |
| IFN-γ production | ||||||||
| Gag peptides | 5 | 6 | 7 | 4 | 3 | 5 | 4 | 5 |
| Nef peptides | 5 | 5 | 2 | 2 | 3 | 4 | 3 | 7 |
| Tat peptides | 1 | 3 | 3 | 3 | 1 | 3 | 1 | 5 |
| p24 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 0 |
| gp160 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 0 |
| Profiliferation | ||||||||
| p24 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 0 |
| gp160 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 0 |
| T. toxoid | 7 | 4 | 0 | 1 | 7 | 7 | 4 | 4 |
| Candida | 6 | 6 | 3 | 5 | 9 | 5 | 5 | 5 |
| PPD | 1 | 1 | 1 | 2 | 2 | 2 | 4 | 0 |
| Streptokinase | 2 | 1 | 0 | 1 | 0 | 1 | 1 | 0 |
| CMV | 7 | 4 | 3 | 6 | 6 | 7 | 8 | 4 |
| HSV | 3 | 5 | 2 | 3 | 1 | 3 | 4 | 3 |
| PHA | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
Samples were taken on the day of HAART discontinuation (day 0 baseline), twice during the off HAART period (V1, day 30; V2, day 63) and within the first month of HAART re-introduction. Responses were measured by both (a) IFN-γ production and (b) 3H thymidine incorporation as described in materials and methods.
Fig. 4.
Representative responder patient from group B. Samples were taken on the day of HAART discontinuation (day 0), twice during the off HAART period (V1 day 28; V2 day 57) and within the first month of HAART re-introduction. Responses to p24 measured by both IFN-γ production and 3H thymidine incorporation as described in materials and methods.
There were responders within each group to overlapping peptide pools, all patients responded to at least one pool at a minimum of one time point. There were no statistically significant changes observed within the groups in responses to peptide pools, using Wilcoxon signed rank test. Moreover, no differences between the groups in terms of responses to pools of Gag, Nef or Tat were observed at any time-point during the study. However, there was a significant correlation, using regression modelling, within groups A and B between the number of pools responded to and the CD8 T-cell count (group A P = 0·0499; group B P = 0·0199).
Discussion
In this study the level of HIV-1 pDNA, immune responses to HIV-1 antigens and peptides, recall and other viral antigens were monitored over the period of PDTI. The aim of the study was to investigate changes, which occur when HAART is discontinued, in two different cohorts of chronically infected patients. Group A had detectable pVL and group B who had pVL below < 50 copies/ml (BLD) at baseline before PDTI.
We have previously shown that HAART initiated during PHI reduces the level of pDNA in PBMC to levels seen in LTNP, however, this is not observed if therapy is initiated in CHI [17]. Here we showed that the level of pDNA detected in the PBMC from group B prior to interruption was consistent with levels observed in our previous study in successfully HAART treated CHI patients [17]. pDNA levels increased significantly in group B to the level seen in group A during the off HAART period. Raised levels of HIV-1 pDNA in group A remained throughout the study, whilst in group B pDNA level decreased after reintroduction of HAART although at a slower rate to that observed for the decrease in pVL. CD4 T-cell counts dropped significantly within the first month of HAART withdrawal in group B as would be expected, which was associated with the increasing pVL.
Previously Lambotte et al. [22] described a model where the lymphocyte HIV-1 pDNA reservoir is portrayed as a dynamic system, which is continually filled with infected, activated lymphocytes reverting to a quiescent state. It has been suggested that lymphocyte archiving of new viral strains depends on the quantity of each viral strain in the blood, its infectiousness and the length of time it is present in circulation. Thus it has been suggested that patients with resistant virus strains should not continue on the same HAART regimen, as resistant strains will be archived to the stable reservoir [22]. We have previously shown that in patients switching from PI-based HAART to NNRTI-based HAART there was no increase in pDNA over the time of the switch despite a slight rebound in plasma RNA levels. pDNA levels in this study were similar to the levels seen in group B and those described in our cohort of CHI [17,18]. Together with the data from the current study showing significantly higher levels of pDNA in virologically nonresponsive patients, our data adds weight to the conclusions of Lambotte et al. [22] that patients with resistant HIV-1 should not continue on the same HAART regimen and that switching regimen where clinically possible at the first sign of failure may protect the lower pDNA levels seen in the successfully treated CHI cohort.
A recent study, showed a benefit of TI for patients who have exhausted all of their therapeutic options among the currently available drug classes. A TI strategy allowed for improved management of the clinical symptoms of virological failure, once drugs were restarted. This strategy may allow the patient to survive until new classes of drugs are available [23]. It must be taken into consideration that the pDNA quantification described here was performed in PBMC, and other HIV-1 reservoir sites require more in-depth analysis in future studies.
During the PDTI we detected only transient increases in responses to HIV-1 antigens, recall and other viral antigens both by IFN-γ and proliferation assays in 5 of 24 patients. In our previous study we detected increases in immune responses when patients switched from PI to NNRTI based HAART [18]. Data presented in this study do not favour the auto-immunization hypothesis, as all responses detected were transient and lost after 2 months of HAART interruption. No differences were seen between the groups in terms of the ability to generate responses to either HIV-1, other viral or recall antigens. Douek et al. [24] have shown that HIV-1-specific memory CD4+ T-cells from HIV-1 infected individuals, contain more viral DNA than other memory CD4+ T-cells, at all stages of HIV-1 disease. Furthermore, they demonstrated that following interruption of antiretroviral therapy and viral rebound, the frequency of HIV-1 DNA in the HIV-1-specific memory CD4+ T-cells pool increases to a greater extent than in other memory CD4+ T-cells. They conclude that HIV-1 in vivo preferentially infects HIV-1-specific CD4+ T-cells and provides a potential mechanism of loss of HIV-1-specific CD4+ T-cell responses, and thus loss of immunological control over HIV-1 replication [24]. This preferential infection of HIV-1-specific CD4+ T-cells seen by Douek et al.[24] may explain the disappearance of the transient HIV-1-specific immune responses in this present study cohort two months after HAART cessation when the viral load had increased in both groups. Only 21% of patients generated a response to HIV-1 antigens, and no direct correlation could be found between viral load, lymphocyte subsets, or pDNA and detection of an anti-HIV-1 response within these patients. However, the increased levels of pDNA have been shown previously to be associated with an increase in the proportion of effector CD4+ cells and a decrease in the proportion of naïve CD4+ and CD8+ T cells [16], this may provide another mechanism by which the provirus will interfere with immune reconstitution in these patients although a direct correlation could not be found. In our switch study where a degree of HIV-1-specific immune reconstitution was seen we did not detect a rise in pDNA [18]. Over the study period, CD8 T-cell count did predict the breadth of the IFN-γ response to pools of overlapping HIV-1 peptides, the higher the CD8 count the more peptides responded to, when analysed using a simple regression model. Based on this and previous data we suggest that switching classes of HAART where available rather than stopping HAART may be more beneficial in terms of inducing and protecting HIV-1-specific immune responses whilst simultaneously protecting against transient increases in levels of pVL in the plasma and the pDNA detectable in PBMC.
Acknowledgments
The authors would like to acknowledge the contribution of the Immunology laboratory staff at the Chelsea and Westminster Hospital for viral load and lymphocyte subset data, the staff and patients of the St Stephen's Centre for taking part in this study and Sundhiya Mandalia for help with statistical analysis. This work was supported by the St Stephen's AIDS Trust and the grants from the Wellcome Trust (No. 058700) and European Union (No. LSHP-CT-2004–503487).
References
- 1.Mocroft A, Devereux H, Kinloch-de-Loes S, et al. Immunological, virological and clinical response to highly active antiretroviral therapy treatment regimens in a complete clinic population: Royal Free Centre for HIV Medicine. AIDS. 2000;14:1545–52. doi: 10.1097/00002030-200007280-00010. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–8. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 4.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–9. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 5.Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881–3. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 6.Lowe SH, Prins JM, Lange JM. Antiretroviral therapy in previously untreated adults infected with the human immunodeficiency virus type I. established and potential determinants of virological outcome. Neth J Medical. 2004;62:424–40. [PubMed] [Google Scholar]
- 7.Li X, Margolick JB, Conover CS, et al. Interruption and discontinuation of highly active antiretroviral therapy in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2005;38:320–8. [PubMed] [Google Scholar]
- 8.Pillay D, Taylor S, Richman DD. Incidence and impact of resistance against approved antiretroviral drugs. Rev Med. 2000;10:231–53. doi: 10.1002/1099-1654(200007/08)10:4<231::aid-rmv290>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Garcia F, Plana M, Ortiz GM, et al. The virological and immunological consequences of structured treatment interruptions in chronic HIV-1 infection. AIDS. 2001;15:F29–F40. doi: 10.1097/00002030-200106150-00002. [DOI] [PubMed] [Google Scholar]
- 10.Garcia F, Plana M, Vidal C, et al. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. AIDS. 1999;13:F79–F86. doi: 10.1097/00002030-199907300-00002. [DOI] [PubMed] [Google Scholar]
- 11.Davey RT, Jr, Bhat N, Yoder C, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96:15109–14. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen J. HIV suppressed long after treatment. Science. 1997;277:1927. doi: 10.1126/science.277.5334.1927. [DOI] [PubMed] [Google Scholar]
- 13.Lisziewicz J, Lori F. Structured treatment interruptions in HIV/AIDS therapy. Microbes Infect. 2002;4:207–14. doi: 10.1016/s1286-4579(01)01529-5. [DOI] [PubMed] [Google Scholar]
- 14.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 15.Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–7. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrowski SR, Katzenstein TL, Thim PT, Pedersen BK, Gerstoft J, Ullum H. Low-level viremia and proviral DNA impede immune reconstitution in HIV-1-infected patients receiving highly active antiretroviral therapy. J Infect Dis. 2005;191:348–57. doi: 10.1086/427340. [DOI] [PubMed] [Google Scholar]
- 17.Pires A, Hardy G, Gazzard B, Gotch F, Imami N. Initiation of Antiretroviral Therapy During Recent HIV-1 Infection Results in Lower Residual Viral Reservoirs. J Acquir Immune Defic Syndr. 2004;36:783–90. doi: 10.1097/00126334-200407010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan AK, Burton CT, Nelson MR, et al. Restoration of human immunodeficiency virus-1-specific responses in patients changing from protease to non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Scand J Immunol. 2003;57:600–7. doi: 10.1046/j.1365-3083.2003.01276.x. [DOI] [PubMed] [Google Scholar]
- 19.Fessel WJ, Krowka JF, Sheppard HW, et al. Dissociation of immunologic and virologic responses to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;23:314–20. doi: 10.1097/00126334-200004010-00005. [DOI] [PubMed] [Google Scholar]
- 20.Imami N, Hardy GA, Nelson MR, et al. Induction of HIV-1-specific T cell responses by administration of cytokines in late-stage patients receiving highly active anti-retroviral therapy. Clin Exp Immunol. 1999;118:78–86. doi: 10.1046/j.1365-2249.1999.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AV, McMichael AJ. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–65. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambotte O, Chaix ML, Gubler B, et al. The lymphocyte HIV reservoir in patients on long-term HAART is a memory of virus evolution. AIDS. 2004;18:1147–58. doi: 10.1097/00002030-200405210-00008. [DOI] [PubMed] [Google Scholar]
- 23.Katlama C, Dominguez S, Gourlain K, et al. Benefit of treatment interruption in HIV-infected patients with multiple therapeutic failures: a randomized controlled trial (ANRS 097) AIDS. 2004;18:217–26. doi: 10.1097/00002030-200401230-00011. [DOI] [PubMed] [Google Scholar]
- 24.Douek DC, Brenchley JM, Betts MR, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]