Abstract
Cytokines are critical molecules necessary for normal lung pathogen host defences. Gamma interferon (IFN-γ) and T1-phenotype immune responses are important components of host defence against Aspergillus. Therefore, we hypothesized that transient overexpression of IFN-γ within the lung could augment host immunity against Aspergillus. Here it was showed that intranasal administration of 5 × 107 colony-forming units (CFU) of Aspergillus fumigatus (Af) induced the expression of IFN-γ. Mice were intranasally (i.n) administrated with 5 × 108 PFU of a recombinant adenovirus vector containing the murine IFN-γ cDNA (AdmIFN-γ), and challenged 24 h later with Af. We observed that i.n. administration of AdmIFN-γ resulted in about a fourfold increase in levels of IFN-γ and IL-12 within the lung, about a 75% reduction in lung fungal contents at day 2 and a more than threefold higher survival rate in the AdmIFN-γ-treated group compared to the controls (P < 0·01). This protection effect was not found when AdmIFN-γ was i.p. administrated. Alveolar macrophages and lung leucocytes isolated from i.n. AdmIFN-γ-treated animals displayed enhanced killing of intracellular Aspergillus organisms ex vivo. These results demonstrate that transient overexpression of IFN-γ could augment host defence against Aspergillus.
Keywords: fungi, invasive pulmonary aspergillosis, gene therapy, interferon γ, adenovirus
Introduction
Aspergillus fumigatus (Af) is a ubiquitous and opportunistic fungal pathogen for humans and animals. Invasive pulmonary aspergillosis (IPA), characterized by hyphal invasion and destruction of pulmonary tissue, is a leading cause of attributable mortality among immunocompromised patients [1]. Although the release of fungal metabolites with immunosuppressive activity may contribute to the pathogenesis of pulmonary aspergillosis [2], local cellular defects in the innate and adaptive immune effector mechanisms are major predisposing factors of the host to IPA. The first and major lines of defence against inhaled Aspergillus conidia is the pulmonary macrophage and neutrophil [3].
Many studies have shown that a T helper (Th)1/Th2 dysregulation and a switch to a Th2-type immune response may contribute to developing a poor outcome of IPA [4–8]. The IFN-γ produced by effector cells such as CD4+ Th1 plays an important role in protection against intracellular pathogens. Studies in experimental animals using recombinant IFN-γ or antibodies to IFN-γin vivo showed that IFN-γ is a pivotal factor for normal host defence against a variety of pulmonary pathogens including Aspergillus[9,10].
Gene therapy may have certain advantages over protein-based therapy. As a gene based approach can be targeted locally to the site of infection and, thus, compartmentalize therapy. This has potential advantages for various cytokine genes because systemic delivery of the protein needs daily administrations, has significant systemic toxicity and the inability to target the cytokine to a specific organ or tissue site [11]. So we examined whether transient overexpression of IFN-γ would promote pulmonary Aspergillus clearance. We found that treatment with AdmIFN-γ i.n. resulted in enhanced Aspergillus clearance. Furthermore, our studies demonstrated that AdmIFN-γ could activate lung cells to kill Aspergillus ex vivo, that the effects of AdmIFN-γ were independent of cell recruitment, and that intrapulmonary rather than systemic IFN-γ expression was required for beneficial effects.
Materials and methods
Mice
Specific pathogen-free BALB/c mice, 8- to 10- week old, were purchased from The Jackson Laboratory (Bar Harbor, ME, USA), and were bred under specific pathogen-free conditions in the animal facility of the Chinese Academy of Science. Procedures involving animals and their care were conducted in conformity with national and international laws and policies. Immunocompromised mice were made by treatment with 200 mg/kg of cyclophosphamide (Sigma, St, Louis, MO, USA), administered i.p. 4 days before intranasal (i.n) Af inoculation.
Micro-organism, culture conditions, and infection
The strain of Af was obtained from a fatal case of IPA at the Institute of Infectious Diseases of Huashan hospital, Fudan University, Shanghai, China. The microorganism was cultured on Sabouraud dextrose agar (Becton Dickinson, Cockeysville, MD, USA) supplemented with chloramphenicol for 4 days at 37°C. The surface of each plate was then washed with 15 ml of sterile 0·1% Tween 80 (Sigma Ultra) in saline. The resulting suspension of conidia was filtered through sterile gauze to remove clumps and hyphal debris. After extensive washing with saline, the conidia were counted using a particle counter and diluted to the desired concentrations. The viability of the conidia was > 95%, as determined by serially diluting and plating out the inoculum on Sabouraud dextrose agar. For i.n. infection, immunocompromised mice were lightly anaesthetized with inhaled diethyl and then a suspension of 5 × 107 conidia in 30 µl of sterile saline were slowly applied to the nostrils by micropipette with a sterile disposable tip. Animals were held in an upright position until the suspension was completely inhaled and normal breathing resumed [12]. Mice succumbing to fungal challenge were routinely necropsied for histopathological confirmation of IPA. For lung histological analysis, whole lungs were fully inflated with 4% paraformaldehyde and dissected and placed in fresh paraformaldehyde for 24 h. Routine histological techniques were used to paraffin embed the entire lung, and 5 mm sections of whole lung were stained with haematoxylin and eosin (H&E), and Gomori methanamine silver (GMS).
Adenovirus vectors
Recombinant adenoviruses encoding mouse IFN-γ (AdmIFN-γ) was a generous gift from Dr Xuetao Cao [13]. The virus was plaque purified, propagated on HEK293 cells (human embryonic kidney cells containing the E1A region of Adenovirus, Microbix, Inc. Ontario, Canada) and purified by CsCl gradient according to standard techniques. Particle titres of all adenovirus were determined by absorbance measurements at 260 nm, and functional PFU titres were determined by plaque assay on HEK293 cells. For experiments using AdmIFN-γ, we used Adenovirus containing green fluorescent protein (AdEGFP) [14] without the transgene as a control.
To overexpress IFN-γ in the lung, AdmIFN-γ was administered through i.n. injection as previously described in Aspergillus infection. Pilot experiments were performed with 107, 108, 5 × 108 AdmIFN-γ given i.n. to mice. Mice were sacrificed 72 h later and IFN-γ was measured from bronchoalveolar lavage fluid (BALF). The amount of AdmIFN-γ chosen for subsequent studies was 5 × 108 PFU since this dose resulted in the highest amount of IFN-γ in the lung without causing systemic toxicity in the mice including lethargy, ruffled fur and loss of body weight (data not shown). To investigate the effect of IFN-γ pretreatment on early pathogen clearance and survival, mice were randomized to i.n. receive 5 × 108 PFU of either AdmIFN-γ or AdEGFP, diluted with phosphate-buffered saline (PBS) to a final volume of 30 µl, 24 h before challenge with Aspergillus. A separate group of mice receiving 30 µl of saline was set up as a control.
Lung harvest and lung myeloperoxidase (MPO) activity assay
At designated time points, lungs of mice were harvested as described by Mehrad [7]. After removal, whole lungs were homogenized in 1·0 ml of PBS with protease inhibitor (Boehringer Mannheim, Indianapolis, Ind, USA) using a tissue homogenizer (Biospec Products, Bartlesville, OK, USA) under a vented hood. Portions of homogenates (10 µl) were inoculated on buffered Sabouraud dextrose agar after serial 1 : 10 dilutions with PBS to determine the number of Colony-forming unit (CFU). The homogenates were also cultured on chocolate agar plates and blood agar plates to rule out superinfection. The remaining homogenates were incubated on ice for 30 min and then centrifuged at 1400 × g for 10 min. Supernatants were collected and stored at −20°C for assessment of cytokine levels. Lung MPO activity was measured as a marker of neutrophil sequestration, as described previously [15].
Collection of bronchoalveolar lavage fluid
For collection of bronchoalveolar lavage fluids, the trachea was exposed and intubated using a 1·7-mm-outer-diameter polyethylene catheter. Bronchoalveolar lavage was performed by instilling PBS containing 5 mm EDTA in 1 ml aliquots. The total volume of lavage was 3 ml per mouse to obtain cells or 1 ml for cytokine analysis. The BALF was centrifuged, and supernatants were removed and immediately stored at −20°C until analysed for cytokine contents.
Total lung leucocyte preparation
Lungs leucocytes were prepared as previously described [16]. Cytospin slides were prepared and stained with Diff-Quik (Dade Behring, Newark, DE, USA) for cell differential. In a subgroup of mice, cells were resuspended in RPMI (Life Technologies, Grand Island, NY, USA) containing 10% fetal calf serum (Gibco, Brl), 2-mercaptoethanol (50 mm), sodium pyruvate (1 mm), HEPES (10 mm), and gentamycin (50 mg/ml) and were plated at a concentration of 2 × 106/ml in 96-well microtitre plate after 48 h of culture, supernatants were removed, and cytokine production was determined by specific ELISAs.
Hyphal damage and alveolar macrophage microbicidal assay
A colourimetric MTT assay [17] was used to study Aspergillus hyphal damage by lung cells. Purified lung cells (106) were added to 105 conidia that had been cultured at 37°C in 5% CO2 with fetal calf serum for 16–18 h, by which time > 95% of the conidia had germinated to hyphae. Antifungal activity (hyphal damage) was calculated with the following formula:
where X is the optical density (OD) of test wells at 2 h and C is the OD of control wells containing hyphae only. Each condition was tested in duplicate or quadruplicate, and the results were averaged.
Alveolar macrophages were isolated from BAL as described above. Briefly, the BALF was spun down at 580 × g for 10 min, the cell pellet was resuspended in RPMI 1640 medium with 5% fetal bovine serum with antibiotics. The cell count was determined using a haemacytometer. Trypan blue staining revealed the cells to be > 95% viable. The cells were cultured for 2 h at 37°C in 5% CO2, nonadherent cells were washed off, and a suspension of Af was added at a multiplicity of infection (MOI) of 1. The cells were incubated with Af for 4 more hours at 37°C. CFUs were counted as described previously [17]. The percentage of CFU inhibition (mean ± SE) was determined as follows:
RNA preparation and reverse transcription–polymerase chain reaction
Reverse transcription PCR (RT-PCR) analysis were done as previously described [18]. The β-actin primers were used as controls for the PCR reactions (sense: 5'-CCA GCC ATG TAC GTT GCT ATC CAG-3' antisense: 5'-GGA ACC GCT CAT TGC CAA TGG TGA-3', giving a 378-bp product). After an initial incubation at 95°C for 2 min, temperature cycling was begun. The cycles consisted of 20 s of denaturation at 94°C, 30 s of primer annealing at 55°C, and 30 s of primer extension at 72°C for 30 cycles. As a control for calibration, an equivalent amount of input cDNA for β-actin was amplified, and all samples were normalized (if necessary) for β-actin cDNA content prior to analysis of cytokine cDNA. The PCR products were analysed by 1·5% agarose gel electrophoresis.
Cytokine assays
The levels of IL-12p70, IFN-γ and TNF-α in BAL fluids, lung homogenates or supernatants were determined by cytokine-specific ELISA kit (R & D Systems, Minneapolis, MN, USA), The sensitivity range for the cytokines was as follows: TNF-α, 15·625–1000 pg/ml; IFN-γ and IL-12, 16–1000 pg/ml.
Statistics
All data were expressed as means+-standard errors. Analysis was performed by SPSS statistical soft ware 10·0. Survival data were analysed by the χ2 test. Student's t-test was used to determine the statistical significance of other values between experimental groups (significance was defined as P < 0·05).
Results
Time- and dose-dependent production of IFN-γ in mice after i.n. AdmIFN-γ administration
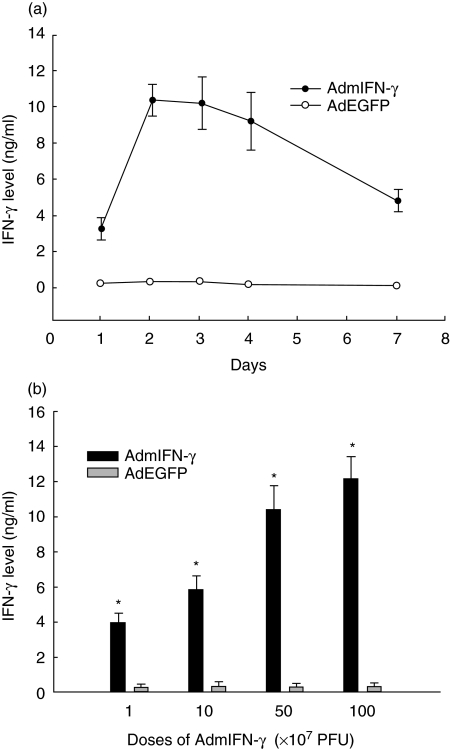
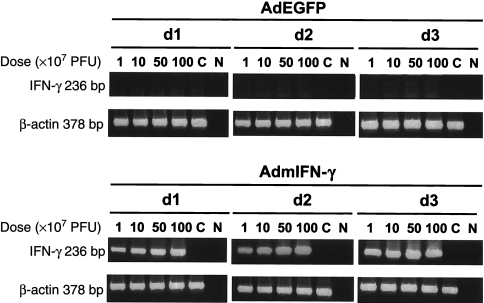
Mice were sacrificed at 24, 48, 72, and 96 h and at 7 days after intratracheal injections of 5 × 108 CFU/mouse of AdmIFN-γ, AdEGFP and an equal volume of saline, and IFN-γ was measured in BALF. A substantial expression of IFN-γ in a time-dependent model after i.n. administration of AdmIFN-γ was detected. IFN-γ levels reached the peak at 48 h after AdmIFN-γ administration with a concentration of 10·37 ng/ml in BALF (Fig. 1a). In contrast, mice treated with AdEGFP or saline had hardly detected levels of IFN-γ in the lung at any time point. In line with the levels in BALF, IFN-γ mRNA was expressed within the lung after i.n. administration of AdmIFN-γ. About a maximal expression of IFN-γ were observed at day 3. In contrast, no IFN-γ mRNA expression was detected in the lungs at any time point after i.n. administration of AdEGFP or saline (Fig. 2). We then administered increasing doses of AdmIFN-γ, which resulted in a significant dose-dependent induction of IFN-γ (Figs 1b and 2). But systemic toxicity (lethargy, ruffled fur and body weight reduction) was observed when the dosage was equal to or larger than 109 PFU. However, levels of IFN-γ in BALF were very low in mice with different doses of AdEGFP. Thus, these data indicate that the i.n. administration of AdmIFN-γ results in a significant induction in the expression of IFN-γ in the lung which is both time and dose dependent.
Fig. 1.
(a) Pharmacokinetics of IFN-γ. BALB/c mice received 5 × 108 PFU/mouse of AdmIFN-γ (•), AdEGFP (○), or an equal volume of saline intratracheally (n = 5). Mice were sacrificed at specific time points and IFN-γ was measured in BALF by ELISA. No IFN-γ was detectable in AdEGFP or saline-treated mice. (b) Dose-dependent IFN-γ expression after i.n. administration of increasing doses of AdmIFN-γ (▪). Equal doses of AdEGFP (□) were used as controls. Two days later, mice were sacrificed for determination of cytokine levels in BALF. The experiment was performed twice (n = 5). *P < 0·01 compared with respective controls.
Fig. 2.
RT-PCR analysis of mRNA expression of β-actin and of cytokine IFN-γ in BALB/c mice treated with AdmIFN-γ. The mice received different PFU/mouse of AdmIFN-γ intranasally and were sacrificed at day 1, day 2, day 3, Lungs were excised and processed for evaluation of cytokine gene expression by RT-PCR. Equal doses of AdEGFP were used as controls. Lanes C, control mRNA from three uninfected wild-type mice; Lanes N, no DNA was added to the amplification mixture during PCR.
Local production of IFN-γ in response to intrapulmonary Aspergillus infection
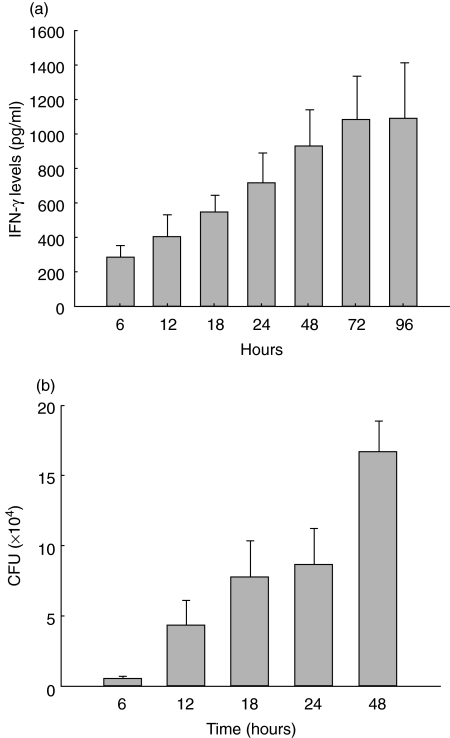
As shown in Fig. 3, the i.n. administration of Af (5 × 107 CFU) resulted in the expression of IFN-γ in the lung. IFN-γ was detectable in BALF 6 h after Aspergillus challenge and was maintained at a high level of about 952·64 pg/ml 48 h and 1095·71 pg/ml 96 h after Aspergillus challenge (Fig. 3a). The IFN-γ level correlated with the pathogen burden closely (Fig. 3b) in this animal model. Despite significant elevated levels of IFN-γ in BALF, IFN-γ was hardly detectable in serum at any time studied after fungal challenge. Moreover, IFN-γ was not detected in BALF or serum in mice inoculated with saline. We did not report IFN-γ data after 48 h because mortality in this model (survival curve shown in Fig. 4) would bias the results to surviving animals.
Fig. 3.
Local production of IFN-γ in BALF (a) and contents of Aspergillus fumigatus in mouse lung homogenates (b) in response to pulmonary Aspergillus challenge. Mice were challenged with 5 × 107 CFU of Aspergillus fumigatus and IFN-γ in BALF was measured at serial time points by ELISA. At the same time, contents of Aspergillus in lung homogenates were measured. The experiment was performed three times (n = 5 animals at 6 h, 12 h, 18 h, 24 h, 48 h and n = 4 animals at 72 h, n = 3 animals at 96 h).
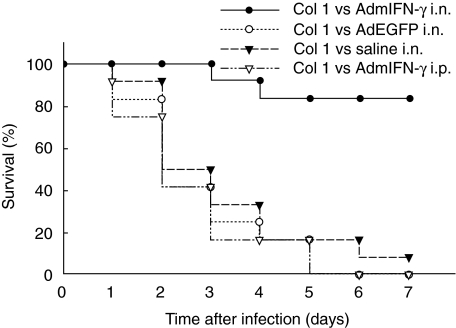
Fig. 4.
Effect of i.n. administration of AdmIFN-γ on mice survival. Animals were treated with AdmIFN-γ (•), AdEGFP (○), saline (▾) i.n. or AdmIFN-γ (▿) i.p. (all adenovirus doses 5 × 108 PFU). 24 h later, Af was i.n. administrated to the animals and survival rate was evaluated over time (n = 12 for all group animals).
Effect of intrapulmonary versus systemic adenoviral IFN-γ gene therapy on the course of IPA
In the study, most of the mice receiving AdEGFP or saline died within 96 h following intrapulmonary challenge with Af (Fig. 4). However, Mice with intranasal administration of AdmIFN-γ demonstrated prolonged survival period, and had a more than threefold higher survival rate than the controls at day 5 (Fig. 4). These data indicated that host pulmonary defences against the pathogen were substantially improved in mice with intrapulmonary IFN-γ transgene expression. In contrast, no significant difference in survival rate was observed in animals receiving AdmIFN-γ i.p. as compared with animals receiving AdEGFP or saline alone (Fig. 4).
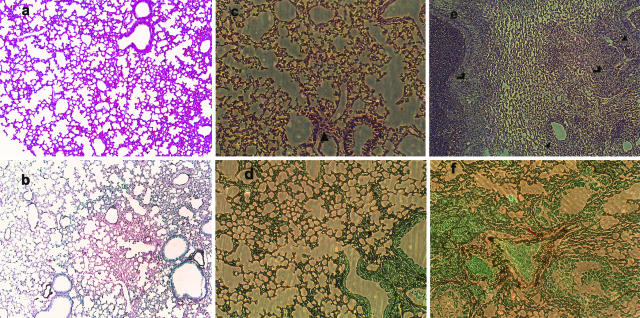
In the lung histopathology, the lung sections from the mice receiving AdEGFP (Fig. 5b) revealed patterns of lesions similar to those observed in saline treated mice (Fig. 5d), characterized by signs of bronchial wall damage, peribronchial necrosis, and the presence of various inflammatory cells (predominantly polymorphonuclear) within both the interstitial and alveolar compartments. Conidia were seen in mice on day 1 and 2, but large numbers of hyphae were noted at day 3 and 5 after inoculation, which indicated the development of IPA. In contrast, these features were not observed in mice with i.n. administration of AdmIFN-γ at day 3, whose lung were characterized by reduced inflammatory cell infiltration, and no evidence of parenchymal destruction or fungal growth (Fig. 5a,c).
Fig. 5.
Representative photomicrographs of haematoxylin and eosin (a, c, e)- and Gomori methanamine silver (GMS) (b, d, f)-stained whole lung sections from BALB/c mice. These mice were untreated (a, b) or treated with AdmIFN-γ (c, d), AdEGFP (e) and saline (f). The mice were i.n. challenged by Aspergillus conidia after being immunosuppressed with cyclophosphamide 4 days before the infection. Lungs were taken 3 days after infection (a, b magnification, × 100. c, d, e, f magnification, × 100). (c, d) few inflammatory cells (▴) infiltrate into peribronchial areas, corresponding to site of conidial deposition. Hyphal forms were not present. In contrast, the sections from the lungs of AdEGFP-treated mice (e) revealed patterns of lesions similar to those observed in saline-treated mice (f), characterized by signs of bronchial wall damage, peribronchial necrosis ( ), and inflammatory cellular infiltrate (▴) peribronchially in areas in which branching hyphae (
), and inflammatory cellular infiltrate (▴) peribronchially in areas in which branching hyphae ( ) were present.
) were present.
Effect of AdmIFN-γ on lung leucocyte influx in mice with IPA
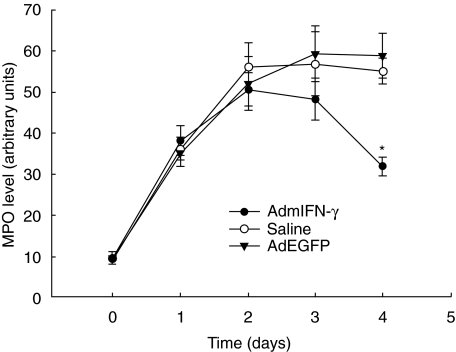
As shown in Table 1, we observed that challenge with Af resulted in a substantial increase in leucocytes in the lungs over the baseline, particularly neutrophils. At day 2 postinfectious challenge, when IFN-γ expression reached its peak in AdmIFN-γ-treated animals, AdmIFN-γ-treated animals did not display significant differences in the total number of neutrophils or mononuclear cells in lung compared to control animals. However, at day 3 there was a high reduction in total leucocytes and polymorphonuclear neutrophil (PMN) in AdmIFN-γ-treated animals campared with the controls. The time course of neutrophil influx into the lungs was determined by measuring lung MPO content (Fig. 6). AdmIFN-γ-treated mice had maximal lung MPO levels 2 days after Af challenge and returned to the baseline of cell count on day 3–4. However, higher MPO levels were sustained until 4 days after Af challenge in mice treated with AdEGFP or saline. These findings were in agreement with the cell counts and differentials in lung homogenates.
Table 1. Cell counts and differentials in lung homogenates after Af challenge in i.n. AdmIFN-γ-treated animals.
| No. (105) of cells/lung | ||||
|---|---|---|---|---|
| Day | Drug given | Total cells | No. of PMN | No. of mononuclear cells |
| Untreated | 1·89 ± 0·31 | 0·72 ± 0·61 | 1·17 ± 0·28 | |
| 2 | Saline | 14·51 ± 2·49 | 11·73 ± 2·06 | 2·78 ± 0·73 |
| AdEGFP | 12·19 ± 2·21 | 9·87 ± 2·09 | 2·32 ± 0·52 | |
| AdmIFN-γ | 12·84 ± 2·23 | 9·86 ± 2·01 | 2·98 ± 0·75 | |
| 3 | Saline | 15·31 ± 2·73 | 12·59 ± 2·62 | 2·72 ± 0·79 |
| AdEGFP | 14·81 ± 2·57 | 11·64 ± 2·33 | 3·17 ± 0·52 | |
| AdmIFN-γ | 8·72 ± 2·04* | 5·66 ± 1·94* | 3·06 ± 0·82 | |
Mice were treated with AdmIFN-γ, AdEGFP, saline 24 h before administration of Af i.n. At day 2 and 3 after Af treatment, mice were sacrificed for lung homogenate. (n = 5 animals per group).
P < 0·01 compared with mice treated with saline and AdEGFP at day 3, respectively.
Fig. 6.
Lung MPO activity. Whole lung MPO activity was measured at various time points after Af challenge in mice treated with AdmIFN-γ (•), saline (○) or AdEGFP (▾). (n = 5 for each group). *P < 0·01 as compared with animals receiving i.n. saline or AdEGFP.
Effect of AdmIFN-γ on intrapulmonary cytokine levels
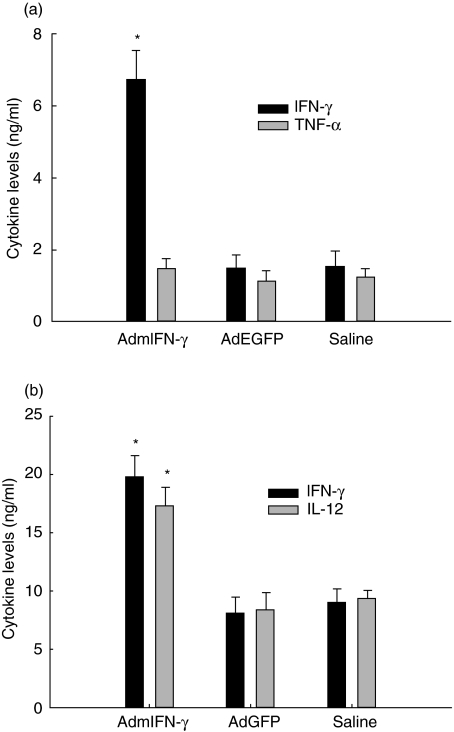
As expected, IFN-γ levels in lung homogenates from AdmIFN-γ-treated mice infected with Af were significantly higher than those from animals treated with AdEGFP or saline (about fourfold increase on day 2; P < 0·01) (Fig. 7a) at all time points studied. These animals had a lower Aspergillus burden in the lungs. Interestingly, a higher level of IL-12 was also detected in the AdmIFN-γ-treated animals compared to the controls (Fig. 7b). Along with this, lung cells from AdmIFN-γ-treated mice produced nearly double the levels of IFN-γ compared to the controls. However, no obvious differences in levels of TNF-α were noted in AdmIFN-γ-treated animals. Based upon these results, we postulated that the induction of IFN-γ and IL-12 may contribute to the beneficial effects of AdmIFN-γ.
Fig. 7.
Cytokine production in lungs of AdmIFN-γ-treated mice. Mice were treated with AdmIFN-γ, AdEGFP, saline 24 h before administration of Af i.n. On day 2 after Af treatment, mice were sacrificed for lung homogenate and lung cells. Lung cells were simply cultured in RPMI for 48 h. (a) IFN-γ (▪) and TNF-α (□) levels in lung homogenates and (b) IFN-γ (▪) and IL-12 (□) levels in culture supernatants of lung cells were measured by ELISA. The experiment was performed three times (n = 5 animals per time point). *P < 0·01 compared with the same cytokine levels in mice treated with saline and AdEGFP, respectively.
Effect of AdmIFN-γ administration on antifungal activity of phagocytic cellsex vivo
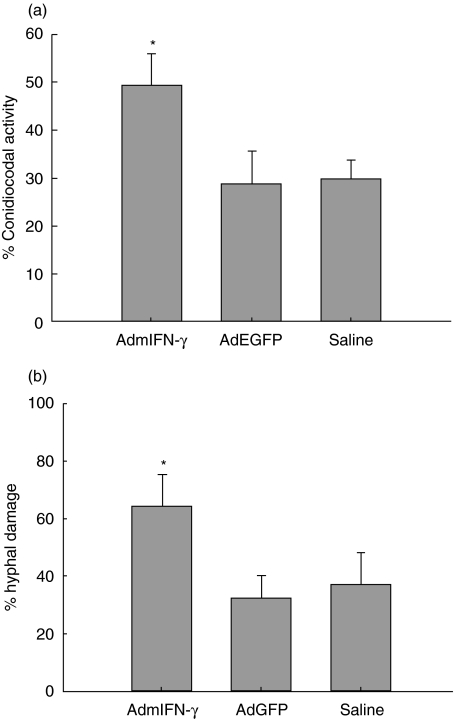
In the study, it was found that both the conidiocidal activity of macrophages and the hyphal damage by lung cells were up-regulated in AdmIFN-γ treated mice, respectively, compared with the control group (Fig. 8). This suggests that intrapulmonary adenoviral IFN-γ gene therapy can enhance the host defence against Aspergillus infection which may act through up-regulation of the ability of phagocytic cells to kill Aspergillus.
Fig. 8.
Conidiocidial activity of alveolar macrophages (a) and hyphal damage by lung cells (b) in mice treated with AdmIFN-γ. Mice were sacrificed at days 2 following i.n. challenge with adenovirus vector. Ability of alveolar macrophages from BALF to kill conidia and of total lung cells to damage hyphae was assessed (see Material and Methods). *P < 0·01 compared with mice treated with saline and AdEGFP, respectively.
Discussion
Given a poor outcome of IPA with current therapy, the precise mechanism of the immune response to Af is of interest, since immunomodulation may be beneficial as adjunctive therapy. With the recent advances in ‘genomics’ and ‘proteomics’, gene therapy is likely to become a reality within the next decades [19]. Currently, there are two main categories of gene therapy vehicles: nonviral and viral. Viral vectors have often been the first choice in gene-transfer studies because of the inherent capacity of certain viruses to enter the cell, translocate into the nucleus, and efficiently express their genes, properties that were acquired through millions of years of evolution and selective pressures. However, there still remains concern over the safety of viral vectors [20]. Cytokine/chemokine gene therapy is a promising approach for inducing efficient immune responses against infectious diseases [10,21,22]. IFN-γ is important in innate host immunity, although there are some detrimental effects if produced in large quantities systemically. IFN-γ administration has a curative effect in Aspergillus infection [23]. Compartmentalized expression of IFN-γ at the site of infection represents an attractive approach to immunotherapy. Previous studies have demonstrated that adenovirus vectors are well suited for gene transfer to lung cells and have been informatively used for experimental study in tumour and infectious models [24–26]. Adenoviral gene transfer has advantages over the use of purified recombinant cytokines in that the transgene can be expressed in a dose-dependent manner in a specific tissue for a limited period of time.
In the present study we used a low dose of the adenoviral construct delivered via the airway to target lung cells and to a certain extent alveolar macrophages [26], since these cell types are probably major targets for Aspergillus infection in vivo. Indeed, mice with IPA administrated AdmIFN-γ i.n. had significantly increased survival rate compared with that of control mice. Moreover, when AdmIFN-γ was given i.p., there was no benefit to the increase of survival rate in mice challenged with Af, which suggests that local, compartmentalized IFN-γ treatment is essential. The lack of benefit with systemic AdmIFN-γ may be partially explained by the inability to achieve appreciable expression of IFN-γ in the lung after i.p. AdmIFN-γ administration, although there was abundant blood levels of IFN-γ after i.p. AdmIFN-γ administration [27]. This observation is similar to a previous report by Su et al. [28]. Thus, cytokine gene transfer can result in compartmentalized effects that can augment host defence and lower the risk of systemic toxicity.
Innate immunity against bacterial pathogens of the lung requires the activation and recruitment of phagocytic cells [29]. Mechanism for increased pathogen clearance from the lung in animals treated with AdmIFN-γ does not appear to be due to the recruitment of leucocytes to the lung. For after administration of AdmIFN-γ, the total cells in the lung tended to decrease together with the pathogen reduction. There was a gradual return to baseline on neutrophil influx into the AdmIFN-γ-treated mice lungs over days 3–4 postinfection according to MPO levels. This was also confirmed histologically. At day 3 after Af treatment, it appears that there was less inflammatory cell infiltration in AdmIFN-γ-treated mice and no fungal growth. This suggested that neutrophil recruitment was resulting from the Af challenge rather than IFN-γ overexpression. Our results also suggest that the enhanced hyphal damage by lung cells contribute to the clearance of the Af. Furthermore, our data indicated that intrapulmonary IFN-γ expression in vivo enhanced the ability of alveolar macrophages to kill Aspergillus conidia when cultured ex vivo. This is supported by previously published findings that recombinant IFN-γ activates alveolar macrophages and other monocytes to limit the growth of intracellular Af in vitro[30]. In vivo, the increased expression of IFN-γ has been associated with protective effects in animal models of aspergillosis [31,32].
The importance of T1-phenotype cytokines, such as IL-12 and TNF-α, in Af and other intracellular infections has previously been demonstrated [33–35]. These cytokines are key modulators of intrapulmonary production of IFN-γ and underscore the significance of a T1-type host response towards promoting bacterial clearance. We found that cytokine measurements in lung homogenates of AdmIFN-γ-treated mice demonstrated significant increases in IFN-γ. AdmIFN-γ treatment resulted in time and dose-dependent increases in IFN-γ in BALF in mice which correlates provisionally with Aspergillus burden. The lung cells cultured ex vivo also produced higher quantities of IFN-γ after in vivo AdmIFN-γ treatment. In addition to IFN-γ, a significant increase in the production of IL-12 in the lung was also detected.
In summary, our studies demonstrate that compartmentalized transgenic IFN-γ expression through adenovirus can augment host lung defence, resulting in improved survival rate, Aspergillus clearance, and production of IL-12 in mice with IPA. These data suggest that in an immunocompromised host, gene therapy may be a useful adjunct to host defence against pathogens.
Acknowledgments
This research was supported by the Shanghai Health Bureau (Bai Ren Project, Grant 98BR030).
References
- 1.Cenci E, Mencacci A, Bacci A, Bistoni F, Kurup VP, Romani L. T cell vaccination in mice with invasive pulmonary aspergillosis. Immunol. 2000;165:381–8. doi: 10.4049/jimmunol.165.1.381. [DOI] [PubMed] [Google Scholar]
- 2.Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–805. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 3.Cenci E, Mencacci A, Casagrande A, Mosci P, Bistoni F, Romani L. Impaired antifungal effector activity but not inflammatory cell recruitment in interleukin-6-deficient mice with invasive pulmonary aspergillosis. J Infect Dis. 2001;184:610–7. doi: 10.1086/322793. [DOI] [PubMed] [Google Scholar]
- 4.Cenci E, Mencacci A, Fe d'Ostiani C, Del Sero G, Mosci P, Montagnoli C, Bacci A, Romani L. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J Infect Dis. 1998;178:1750–60. doi: 10.1086/314493. [DOI] [PubMed] [Google Scholar]
- 5.Del Sero G, Mencacci A, Cenci E, et al. Antifungal type 1 responses are upregulated in IL-10-deficient mice. Microbes Infect. 1999;1:1169–80. doi: 10.1016/s1286-4579(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 6.Cenci E, Mencacci A, Del Sero G, et al. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J Infect Dis. 1999;180:1957–68. doi: 10.1086/315142. [DOI] [PubMed] [Google Scholar]
- 7.Mehrad B, Strieter RM, Standiford TJ. Role of TNF-α in pulmonary host defense in murine invasive aspergillosis. J Immunol. 1999;162:1633–40. [PubMed] [Google Scholar]
- 8.Pahl HL, Krauss B, Schultze-Osthoff K, et al. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-κB. J Exp Med. 1996;183:1829–40. doi: 10.1084/jem.183.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolls JK, Habetz S, Shean MK, et al. IFN-gamma and CD8+ T cells restore host defenses against Pneumocystis carinii in mice depleted of CD4+ T cells. J Immunol. 1999;162:2890–4. [PubMed] [Google Scholar]
- 10.Deng JC, Tateda K, Zeng X, Standiford TJ. Transient overexpression of gamma interferon promotes Legionella pneumophila clearance in immunocompetent hosts. Infect Immun. 2001;69:6382–90. doi: 10.1128/IAI.69.10.6382-6390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson S, Summer WR. Innate immunity, cytokines, and pulmonary host defense. Infect Dis Clin North Am. 1998;12:555–67. doi: 10.1016/s0891-5520(05)70198-7. [DOI] [PubMed] [Google Scholar]
- 12.Wolach B, Alon E, Gottesman G, Yellin A. Pulmonary aspergillosis in a child with hyperimmunoglobulin E syndrome. J Infect Dis. 1998;26:204–5. doi: 10.1086/516254. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Mi J, Yu Y, Yao H, Chen H, Li M, Cao X. IFN-γ gene therapy by intrasplenic hepatocyte transplantation: a novel strategy for reversing hepatic fibrosis in Schistosoma japonicum-infected mice. Parasite Immunol. 2001;23:11–7. doi: 10.1046/j.1365-3024.2001.00349.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZL, Zou WG, Luo CX, Li BH, Wang JH, Sun LY, Qian QJ, Liu XY. An armed oncolytic adenovirus system, ZD55-gene, demonstrating potent antitumoral efficacy. Cell Res. 2003;13:481–9. doi: 10.1038/sj.cr.7290191. [DOI] [PubMed] [Google Scholar]
- 15.Mehrad B, Strieter RM, Moore TA, Tsai WC, Lira SA, Standiford TJ. CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J Immunol. 1999;163:6086–94. [PubMed] [Google Scholar]
- 16.Moore TA, Moore BB, Newstead MW, Standiford TJ. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol. 2000;165:2643–50. doi: 10.4049/jimmunol.165.5.2643. [DOI] [PubMed] [Google Scholar]
- 17.Roilides E, Dimitriadou-Georgiadou A, Sein T, Kadiltsoglou I, Walsh TJ. Tumor necrosis factor alpha enhances antifungal activities of polymorphonuclear and mononuclear phagocytes against Aspergillus fumigatus. Infect Immun. 1998;66:5999–6003. doi: 10.1128/iai.66.12.5999-6003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sisto F, Miluzio A, Leopardi O, Mirra M, Boelaert JR, Taramelli D. Differential cytokine pattern in the spleens and livers of BALB/c mice infected with Penicillium marneffei: protective role of gamma interferon. Infect Immun. 2003;71:465–73. doi: 10.1128/IAI.71.1.465-473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mountain A. Gene therapy: the first decade. Trends Biotechnol. 2000;18:119–28. doi: 10.1016/s0167-7799(99)01416-x. [DOI] [PubMed] [Google Scholar]
- 20.Alesci S, Ramsey WJ, Bornstein SR, Chrousos GP, Hornsby PJ, Benvenga S, Trimarchi F, Ehrhart-Bornstein M. Adenoviral vectors can impair adrenocortical steroidogenesis: clinical implications for natural infections and gene therapy. Proc Natl Acad Sci USA. 2002;99:7484–9. doi: 10.1073/pnas.062170099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehrad B, Wiekowski M, Morrison BE, Chen SC, Coronel EC, Manfra DJ, Lira SA. Transient lung-specific expression of the chemokine KC improves outcome in invasive aspergillosis. Am J Respir Crit Care Med. 2002;166:1263–8. doi: 10.1164/rccm.200204-367OC. [DOI] [PubMed] [Google Scholar]
- 22.Zeng X, Moore TA, Newstead MW, Hernandez-Alcoceba R, Tsai WC, Standiford TJ. Intrapulmonary expression of macrophage inflammatory protein 1alpha (CCL3) induces neutrophil and NK cell accumulation and stimulates innate immunity in murine bacterial pneumonia. Infect Immun. 2003;71:1306–15. doi: 10.1128/IAI.71.3.1306-1315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolls JKYeP, Shellito JE. Gene therapy to modify pulmonary host defenses. Semin Respir Infect. 2001;16:18–26. doi: 10.1053/srin.2001.22725. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Snider DP, Hewlett BR, Lukacs NW, Gauldie J, Liang H, Xing Z. Overexpression of granulocyte-macrophage colony-stimulating factor induces the differentiation and activation of a novel dendritic cell population in the lung. Blood. 2000;95:2337–45. [PubMed] [Google Scholar]
- 25.Matsukawa A, Lukacs NW, Standiford TJ, Chensue SW, Kunkel SL. Adenoviral-mediated overexpression of monocyte chemoattractant protein-1 differentially alters the development of Th1 and Th2 type responses in vivo. J Immunol. 2000;164:1699–704. doi: 10.4049/jimmunol.164.4.1699. [DOI] [PubMed] [Google Scholar]
- 26.Lei XF, Ohkawara Y, Stampfli MR, Gauldie J, Croitoru K, Jordana M, Xing Z. Compartmentalized transgene expression of granulocyte-macrophage colony-stimulating factor (GM-CSF) in mouse lung enhances allergic airways inflammation. Clin Exp Immunol. 1998;113:157–65. doi: 10.1046/j.1365-2249.1998.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen GH, Reddy RC, Newstead MW, Tateda K, Kyasapura BL, Standiford TJ. Intrapulmonary TNF gene therapy reverses sepsis-induced suppression of lung antibacterial host defense. J Immunol. 2000;165:6496–503. doi: 10.4049/jimmunol.165.11.6496. [DOI] [PubMed] [Google Scholar]
- 28.Su H, Messer R, Whitmire W, Fischer E, Portis JC, Caldwell HD. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J Exp Med. 1998;188:809–18. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P, Summer WR, Bagby GJ. Innate immunity and pulmonary host defense. Immunol Rev. 2000;173:39–51. doi: 10.1034/j.1600-065x.2000.917306.x. [DOI] [PubMed] [Google Scholar]
- 30.Duong M, Ouellet N, Simard M, Bergeron Y, Olivier M, Bergeron MG. Kinetic study of host defense and inflammatory response to Aspergillus fumigatus in steroid-induced immunosuppressed mice. J Infect Dis. 1998;178:1472–82. doi: 10.1086/314425. [DOI] [PubMed] [Google Scholar]
- 31.Nagai H, Guo J, Choi H. Interferon-ã and tumor necrosis factor-α protect mice from invasive aspergillosis. J Infect Dis. 1995;103:1554–60. doi: 10.1093/infdis/172.6.1554. [DOI] [PubMed] [Google Scholar]
- 32.Bozic CR, Kolakowski LF, Gerard NP, et al. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154:6048–57. [PubMed] [Google Scholar]
- 33.Brieland JK, Jackson C, Hurst S, et al. Immunomodulatory role of endogenous interleukin-18 in gamma interferon-mediated resolution of replicative Legionella pneumophila lung infection. Infect Immun. 2000;68:6567–73. doi: 10.1128/iai.68.12.6567-6573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai G, Kastelein R, Hunter CA. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infect Immun. 2000;68:6932–8. doi: 10.1128/iai.68.12.6932-6938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahuja SS, Reddick RL, Sato N, et al. Dendritic cell (DC)-based anti-infective strategies: DCs engineered to secrete IL-12 are a potent vaccine in a murine model of an intracellular infection. J Immunol. 1999;163:3890–7. [PubMed] [Google Scholar]