Abstract
Ocular chlamydial disease is clinically diagnosed by the appearance of characteristic inflammatory changes and development of lymphoid follicles in the conjunctiva. Nucleic acid amplification tests and relatively non-invasive methods of sampling the conjunctival surface can be used to quantify the expression of chlamydial and host genes. Using quantitative real-time polymerase chain reaction to detect the presence of Chlamydia trachomatis (CT) 16S rRNA and human interleukin (IL)-1β, IL-10, IL-12p40, interferon (IFN)-γ and tumour necrosis factor (TNF)-α transcripts we examined the immune response at the conjunctival surface in a cohort of children living in a trachoma-endemic village in The Gambia. Elevated cytokine transcript levels were associated with the presence of CT 16S rRNA. Subclinical infection (CT infection without clinical signs of disease) elicited an immune response that is proinflammatory in nature, with elevations in the transcription of IL-1β, IFN-γ and IL-12p40. Clinically apparent infections were associated with the elevation of mRNA for the multi-functional cytokine TNF-α (fibrotic, type 1 inflammatory and regulatory) and the counter regulatory cytokine, IL-10, in addition to the other proinflammatory cytokines. A positive correlation between IFN-γ transcript levels and the amount of CT 16S rRNA expressed in conjunctiva was found.
Keywords: chlamydia, conjunctiva, cytokine, immunity, trachoma
Introduction
Ocular Chlamydia trachomatis infection often results in the inflammation of the conjunctiva as in follicular trachoma (TF) and intense inflammatory trachoma (TI). The World Health Organization estimates that 500 million people (almost all in developing countries) are at risk of trachoma, with about 7 million already blind as a result [1]. Active trachoma is found mainly in young children, with the highest prevalence in children under 5 years (15–20% in Gambian children) [2]. The adverse sequelae that characterize trachoma; trichiasis (in-turning of the eyelashes against the cornea), entropion (in-turning of the eyelid margin) and corneal opacity, manifest in adult life in only a small proportion of those who experience active trachoma as children. In addition the prevalence of active trachoma and of ocular C. trachomatis infection in endemic areas decreases with age [3], as does the duration of disease episodes [4], suggesting the development of a degree of protective immunity to ocular C. trachomatis infection.
Studies of the immune response to chlamydiae have linked much of the pathology and sequelae of infection back to the immune response. The processes that lead to disease and its sequelae are not fully understood, but are thought to be due to dysregulation of the immune response which normally resolves infection and controls tissue fibrosis. Alternative views which obviate the requirement or contribution of the adaptive immune response in these processes in favour of local epithelial cell responses have been put forward [5]. However, probably both the adaptive immune response and the conjunctival epithelial cell response contribute to the homeostatic balance between proinflammatory and anti-inflammatory responses in ocular tissue following chlamydial disease.
We conducted a longitudinal study of ocular C. trachomatis infection in which children living in a trachoma-endemic area were examined every 2 weeks over a 6-month period. A limited group of cytokines representative of the pro- and anti-inflammatory network were selected (the roles of these cytokines in relation to chlamydial diseases have been reviewed by others [6–8]) and the expression of these cytokines in the conjunctiva was investigated using quantitative real-time reverse transcriptase-polymerase chain reaction (PCR). The level of expression of human cytokine mRNAs was correlated to the load of ‘metabolically active’ bacteria at the conjunctival surface and to the development of disease.
Materials and methods
Ethical approval
The study and its procedures were approved by the Gambia Government/MRC joint Ethics Committee and by the Ethics Committee of LSHTM.
Recruitment and follow-up
School children in Sandali Primary School, Western Division, The Gambia were examined for active trachoma. A subset of children including some with active trachoma and some with normal conjunctivae were recruited and visited approximately every 2 weeks (10–19-day intervals) for a period of 24 weeks. At the end of the study each member of the household in which a diseased subject was resident were treated by administration of 20 mg/kg oral azithromycin.
At recruitment and at each subsequent visit, subjects were examined by a trained fieldworker, and a clinical diagnosis made according to the WHO Simplified Trachoma Grading system [9]. Digital photographs of each eyelid were taken and anaesthetic eye drops (Proxymetacaine 0·5%, Minims®, Chauvin Pharmaceuticals, Romford, UK) administered. A sterile polyester-tipped swab (Dacron®, Hardwood Products Company, Guildford, ME, USA) was used to obtain cellular material from the upper tarsal conjunctiva of the right eye, by rubbing the swab horizontally four times against the surface of the conjunctiva, turning the swab through 90° each time. The swab was collected into RNAlater (Ambion Europe Ltd, Huntingdon, UK), stored on ice and subsequently at −20°C.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
Swabs collected into RNAlater were extracted using the Qiagen RNeasy Kit, according to the manufacturer's protocol, with the following modification: swabs were transferred to a 2-ml screw-cap tube containing Qiagen RNeasy sample lysis buffer (RLT), vortexed vigorously and the swab discarded. DNAse I digestion was carried out during the extraction process to remove contaminating DNA.
In vitro experiments using DNA and RNA derived from cultured C. trachomatis demonstrated repeatedly that a single DNase I digestion reduced 1 × 107 copies to undetectable levels, provided that Qiagen RNeasy columns were not overloaded with > 10 µg total nucleic acid. Conjunctival epithelial swabs typically yield approximately 1–2 µg nucleic acid (data not shown). Initial experiments demonstrated no carry-through genomic DNA contamination when primer pairs that amplified genomic DNA were used.
The RNA was eluted into 50 µl of AE buffer and stored at −20°C. Twenty-five µl of extracted RNA were reverse-transcribed using an oligo-dT15 primer and the Omniscript® reverse transcription system according to the manufacturer's protocol and the complementary DNA (cDNA) stored at −20°C. The efficiency of DNase I digestion could not be checked formally by the inclusion of a reverse-transcriptase negative control reaction for each sample due to limitations in the quantity of RNA available for subsequent assays.
Quantification of chlamydial 16S rRNA
Three µl of RNA extracted from the ocular swabs were reverse transcribed and amplified in duplicate by PCR using primers specific for chlamydial 16S ribosomal RNA cDNA and the Quantitect™ SYBR® Green I one-step RT-PCR kit (Qiagen Ltd, Crawley, UK), described previously [10]. The amount of CT 16S rRNA per sample was estimated from standard curves generated with each qRT-PCR assay.
Quantification of human mRNA
Total RNA was first reverse-transcribed using the Omniscript RT kit (Qiagen) and 3 µl of the cDNA obtained amplified in duplicate by real-time PCR using intron-spanning primers specific for the cytokine transcripts [interleukin (IL)-1β, IL-10, IL-12p40, tumour necrosis factor (TNF)-α and interferon (IFN)-γ] and human hypoxanthine guanine phosphoribosyl transferase transcript (HPRT), to preclude amplification of genomic DNA. Primer sequences and reaction conditions are given elsewhere [10]. The copy number of HPRT and cytokines per sample were estimated from standard curves included in each assay [10]. Studies using HPRT as a housekeeping gene have shown no variation in the expression of HPRT with infection or disease and thus would not affect the relative expression of cytokine mRNA [10].
Statistical analysis
For the construction of disease duration data, concordant observations of disease/infection status at 2-weekly follow-up were taken to imply the state was persistent between the observations. Where a single observation was missing between concordant observations continuity was assumed, and where a missing observation intervened between a change of state, the state was assumed to change midway between the observations that were actually made. In analysis, these assumptions are used to estimate duration data. For preliminary analysis of episodes of disease and infection in relation to cytokine response, it is assumed that the observations in an individual during follow-up are independent of each other. Individual observations are grouped into four disease/infection states: normal (no disease or infection), infection without clinical signs (CT 16S rRNA detected but no clinical signs of active trachoma), clinical signs (active trachoma disease signs but no infection) and clinical signs with infection (active trachoma disease signs and CT 16S rRNA).
Cytokine mRNA expression was normalized against the expression of HPRT. For comparisons of the cytokine gene expression in the four disease/infection states, the follow-up time was divided according to phenotype (based on the presence of clinical signs of disease and the detection of CT 16S rRNA) and the geometric mean cytokine expression for each state calculated. As not all individuals could contribute to each of the four categories, unpaired t-test on cytokine mRNA expression levels normalized by log transformation was used to make comparisons between the categories. Spearman's rank correlation test was used to determine correlations between cytokine expression, duration of infection and chlamydial load in infection.
Study data were managed using Microsoft Access and Excel packages and statistical analyses carried out using Stata 7.
Results
Completeness of follow-up
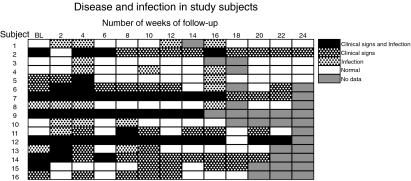
Sixteen children were recruited into the study, eight female and eight male, with a median age of 9 years (6–14 years). The median duration of follow-up was 24 weeks (4–24 weeks). Six children were absent on one or more visits; median number of missed visits 1 (one to four visits) and as such could not be examined or sampled at that visit. One child was lost to follow-up at the very end of the study (Fig. 1).
Fig. 1.
Clinical signs of disease (follicular trachoma and/or intense inflammatory trachoma) and infection (detection of Chlamydia trachomatis 16S rRNA by polymerase chain reaction) at each follow-up visit. An episode is assumed to continue if there is no change in the infection and/or disease state between visits. BL, baseline.
Clinical signs of trachoma
At the time of recruitment, nine of the children showed signs of clinical disease, five with follicular trachoma (TF) (subjects 7, 12, 14, 15 and 16) and four with both TF and intense inflammatory trachoma (TI) (subjects 2, 5, 6 and 9) (Fig. 1). In five children, disease persisted throughout the follow-up period, with three children showing evidence of both severe and mild conjunctival inflammation (TI and TF) at different time-points (subjects 2, 7, 9, 12 and 14) (Fig. 1). Five children were disease-free throughout the period (subjects 1, 3, 4, 8 and 10) while six children were able to resolve disease during follow-up (subjects 5, 6, 11, 13, 15 and 16) (Fig. 1). There were 20 episodes of disease observed during follow-up, with a median of one episode/child (0–3 episodes/child) (Fig. 1). The median duration of an episode of disease was 28 days (13·5–193 days). In the 10 episodes in which both acquisition and resolution of disease could be observed, the median duration of disease was 17·5 days (14–28 days).
Ocular C. trachomatis infection
Chlamydial infection is defined as the detection of one or more copies of chlamydial 16S rRNA per reaction in a quantitative RT-PCR assay, which is equivalent to an estimated 16 or more copies per swab. Eight of the 16 children were infected at baseline (subjects 2, 7, 8, 9, 12, 13, 14 and 15) (Fig. 1). In contrast to the observations of clinical signs, infection was detected in all but one child (subject 16), and no infections persisted for the entire study period (Fig. 1). During follow-up, 27 separate episodes of ocular infection (CT 16S rRNA PCR positive) were observed in these 16 children, with a median duration of 20·5 days (range 13·5–130 days) and a median of 2 episodes per child (0–3 episodes/child). Both acquisition and resolution of infection could be observed in 20 of these episodes of infection, and in these infections the median duration was 14 days (range 13–41·5 days). Eight of these 27 infections were of ‘long’ duration, i.e. > 4 weeks.
Clinical signs of trachoma and infection
At baseline, infection and the presence of clinical signs were concurrent in six children (subjects 2, 7, 9, 12, 14 and 15); one child resolved both infection and disease (subject 15) and three children resolved and reacquired infection (subjects 2, 12 and 14) (Fig. 1). In five of these six children clinical signs persisted for the duration of follow-up, and in four the concurrent infection was of ‘long’ duration, > 4 weeks (subjects 2, 7, 9 and 12) (Fig. 1). There was no association between number of infections and the duration of a disease episode (Mann–Whitney, P < 0·20).
Six children experienced infections that did not precede or coincide with the presence of clinical signs, suggesting that a proportion of infections are subclinical (subjects 1, 3, 4, 6, 8 and 10) (Fig. 1). These clinically inapparent infections tended to be shorter in duration than those in which clinical signs of disease coincident with infection became apparent; however, this difference in duration did not attain statistical significance (Fig. 1). Four children experienced disease episodes that were not preceded by or coincident with a detectable infection (subjects 6, 11, 15 and 16) (Fig. 1).
Chlamydial load in infection
The distribution of the chlamydial load in infection was very highly skewed to the right (GM 16S rRNA = 641 copies, range: 16·39–901 441 copies). The majority of infections had relatively low levels of CT 16S rRNA copies, and only nine of all detected ocular infections had more than 1000 copies of CT 16S rRNA. There was a significant correlation between the ocular chlamydial load in infection and the duration of the infection (Spearman's R = 0·50, P = 0·0076). In those disease episodes with concurrent chlamydial infection, there was also a correlation between the CT 16SrRNA load and the duration of the disease episode (Spearman's R = 0·71, P = 0·022).
Cytokine gene expression in infection and disease
Cytokine gene expression during periods of infection (CT 16S rRNA detected but no clinical signs), clinical signs (clinically active trachoma but no detectable CT 16S rRNA) and clinical signs with infection (clinically active trachoma plus detectable infection) were compared to periods when subjects had no disease or infection (normal conjunctiva).
All cytokine transcripts that were examined in this study (IL-1β, IL-10, IL-12p40, IFN-γ and TNF-α) were detectable in the conjunctiva in periods where no disease or infection was evident. However, significant up-regulation of the different cytokines was observed during periods of disease (TF and/or TI), infection (CT 16S rRNA) or infection with disease (both TF/TI and CT 16S rRNA).
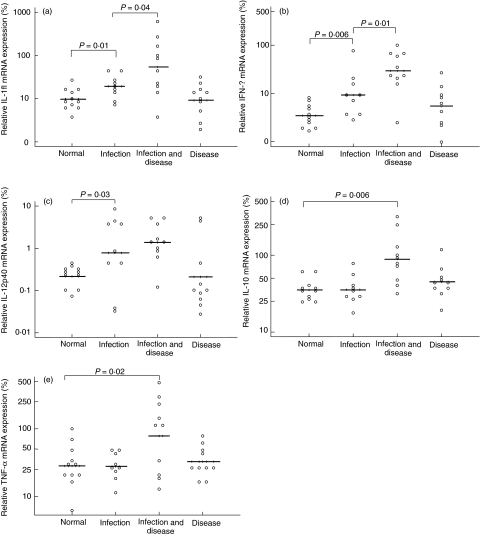
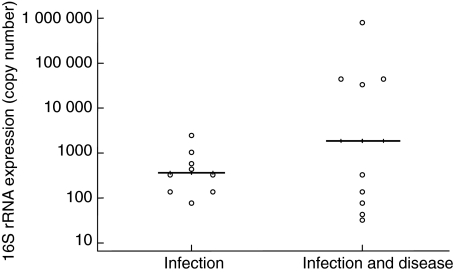
The expression of proinflammatory cytokine mRNAs, IFN-γ, IL-1β and IL-12p40 was found to be significantly up-regulated when subjects were infected with C. trachomatis, but had no clinical signs of active trachoma compared to periods when they were not infected (Fig. 2a–2c). When the presence of C. trachomatis coincided with the presence of clinical signs, the gene expression of all the cytokines assayed was significantly up-regulated when compared to disease and infection free periods. The expression of IFN-γ and IL-1β remained higher than in periods of subclinical infection (Fig. 2a–2e). There was a trend towards an increase in the CT 16S rRNA load of the infection in periods when clinical signs (TF/TI) were present compared to periods when they were absent; however, this did not attain statistical significance (Fig. 3). When subjects were diseased but not infected, none of the cytokines assayed were up-regulated compared to when they were ‘normal’ (Fig. 2a–2e).
Fig. 2.
Conjunctival cytokine mRNA expression is elevated in the presence of infection. The follow-up time of the subject is categorized according to the infection and disease status. Each circle represents the geometric mean expression for the period of follow-up time that the disease status of the individual falls into one of the four categories. Solid lines indicate the geometric mean expression of cytokine mRNA for each category.
Fig. 3.
Conjunctival Chlamydia trachomatis (CT) 16S rRNA expression in subclinical and clinical infections. Each circle represents the geometric mean CT 16S rRNA copy number in periods of infection without active trachoma (subclinical) and in periods of infection with active trachoma (clinical) for a given individual. Solid lines indicate the geometric mean CT 16S rRNA copy number for each type of infection.
Cytokine mRNA expression, the chlamydial load and duration of infection
The correlation between the level of cytokine mRNA expression and the amount of C. trachomatis present during infection (CT 16S rRNA load) and the persistence of C. trachomatis (duration of CT 16S rRNA positivity) was examined. Only IFN-γ was significantly correlated both with the amount of C. trachomatis present and with the duration of infection (Table 1). Interestingly, with the exception of IL-12p40 mRNA, the expression of IL-1β, IL-10, TNF-α and IFN-γ mRNAs were all associated with duration of infection (Table 1).
Table 1.
Correlation between cytokine mRNA expression in infection, the duration of infection and the chlamydial load in individual infection episodes. Number of comparisons = 10.
| Duration of infection | Chlamydial load in infection | |||
|---|---|---|---|---|
| Cytokine | Spearman's Rho | P-value | Spearman's Rho | P-value |
| TNF-α | 0·48 | 0·0120 | 0·32 | 0·108 |
| IFN-γ | 0·63 | 0·0005 | 0·52 | 0·0054 |
| IL-1β | 0·37 | 0·0154 | 0·26 | 0·188 |
| IL-10 | 0·48 | 0·0109 | 0·33 | 0·090 |
| IL-12p40 | 0·25 | 0·2026 | 0·12 | 0·552 |
Discussion
In this study, each child had, on average, one episode of ocular infection every 12 weeks where infection was apparent by CT 16S rRNA. These episodes of infection were of relatively short duration, analogous to that described by Bailey et al. in The Gambia [4]. In the study by Bobo et al. in Tanzania, a higher proportion of children (21/35) experienced ‘persistent’ infection (duration in excess of 6 weeks) [11]. This difference in duration between the two study sites may reflect a difference in prevalence of disease and by inference the rate of transmission of infection.
A third of children in this study were unable to resolve disease over the 6-month observation period, and their clinical episodes were characterized by long periods of coincident infection with high CT 16S rRNA loads, rather than several short infections. These subjects are likely to be reservoirs of infection. In contrast, in a few children high load infections were transient. These children may represent a group in whom a rapid response against ocular chlamydial infection is well developed and chlamydial replication is efficiently controlled.
The data suggest further that in periods of clinically inapparent infection an inflammatory response remains predominant (up-regulation of IL-1β, IFN-γ and IL-12p40) and when the specific signs of active disease are visible the degree of inflammation is further increased (further up-regulation of IL-1β and IFN-γ).
Interestingly, there was no up-regulation of any of the cytokines assayed in association with clinical signs (in the absence detectable CT 16S RNA). This is in contrast to a study in a Tanzanian trachoma endemic population which found lower levels in uninfected subjects compared to those who were infected [12]. Previously in a cross-sectional study we found elevated mRNA levels of TNF-α, IL-1β and IL-10 in individuals with clinically active trachoma in whom CT 16S rRNA could not be detected [10]. As latter studies were cross-sectional, it is plausible that the resolution of infection may have occurred sufficiently close to the time of sampling to indicate a difference in cytokine profile when compared to clinically normal individuals.
Proinflammatory cytokines are important in the control of chlamydial infection, as early expression of IL-1β can promote the development of a Th1 response. In a murine model of chlamydial genital infection, the rapid production of TNF-α and IL-1β produced by macrophages is associated with earlier clearance of infection [13]. Immunohistological studies have shown a marked increase in the expression of IL-1α and IL-1β by conjunctival epithelial cells of individuals with trachoma, compared to controls [14]. These studies support our finding of an up-regulation of IL-1β in subclinical and clinical chlamydial infections and suggest that this cytokine plays an important role in disease outcome.
IL-12 induces the synthesis of IFN-γ and TNF-α and acts to stimulate Th1 proliferation and differentiation of activated CD8+ T cells into cytotoxic T lymphocytes. In murine models, IL-12 administration has been shown to confer resistance to naïve mice against challenge with C. trachomatis, which is paralleled by IFN-γ secretion in vivo[15]. The role of IFN-γ in the control of murine chlamydial infections has been well established [16]. Evidence also shows that in secondary infection in mice depleted of CD4+ and CD8+ T cells, other cells produce IFN-γ, and this is sufficient to protect mice [17]. In the conjunctiva, IL-12p40 expression did not correlate with duration of infection, suggesting that its expression is uncoupled from the other proinflammatory cytokines, e.g. IFN-γ.
One of the mechanisms by which IFN-γ mediates the inhibition chlamydial replication in epithelial cells is by up-regulation of indoleamine-2,3-dioxygenase (IDO); IDO is an enzyme that catalyses the conversion of l-tryptophan (Trp) to N-formylkynurenine, thereby limiting the availability of Trp, which is an essential amino acid for C. trachomatis[18]. Depletion of Trp by IDO in APC also impacts on the immune response by modulating the stimulatory behaviour of these cells [19,20]. However, ocular isolates of C. trachomatis lack a functional Trp synthase gene [21] and in vitro studies demonstrate that ocular strains of C. trachomatis are sensitive to IFN-γ-mediated inhibition. It is a paradox, then, that data from cross-sectional studies in trachoma-endemic populations and these data show a positive correlation between the levels of IFN-γ and the amount and persistence (duration of infection) of C. trachomatis in the conjunctiva. Thus, although ocular strains have a non-functional tryptophan synthase enzyme, they may possess an alternative means of withstanding IFN-γ-mediated limitations in tryptophan bioavailability.
When clinical signs of active trachoma (TI/TF) coincide with detectable levels of viable C. trachomatis, proinflammatory cytokine expression is enhanced further along with expression of IL-10 and TNF-α. The importance of IL-10 as a regulatory cytokine in chlamydial infection has long been known [22,23] and the recent expansion of research on T regulatory cells (TR) has established its position as a key cytokine of some TR[24]. The elevation of IL-10 at the conjunctival surface may be a response to the high levels of proinflammatory cytokine expression in periods of clinically apparent infection. Although the associated inflammation may be required to resolve infection, the extent of this response must regulated in order to limit pathology. The specific phenotype of cells responsible for the production of conjunctival IL-10 remains unknown in chlamydial infection.
The elevated expression of TNF-α in periods of clinically apparent infection may be part of the counter-regulatory mechanism [25] or may be important in the maintenance of inflammation and the later development of fibrosis [26,27]. Elevated levels of TNF-α in tear fluid [10,12,28] and polymorphisms in the promoter region of the TNF-α gene are associated with trachomatous scarring [28].
Previous work by Bailey et al. [4], using a less sensitive method of chlamydial detection, chlamydial antigen detection, suggested that the median duration of disease and infection was much shorter in adults than children. This study suggests that in some children the duration of infection is as short as that of more ‘antigen-experienced’ adults possibly due to high exposure.
The method of conjunctival sampling used in this study precludes the identification of the cellular sources of cytokines. Histopathological and impression cytology studies have demonstrated the presence of inflammatory cells (dendritic cells, Langerhans cells and IEL) in the human conjunctiva [29,30]. The cytokines assayed in this study are expressed by these cells, thus it is highly likely that some of these immune cells are collected in the sampling process.
This study highlights the importance of observation of the immune response in clinically inapparent chlamydial infections. At that time it is arguably the best example of effective protection against C. trachomatis and defining its characteristics may contribute to the elucidation of immune responses that vaccines should aim to elicit.
References
- 1.Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull WHO. 1987;65:477–83. [PMC free article] [PubMed] [Google Scholar]
- 2.Faal H, Minassian D, Sowa S, Foster A. National survey of blindness and low vision in The Gambia: results. Br J Ophthalmol. 1989;73:82–7. doi: 10.1136/bjo.73.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mabey DC, Robertson JN, Ward ME. Detection of Chlamydia trachomatis by enzyme immunoassay in patients with trachoma. Lancet. 1987;2:1491–2. doi: 10.1016/s0140-6736(87)92623-7. [DOI] [PubMed] [Google Scholar]
- 4.Bailey R, Duong T, Carpenter R, Whittle H, Mabey D. The duration of human ocular Chlamydia trachomatis infection is age dependent. Epidemiol Infect. 1999;123:479–86. doi: 10.1017/s0950268899003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephens RS. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 2003;11:44–51. doi: 10.1016/s0966-842x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 6.Kelly KA. Cellular immunity and Chlamydia genital infection: induction, recruitment, and effector mechanisms. Int Rev Immunol. 2003;22:3–41. doi: 10.1080/08830180305229. [DOI] [PubMed] [Google Scholar]
- 7.Yang X. Role of cytokines in Chlamydia trachomatis protective immunity and immunopathology. Curr Pharm Des. 2003;9:67–73. doi: 10.2174/1381612033392486. [DOI] [PubMed] [Google Scholar]
- 8.Rottenberg ME, Gigliotti-Rothfuchs A, Wigzell H. The role of IFN-gamma in the outcome of chlamydial infection. Curr Opin Immunol. 2002;14:444–51. doi: 10.1016/s0952-7915(02)00361-8. [DOI] [PubMed] [Google Scholar]
- 9.Dawson CR, Jones BR, Tarizzo ML. Guide to trachoma control in programs for the prevention of blindness. Geneva: World Health Organization; 1981. p. 56. [Google Scholar]
- 10.Burton MJ, Bailey RL, Jeffries D, Mabey DC, Holland MJ. Cytokine and fibrogenic gene expression in the conjunctivas of subjects from a Gambian community where trachoma is endemic. Infect Immun. 2004;72:7352–6. doi: 10.1128/IAI.72.12.7352-7356.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bobo LD, Novak N, Munoz B, Hsieh YH, Quinn TC, West S. Severe disease in children with trachoma is associated with persistent Chlamydia trachomatis infection. J Infect Dis. 1997;176:1524–30. doi: 10.1086/514151. [DOI] [PubMed] [Google Scholar]
- 12.Bobo L, Novak N, Mkocha H, Vitale S, West S, Quinn TC. Evidence for a predominant proinflammatory conjunctival cytokine response in individuals with trachoma. Infect Immun. 1996;64:3273–9. doi: 10.1128/iai.64.8.3273-3279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darville T, Andrews CW, Jr, Sikes JD, Fraley PL, Rank RG. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect Immun. 2001;69:3556–61. doi: 10.1128/IAI.69.6.3556-3561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu el-Asrar AM, Geboes K, Tabbara KF, al-Kharashi SA, Missotten L, Desmet V. Immunopathogenesis of conjunctival scarring in trachoma. Eye. 1998;12:453–60. doi: 10.1038/eye.1998.104. [DOI] [PubMed] [Google Scholar]
- 15.Lu H, Zhong G. Interleukin-12 production is required for chlamydial antigen-pulsed dendritic cells to induce protection against live Chlamydia trachomatis infection. Infect Immun. 1999;67:1763–9. doi: 10.1128/iai.67.4.1763-1769.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lampe MF, Wilson CB, Bevan MJ, Starnbach MN. Gamma interferon production by cytotoxic T lymphocytes is required for resolution of Chlamydia trachomatis infection. Infect Immun. 1998;66:5457–61. doi: 10.1128/iai.66.11.5457-5461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison SG, Morrison RP. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect Immun. 2001;69:2643–9. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beatty WL, Belanger TA, Desai AA, Morrison RP, Byrne GI. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62:3705–11. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellor AL, Chandler P, Baban B, et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16:1391–401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 20.Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell HD, Wood H, Crane D, et al. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. J Clin Invest. 2003;111:1757–69. doi: 10.1172/JCI17993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Gartner J, Zhu L, Wang S, Brunham RC. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection. J Immunol. 1999;162:1010–7. [PubMed] [Google Scholar]
- 23.Yang X, HayGlass KT, Brunham RC. Genetically determined differences in IL-10 and IFN-gamma responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J Immunol. 1996;156:4338–44. [PubMed] [Google Scholar]
- 24.Mittrucker HW, Kaufmann SH. Mini-review: regulatory T cells and infection: suppression revisited. Eur J Immunol. 2004;34:306–12. doi: 10.1002/eji.200324578. [DOI] [PubMed] [Google Scholar]
- 25.Zganiacz A, Santosuosso M, Wang J, et al. TNF-alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J Clin Invest. 2004;113:401–13. doi: 10.1172/JCI18991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou DH, Lee W, McCulloch CA. TNF-alpha inactivation of collagen receptors: implications for fibroblast function and fibrosis. J Immunol. 1996;156:4354–62. [PubMed] [Google Scholar]
- 27.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive Type 1 and Type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–16. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 28.Conway DJ, Holland MJ, Bailey RL, et al. Scarring trachoma is associated with polymorphism in the tumor necrosis factor alpha (TNF-alpha) gene promoter and with elevated TNF-alpha levels in tear fluid. Infect Immun. 1997;65:1003–6. doi: 10.1128/iai.65.3.1003-1006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knop N, Knop E. Conjunctiva-associated lymphoid tissue in the human eye. Invest Ophthalmol Vis Sci. 2000;41:1270–9. [PubMed] [Google Scholar]
- 30.Brignole-Baudouin F, Ott AC, Warnet JM, Baudouin C. Flow cytometry in conjunctival impression cytology: a new tool for exploring ocular surface pathologies. Exp Eye Res. 2004;78:473–81. doi: 10.1016/j.exer.2003.08.005. [DOI] [PubMed] [Google Scholar]