Abstract
Aspergillus fumigatus (Af) is a fungus associated with allergic bronchopulmonary aspergillosis (ABPA) and other allergic diseases. Immune responses in these diseases are due to T and B cell responses. T cell activation requires both Af-specific engagement of the T-cell-receptor as well as interaction of antigen independent costimulatory molecules including CD28-CD80/CD86 and OX40–OX40L interactions. Since these molecules and their interactions have been suggested to have a potential involvement in the pathogenesis of ABPA, we have investigated their role in a model of experimental allergic aspergillosis. BALB/c mice were primed and sensitized with Af allergens, with or without exogenous IL-4. Results showed up-regulation of both CD86 and CD80 molecules on lung B cells from Af-sensitized mice (79% CD86+ and 24% CD80+) and Af/rIL-4-treated mice (90% CD86+ and 24% CD80+) compared to normal controls (36% and 17%, respectively). Lung macrophages in Af-sensitized mice treated or not with IL-4 showed enhanced expression of these molecules. OX40L expression was also up-regulated on lung B cells and macrophages from both Af-sensitized and Af/rIL-4 exposed mice as compared to normal controls. All Af-sensitized animals showed peripheral blood eosinophilia, enhanced total serum IgE and allergen-specific IgG1 antibodies and characteristic lung inflammation. The up-regulation of CD80, CD86 and OX40L molecules on lung B cells and macrophages from Af-allergen exposed mice suggests a major role for these molecules in the amplification and persistence of immunological and inflammatory responses in ABPA.
Keywords: Aspergillus induced allergy, T cells, cell surface molecules, Th1/Th2 cells, murine model
Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is a hypersensitivity lung disease caused by the ubiquitous fungus Aspergillus fumigatus (Af) [1]. ABPA develops from inhalation of Aspergillus allergens present in the environment or from the limited growth of Aspergillus in the bronchi and is characterized by eosinophilia, fleeting pulmonary infiltrates, central bronchiectasis, elevated serum IgE, and Aspergillus-specific IgG and IgE [2]. The lung injury associated with ABPA is considered to be the result of IgE mast cell degranulation. The pathogenesis of ABPA, although not fully known, is thought to be due to CD4+ T-helper 2 (Th2) cells and is mediated by the production of IL-4, IL-5, IL-10 and IL-13 [1–3].
Optimal T cell activation requires antigen-specific engagement of the T cell receptor and additional antigen–independent costimulatory molecule interactions [4,5]. Interaction between CD28 and its ligands CD80 and CD86 has been found to be a dominant costimulatory pathway in allergy [6–12]. B7-2 (CD86) molecules are constitutively expressed on dendritic cells (DC) and are rapidly induced on B cells following activation. B7-1 (CD80) molecules are also constitutively expressed on antigen presenting cells (APC) including DCs and macrophages, but at lower levels than CD86, and following activation CD80 expression is up-regulated by cytokines [13]. Differentiation of Th1/Th2 cells, expansion of committed T cells, and the migration of T cells into target tissues are some of the mechanisms resulting from the CD28/B7-mediated T cell costimulation in asthma [4]. During sensitization of mice with ovalbumin treatment with CTLA-4-Ig or anti-B7 antibody inhibited airway hyperresponsiveness, inflammatory cell infiltration, expansion of lymphocytes and allergen-specific responsiveness of pulmonary T cells [14,15].
The OX40 (CD134)-OX40L costimulatory pathway also has been shown to have an important role in allergic inflammation [16–19]. OX40 is present on activated T cells and binds to OX40L on activated B cells, DC and other APC. OX40 is expressed on memory CD4 T cells, controlling the pivotal memory Th2 cells that regulate lung inflammation [20]. Blocking OX40/OX40L interactions by the administration of neutralizing anti-OX40L mAb during allergen sensitization abolishes the induction of allergic responses, indicating a critical role for these interactions in the early phase of the immune response [21]. Ablation of OX40 or its ligand, OX40L, has been shown to diminish lung inflammation, reduce eosinophilia and mucus production, and significantly attenuate airway hyperreactivity [22,23]. Recently, it was shown that the cytokine IL-4 specifically down regulates the expression of OX40L on DC, B and T cells, and thereby influences the Th1/Th2 ratio and resulting immune response [24].
In this study, we investigated whether during Af sensitization the addition of exogenous IL-4 to the lung microenvironment, could influence the expression of costimulatory molecules and subsequent inflammatory and immunological responses using a previously developed murine model of ABPA [25,26]. We demonstrated that costimulatory molecules play a significant role in the onset, persistence and progression of immune responses in the induced murine disease. Our results indicate that both B7 (CD80/CD86) and OX40L costimulatory molecules are up-regulated in response to A. fumigatus allergens. Additionally, rIL-4 administration during sensitization further enhances the allergic response. We believe that these findings have significant implications for developing control measures to prevent progression of the disease.
Materials and methods
Animals
Six–8-week-old female BALB/c (H-2d) mice were purchased from Charles River Laboratories (Wilmington, MA, USA). The mice were housed in the Veterinary Medical Unit at the Veterans Administration Medical Center, Milwaukee, WI, USA. All procedures were conducted according to the protocol approved by the Institutional Animal Care and Use Committee.
Allergens
A mixture of soluble A. fumigatus (Af) antigens released in broth during culture was combined with an extract from whole mycelium grown in aerated cultures for 96 h [26]. Recombinant proteins of Af (Asp f2, f3, f4 and f6) were expressed using procedures previously described [27]. All antigens were quality controlled for their reactivity and immunochemical characteristics as previously described [28,29].
Sensitization protocol
Mice were injected intraperitoneally (i.p) once per week for two weeks with 100 µg of Af antigens in PBS. Thereafter, the animals were challenged with 50 µg of Af antigen intranasally (i.n) twice per week for four consecutive weeks (Group 2). Mice were anaesthetized by Halothane inhalation (Halocarbon Laboratory, NJ, USA) and 50 µl of Af antigen in PBS was applied to both nostrils [26]. Some of the antigen-exposed animals were also treated with recombinant IL-4 (rIL-4) (Pharmingen, 100 ng per mouse/dose, Group 3). Finally the mice were challenged i.p and i.n. with either Af or Af/rIL-4, 24 hours before euthanasia for final evaluation. Control groups of mice received only IL-4 (Group 4) or PBS (Group 1).
Eosinophils in peripheral blood
Animals were bled from the tail vein and eosinophils were enumerated on a haemacytometer after staining with Eosin Y as previously described [26].
Serum antibody detection by ELISA
Total serum IgE was measured by ELISA using a rat anti-mouse IgE monoclonal antibody as described previously [30]. Quantification was carried out by converting the optical density (O.D) values to nanograms using a standard curve. Af specific IgG1 and IgG2a antibodies in serum samples were determined by ELISA using biotinylated goat anti-mouse IgG (Sigma, St Louis, MO, USA) or rabbit anti-mouse IgG1 and IgG2a subclasses (Zymed, San Francisco, CA, USA) as described previously [27]. A cut off titre was determined by studying large numbers of normal sera (mean +3 SD). Determination of Af specific antibodies was carried out using antibody titres calculated as the reciprocal of the last dilution that gave an OD490 above the cut off value [31].
Antigen specific T cell proliferation
Spleen cells (1 × 105/well) were cultured for 7 days in 200 µl/well of complete RPMI medium (RPMI-1640 containing glutamine, sodium pyruvate, penicillin-streptomycin and 10% heat inactivated fetal bovine serum (FBS); (Invitrogen, Carlsbad, CA, USA) with Af antigens or with a mixture of Af recombinant proteins (Asp f2, f3, f4 and f6) at different concentrations. Cell proliferation was assessed by [3H] thymidine (Amersham Biosciences, Piscataway, NJ, USA) uptake during the final 8 h of culture by adding 1 µCi of[3H] thymidine to each well. Incorporated radioactivity was measured on a liquid scintillation counter (Packard Instrument Co., Inc., Meriden, CT, USA). The stimulation index (SI) was calculated as: [3H] thymidine uptake in the presence of Af antigen/[3H] thymidine uptake by unstimulated cells. The results were expressed as the mean ± SEM [32,33].
Cytokine production in vitro by antigen-stimulated spleen cells
Spleen cells were cultured in 1 ml of complete RPMI medium at a concentration of 107/ml for 60 h with Af antigens (5 µg/ml) or a mixture of recombinant Af proteins (Asp f2, f3, f4 and f6, 5 µg/ml each). Cell-free culture supernatants were collected and assayed for the presence of cytokines (IFN-γ, IL-4, IL-5 and IL-10) by ELISA according to the manufacturer's (Pharmingen, San Diego, CA, USA) instructions. Briefly, anti-IFN-γ (R4–6A2), anti-IL-4 (11B11), anti-IL-5 (TRFK5) and anti-IL-10 (JES5–2A5) monoclonal antibodies were used to coat the ELISA plates. Culture supernatants were added to respective wells and incubated overnight at 4 °C. Biotinylated rat-antimouse IFN-γ (XMG1·2), IL-4 (BVD6–24G2), IL-5 (TRFK4) and IL-10 (SXC-1) were used as detection antibodies. HRP-Streptavidin (Sigma, St Louis, MO, USA) at a dilution of 1 : 4000 was added followed by OPD (ortho-phenylenediamine, Sigma) substrate. OD values were read at 490 nm using an automatic ELISA reader (Bio-Tek Instruments, Inc, Winooski, VT, USA) [34]. The concentration (pg/ml) of each cytokine was determined by standard curves. Antigen-specific cytokine secretion was then calculated by subtracting the cytokine content of the supernatants from supernatants of spleen cells incubated with RPMI alone [30,31].
Histology
Lungs removed from euthanized mice were insufflated with 10% buffered formalin, fixed in formalin solution and processed. Section were cut at 6 micron of thickness and stained with Hematoxylin and Eosin (HE) or Periodic Acid Schiff (PAS) stains. Lung sections were evaluated microscopically for morphological changes according to published criteria [34]. Morphological changes including perivascular inflammatory cell infiltration of eosinophils, lymphocytes, macrophages, neutrophils and plasma cells, bronchial epithelial hyperplasia, presence of goblet cells, cellular infiltration, alveolar septal thickening and interstitial oedema were assessed.
Flow cytometric analysis
Lungs were removed from euthanized mice 24 h after the last antigen challenge. The lungs were cut into small pieces, enzymatically digested with 120 µg/ml of Dispase (Invitrogen) and 120 µg/ml of collagenase type IA (Sigma) and incubated with shaking at 37 °C for 1 h. The tissue was gently homogenized in a tissue grinder. Homogenates were washed several times, cell numbers determined and the pooled lung cells were incubated with anti-CD80-PE, anti-CD86-PE or anti-OX40L-PE (eBioscience, San Diego, CA, USA), and anti-B220-FITC or anti-MAC (CD11b)-FITC (BD Pharmingen) monoclonal antibodies [35]. All stained samples were analysed on a flow cytometric cell sorter (FACSCalibur; Becton Dickinson, Mountain View, CA, USA). Pooled cells of each group were analysed and the values presented as a percentage of positive cells (macrophages or B cells) expressing CD86, CD80 or OX40L costimulatory molecules. The representative histograms and the percent positive cells are based on electronically gated B cell (B220 +) and macrophage (Mac-1 +) populations.
Statistical analysis
Serum antibody levels, peripheral blood eosinophils, cytokines and antigen-induced proliferation values were compared by the Student ‘t’ test with unequal variance. The results are expressed as means and ± SEM for each group of animals tested. For all statistical comparisons P < 0·05 was considered as significant.
Results
Peripheral blood eosinophilia in animals sensitized with Af and Af/rIL-4
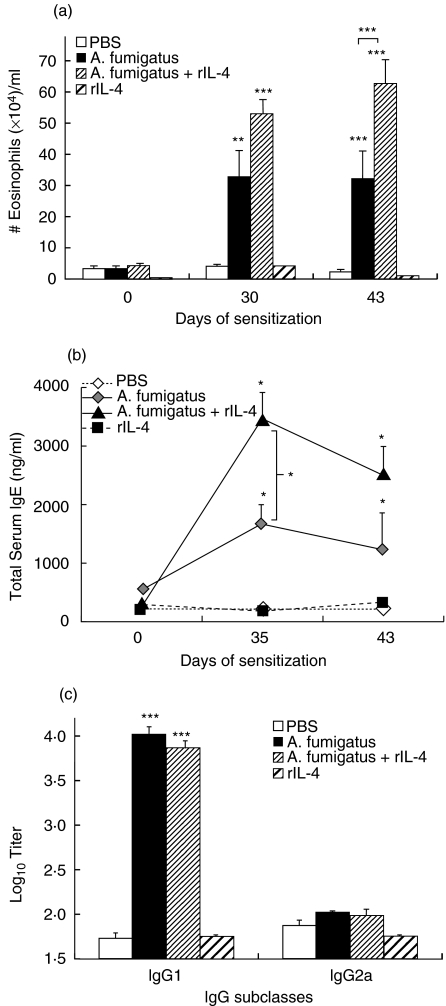
Mice sensitized via i.p. injection with Af allergen alone or Af allergen/rIL-4 demonstrated marked peripheral blood eosinophilia after intranasal challenge (Group 2, Af alone: 32·3 × 104 ± 4·5 × 104 and Group 3, Af/rIL-4: 62·9 × 104 ± 8 × 104) as compared to the PBS controls (Group 1: 2·2 × 104 ± 0·7 × 104) (P < 0·0001) (Fig. 1a). Af/rIL-4 treated animals (Group 3) had the highest number of eosinophils, which were significantly elevated on day 43 as compared to Af-sensitized mice (P < 0·0001). Mice treated with rIL-4 alone failed to show any difference from the PBS control mice (Group 4: 1·1 × 104 ± 0·4 × 104).
Fig. 1.
Peripheral blood eosinophils and total serum IgE and Af-IgG antibodies levels detected in Af treated and control mice. (a) Eosinophils in the peripheral blood of mice treated with Af antigens, Af/rIL-4, rIL-4 alone or PBS (normal controls) before treatment (day 0), on day 30 after initial treatment and after the last Af antigen challenge (day 43). (b) Total serum IgE responses in the sera of mice before treatment (day 0), during treatment (day 35) and after last Af antigen challenge (day 43). (c) Aspergillus-specific IgG1 and IgG2a on day 43 post challenge. IgG values are presented as Log10 titre. All pretreatment sera had < 1·6 Log10 titre. The data is presented as mean + SEM, and P-values are designated as *P < 0·05, **P < 0·001 and ***P < 0·0001 versus the PBS controls. Five to 10 animals from each group were analysed. Data are representative of two identical experiments.
Elevated levels of total IgE and Af-IgG antibodies were detected in the sera of Af sensitized mice
Af-exposed mice had significantly elevated total serum IgE levels on day 35 after challenge as compared to normal PBS treated controls (1668 ng/ml ± 334 versus 221 ng/ml ± 17) (P < 0·05) (Fig. 1B), while Af/rIL-4 treated mice demonstrated a further increase (3462 ng/ml ± 453) (P < 0·001). Af/rIL-4 treated animals had significantly higher levels of total serum IgE as compared to mice sensitized only with Af (P < 0·05). Recombinant IL-4 treated mice without Af had low levels of total serum IgE (185 ng/ml ± 72). Higher titres of Af-specific IgG1 antibody were present in the sera of mice sensitized with Af (Group 2: 4·01 ± 0·1 Log10 titre) as compared to PBS control animals (Group 1: 1·74 ± 0·03 Log10 titre) (P < 0·0001) (Fig. 1c). Similar high values were also detected in sera of mice coinjected with Af/rIL-4 (Group 3: 3·85 ± 0·1 Log10 titre). However, Af/rIL-4 treated animals failed to show any difference in Af-specific IgG1 levels compared to Af-sensitized mice. Serum from mice injected with rIL-4 did not contain Af-specific serum IgG1 antibodies (Group 4: 1·76 ± 0·02 Log10 titre). The Af treated groups of mice did not have significant levels of serum Af-specific IgG2a antibody (Fig. 1c). The results thus demonstrate significant increase in Af-specific IgG1 levels but not Af-specific IgG2a in the sera of Af-treated mice with or without rIL-4. Neither dilution of the mouse serum nor adsorption of IgG1 using rat anti-mouse IgG1 antibody (data not shown) made any difference in the IgG2a titres.
Enhanced antigen-specific lymphocyte proliferation of spleen cells from mice sensitized with Af allergens
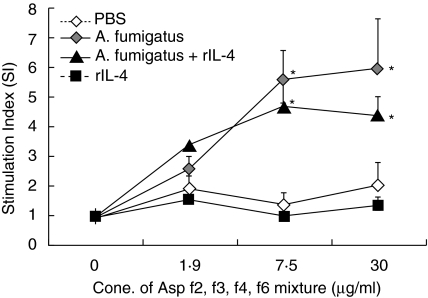
A mixture of recombinant Af proteins (Asp f2, f3, f4 and f6) was used to stimulate spleen cells from mice sensitized with Af allergens or exposed to Af/rIL-4 (Fig. 2). Both sensitized groups showed significantly enhanced cell proliferation, measured as stimulation index (SI) compared to PBS control animals (Group 2: 5·98 ± 1·5, Group 3: 4·43 ± 0·52 versus Group 1: 2·04 ± 0·67) (P < 0·05). The SI from Af/rIL-4 treated mice was not significantly different from the Af-sensitized group at any of the antigen concentrations studied. Spleen cells from IL-4 treated mice did not show any Af-specific proliferative response (Group 4: 1·31 ± 0·33).
Fig. 2.
Proliferative responses of spleen cells from Af sensitized and nonsensitized mice to A. fumigatus recombinant proteins, Asp f2, f3, f4 and f6. SI values are shown as means ± SEMs. The P-values less than <0·05 of sensitized versus PBS control mice were considered to be significant (*). Five animals from each group were studied. Data are representative of two identical experiments.
Elevated cytokine production by spleen cells of Af sensitized mice
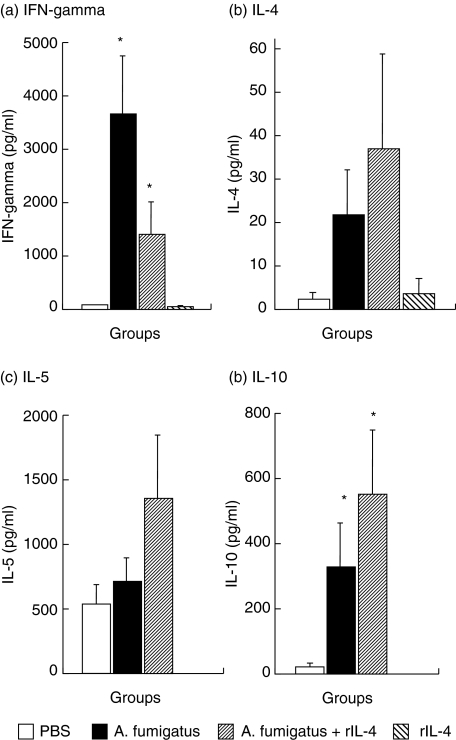
Significant levels of IFN-γ were detected in the culture supernatants of Af allergen-stimulated spleen cells from Af sensitized mice (3697 pg/ml ± 1063) and Af/rIL-4 treated animals (1385 pg/ml ± 620) as compared to PBS control mice (60 pg/ml ± 10) and rIL-4 treated mice (27 pg/ml ± 17) (P < 0·05) (Fig. 3a). A tendency towards reduced IFN-γ levels was detected in the spleen cell cultures of Af/rIL-4 treated animals compared to mice treated only with Af. Cells from Af/rIL-4 exposed mice produced the highest concentrations of IL-4, IL-5 and IL-10 (Figs 3b–3d).
Fig. 3.
Cytokine production by spleen cells from Af sensitized and control animals in response to Af allergens. (a) IFN-gamma, (b) IL-4, (c) IL-5 and (d) IL-10 were quantified by ELISA. Antigen induced cytokine expression was quantified after comparison with reference standards of mouse cytokines. Data represent the mean ± SEM of two separate experiments, and 5–10 mice were used in each group. *P-values < 0·05 were considered to be significant.
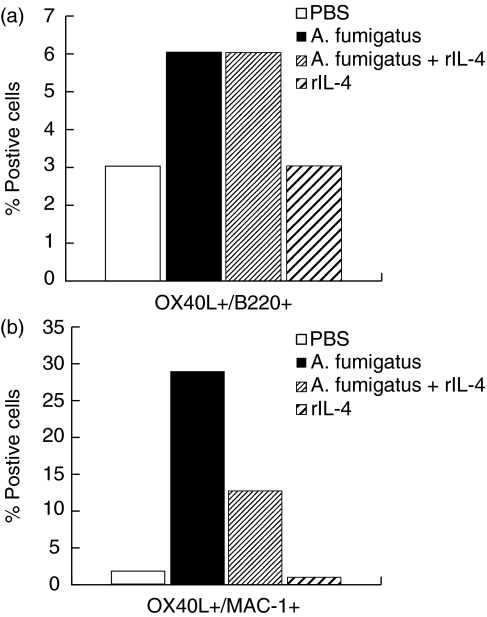
Increased expression of costimulatory molecules in sensitized mice
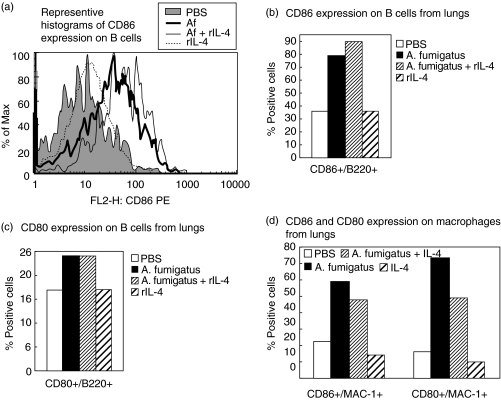
Cell surface expression of the costimulatory molecules CD80, CD86 and OX40L on B cells and macrophages from lungs of mice were analysed by flow cytometry (Figs 4 and 5). Up-regulation of both CD86 and CD80 molecules on B cells was detected after sensitization with Af and Af/rIL-4 (Fig. 4a–4c and data not shown). Representative histograms depicting CD86 expression on lung B cells from the different treatment groups are shown in Fig. 4a. Elevated percentages of B cells expressing CD86 were detected in lungs of both Af and Af/rIL-4 exposed mice (79% and 90%, respectively, versus 36% in PBS control animals) (Fig. 4b), while only modest increases in the percentages of CD80+ Bcells were seen (24% and 24%, respectively, versus 17% in the PBS control group) (Fig. 4c). Two to five fold increases in the percentages of CD86+ and CD80+ lung macrophages were detected in Af and Af/rIL-4 treated mice (Fig. 4d). Recombinant IL-4 treatment alone (Group 4) failed to induce any change in expression of CD80 and CD86 on both lung B cells and macrophages.
Fig. 4.
Expression of CD86 and CD80 costimulatory molecules on cells from the lungs of sensitized and nonsensitized mice. Lung cells were pooled from mice in each group and analysed by flow cytometry. (a) Representative histograms comparing CD86 expression on gated B cells from the four groups tested are shown. (b) CD86 expression and (c) CD80 expression on lung B cells are depicted as percent positive cells. (d) Similarly, CD86 and CD80 expression on lung macrophages from experimental and control groups are shown. The results are from one of two independent experiments.
Fig. 5.
Expression of OX40L molecules on pooled lung B cells and macrophages from four groups of mice. The percentages of (a) B cells and (b) macrophages expressing OX40L by flow cytometric analysis are shown. The data are representative from two independent experiments.
Enhanced expression of the costimulatory molecule OX40L in lung B cells and macrophages was observed in Af and Af/rIL4 treated mice (Fig. 5a,b). The percentages of OX40L+ macrophages in the lungs of Af and Af/rIL-4 treated mice were increased by more than six fold as compared to PBS treated mice. Interestingly, the increased expression of OX40L was observed throughout the Af sensitization period (data not shown).
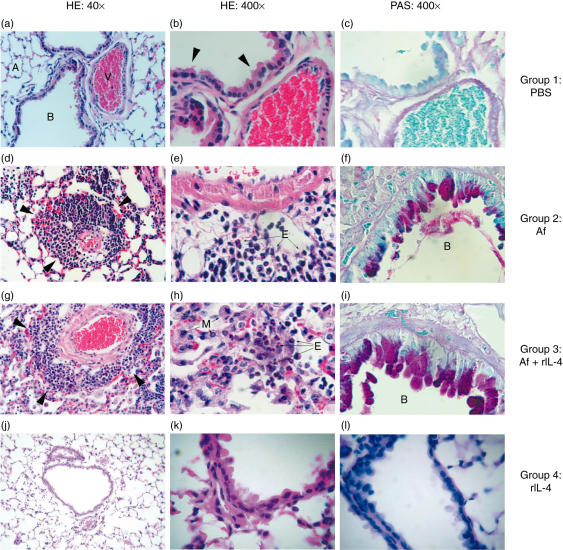
Histopathology
Lung histology from the different groups of mice is shown in Fig. 6. No pathological abnormalities were observed in the lungs of PBS-treated or rIL-4-treated control mice (Figs 6a–6c and 6j–l, respectively). Mice sensitized with Af demonstrated bronchiolar epithelial hyperplasia and increased numbers of goblet cells (11–30/high power field (hpf)) (Fig. 6f). Few (4–10/hpf) mononuclear and PMN cells were observed in the bronchial submucosa. Minimal perivascular oedema and moderate perivascular cuffing with infiltration of eosinophils, mononuclear and PMN cells were also observed in Af-sensitized animals (Fig. 6d,e). The Af/rIL-4 treated mice had more severe histopathological findings including moderate to severe bronchial epithelial hyperplasia containing few (4–10/hpf) eosinophils, numerous (> 30/hpf) lymphocytes, and goblet cells containing mucus (Fig. 6i). The alveolar septae were thickened and contained neutrophils, lymphocytes, occasional eosinophils and multinucleated giant cells. Mononuclear cells, occasional eosinophils (1–3/hpf) and a few macrophages (4–10/hpf) were located within the alveoli. Severe perivascular oedema and cuffing comprised of numerous lymphocytes and eosinophils were present in the lungs of these mice (Fig. 6g,h).
Fig. 6.
Inflammatory changes in the lungs of Af sensitized mice. (a) Normal mouse lung architecture (40× HE stain). (b) Higher magnification of normal lung tissue shows an absence of inflammatory cell infiltration around vessels and bronchi, with a single layer of ciliated columnar epithelium (arrows), 400× HE stain. (c) Depicts the absence of goblet cells in the bronchial epithelium of lung tissue from PBS controls (400× PAS). (d) The lung tissue from an A. fumigatus antigen treated mouse showing the degree of perivascular cuffing (arrows), similar to that seen in Af/rIL-4 treated mice (6G). Few macrophages are present in alveoli or alveolar septae (40× HE stain). (e) A higher magnification of the lung tissue shows numerous eosinophils located in the perivascular interstitium (400× HE stain). Fewer PAS staining goblet cells are located in the mucosa lining the bronchial lumen in Af exposed mouse (f) as compared to Af/rIL-4 treated mouse (i) (400× PAS). Severe perivascular cuffing (arrows) with eosinophils, macrophages and lymphocytes are present in Af/rIL-4 treated mice (g,h). Lungs of rIL-4 treated mice did not show any abnormal histological changes (6 j–l). A, alveolar space; B, bronchial lumen; V, bronchial artery; M, macrophages; E, eosinophils.
Discussion
Individuals with ABPA and Cystic Fibrosis patients with ABPA have been shown to have significant levels of serum IgE and IgG antibodies against A. fumigatus antigens, elevated total serum IgE, a predominant Th2 response, and increased sensitivity to IL-4 [36,37]. In the present study, we investigated the expression of the CD80, CD86 and OX40L costimulatory molecules in Af-sensitized mice and the effect of exogenous IL-4 in the modulation of these costimulatory molecules. IL-4 is a key cytokine that plays a central role in the development of allergic inflammatory responses in ABPA [38,39].
The results demonstrated significant increases in the levels of total IgE and Af-specific IgG1 antibodies in the sera of mice sensitized with Af. A further enhancement of total serum IgE was detected in animals treated with rIL-4 and Af. It has been shown that production of IgE by human B cells requires interaction with T cells in the presence of IL-4 and/or IL-13 [40]. The B7-CD28 pathway was also reported to be involved in this process [41–43]. We found increased percentages of lung B cells and macrophages expressing CD86 and CD80 molecules in both groups of sensitized mice. The enhanced expression of these molecules may suggest that these costimulatory molecules contribute to the immunological and inflammatory responses in allergic aspergillosis.
It has been reported that ABPA and atopic patients (individuals that produce IgE in response to inhaled antigens), show increased expression of CD86 on B cells from peripheral blood [36]. PBMCs from ABPA and atopic patients showed enhanced expression of CD86 on B cells when stimulated in vitro with IL-4 as compared to similarly treated PBMCs from nonatopic individuals [36]. Increased sensitivity to IL-4 was also demonstrated in Cystic Fibrosis patients with ABPA [37]. High expression of CD86 on B cells reported in atopic dermatitis patients also suggests the potential role of this molecule in the modulation of IgE synthesis [44].
The sensitization of mice with Af or Af/rIL-4 induced the production of Th2 cytokines including IL-4, IL-5 and IL-10 from cultured spleen cells. This suggests that in vivo administration of rIL-4 in the lung plays an important role in the exacerbation of allergic reactions. The local rIL-4 challenge resulted in the induction of a strong Th2 response as indicated by elevated total serum IgE, Af-specific IgG1 and peripheral blood eosinophilia. These responses are dependent on activation signals provided through CD28 ligation with costimulatory molecules CD80 and CD86 [41–43]. Our data suggests that up-regulation of CD80 and CD86 molecules may contribute to the increased activation of T cells that produce Th2 cytokines.
OX40 ligand has been described as a potent costimulatory molecule contributing in the development of Th2 responses driving lung inflammation [16–23]. Recently, it has been reported that the addition of IL-4 down regulates the expression of OX40L on activated DC, B cells and T cells in vitro; we investigated the effect of exogenous IL-4 during Af-sensitization [24]. Our results demonstrate that the OX40L molecule was up-regulated on lung macrophages and B cells from both groups of Af-sensitized mice; however, this increase was more pronounced with macrophages from Af exposed animals as compared to Af/rIL-4 challenged mice. Kim et al. [24] found that IL-4 down-regulated the expression of OX40L on activated splenic B cells. However, we failed to detect any down regulation of OX40L on B cells, in the present study and our results cannot be compared to those of Kim et al. [24] due to the differences in experimental design as we analysed freshly isolated lung cells without further stimulation while they have analysed the spleen cells after activation in vitro. Results of our study suggest that B7-CD28 and OX40–OX40L interactions may contribute to the development and regulation of allergic inflammation caused by Af.
Thus, sensitization with Af resulted in elevated expression of CD80, CD86 and OX40L costimulatory molecules on lung macrophages and B cells. The addition of rIL-4 during Af sensitization further enhanced the allergic and inflammatory responses. These results suggest that blocking the B7/CD28 and OX40L/OX40 pathways may be of value in modulating the disease process of allergic aspergillosis and related diseases.
Acknowledgments
This study was supported by Veterans Affairs Medical Research. The technical assistance of Nancy Elms, Laura Castillo and Abe Resnick are highly appreciated.
References
- 1.Greenberger PA. Middletonís Allergy Principles and Practice. In: Adkinson NF Jr, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. St Louis: Mosby; 2003. pp. 1353–71. [Google Scholar]
- 2.Knutsen AP, Chauhan B, Slavin RG. Cell-mediated immunity in allergic bronchopulmonary aspergillosis. In: Kurup VP, Apter AJ, editors. Immunology and Allergy Clinics of North America. Philadelphia: W.B. Saunders; 1998. pp. 575–99. [Google Scholar]
- 3.Kurup VP, Madan T, Sarma UP. Allergic bronchopulmonary aspergillosis. Recent concepts and considerations. In: Domer JE, Kobayashi GS, editors. The Mycota XII. Human Fungal Pathogens. Berlin: Springer-Verlag; 2004. pp. 225–41. [Google Scholar]
- 4.Abbas AK. The control of T cell activation vs. tolerance. Autoimmun Rev. 2003;2:115–8. doi: 10.1016/s1568-9972(03)00028-4. [DOI] [PubMed] [Google Scholar]
- 5.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 6.Chapoval SP, David CS. CD28 costimulation is critical for experimental allergic asthma in HLA-DQ8 transgenic mice. Clin Immunol. 2003;106:83–94. doi: 10.1016/s1521-6616(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 7.Coyle AJ, Gutierrez-Ramos JC. The role of ICOS and other costimulatory molecules in allergy and asthma. Springer Semin Immunopathol. 2004;25:349–59. doi: 10.1007/s00281-003-0154-y. [DOI] [PubMed] [Google Scholar]
- 8.Deurloo DT, van Berkel MA, van Esch BC, Hofhuis F, Nijkamp FP, Oosterwegel MA, van Oosterhout AJ. CD28/CTLA-4 double deficient mice demonstrate crucial role for B7 co-stimulation in the induction of allergic lower airways disease. Clin Exp Allergy. 2003;33:1297–304. doi: 10.1046/j.1365-2222.2003.01757.x. [DOI] [PubMed] [Google Scholar]
- 9.El Biaze M, Boniface S, Koscher V, et al. T cell activation, from atopy to asthma: more a paradox than a paradigm. Allergy. 2003;58:844–53. doi: 10.1034/j.1398-9995.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- 10.Mark DA, Donovan CE, De Sanctis GT, et al. B7–1 (CD80) and B7–2 (CD86) have complementary roles in mediating allergic pulmonary inflammation and airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2000;22:265–71. doi: 10.1165/ajrcmb.22.3.3747. [DOI] [PubMed] [Google Scholar]
- 11.Mathur M, Herrmann K, Qin Y, et al. CD28 Interactions with either CD80 or CD86 are sufficient to induce allergic airway inflammation in mice. Am J Resp Cell Mol Biol. 1999;21:498–509. doi: 10.1165/ajrcmb.21.4.3714. [DOI] [PubMed] [Google Scholar]
- 12.Zdolsek HA, Jenmalm MC. Expression of the T-cell markers CD2 and CD28 in healthy and atopic children during the first 18 months of life. Pediatr Allergy Immunol. 2003;14:169–77. doi: 10.1034/j.1399-3038.2003.00016.x. [DOI] [PubMed] [Google Scholar]
- 13.Bugeon L, Dallman MJ. Costimulation of T Cells. Am J Resp Crit Care Med. 2000;162:S164–8. doi: 10.1164/ajrccm.162.supplement_3.15tac5. [DOI] [PubMed] [Google Scholar]
- 14.Haczku A, Takeda K, Redai I, et al. Anti-CD86 (B7.2) treatment abolishes allergic airway hyperresponsiveness in mice. Am J Respir Crit Care Med. 1999;159:1638–43. doi: 10.1164/ajrccm.159.5.9711040. [DOI] [PubMed] [Google Scholar]
- 15.Krinzman SJ, De Sanctis GT, Cernadas M, et al. Inhibition of T cells costimulation abrogates airway hyperresponsiveness in a murine model. J Clin Invest. 1996;98:2693–9. doi: 10.1172/JCI119093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gramalia IA, Weinberg D, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–7. [PubMed] [Google Scholar]
- 17.Murata K, Ishii N, Takano H, Miura S, Ndhlovu LC, Nose M, Noda T, Sugamura K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J Exp Med. 2000;191:365–74. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess JK, Carlin S, Pack RA, Arndt GM, Au WW, Johnson PR, Black JL, Hunt NH. Detection and characterization of OX40 ligand expression in human airway smooth muscle cells: a possible role in asthma? J Allergy Clin Immunol. 2004;113:683–9. doi: 10.1016/j.jaci.2003.12.311. [DOI] [PubMed] [Google Scholar]
- 19.Burgess JK, Blake AE, Boustany S, Johnson PR, Armour CL, Black JL, Hunt NH, Hughes JM. CD40 and OX40 ligand are increased on stimulated asthmatic airway smooth muscle. J Allergy Clin Immunol. 2005;115:302–8. doi: 10.1016/j.jaci.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Salek-Ardakani S, Song J, Halteman BS, Jember AG, Akiba H, Yagita H, Croft M. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003;198:315–24. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoshino A, Tanaka Y, Akiba H, et al. Critical role for OX40 ligand in the development of pathogenic Th2 cells is a murine model of asthma. Eur J Immunol. 2003;33:861–9. doi: 10.1002/eji.200323455. [DOI] [PubMed] [Google Scholar]
- 22.Arestides RS, He H, Westlake RM, Chen AI, Sharpe AH, Perkins DL, Finn PW. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur J Immunol. 2002;32:2874–80. doi: 10.1002/1521-4141(2002010)32:10<2874::AID-IMMU2874>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Jember AG, Zuberi R, Liu FT, Croft M. Development of Allergic Inflammation in a Murine Model of Asthma is Dependent on the Costimulatory Receptor OX40. J Exp Med. 2001;193:387–92. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MY, Bekiaris V, McConnell FM, Gaspal FM, Raykundalia C, Lane PJ. OX40 Signals during Priming on Dendritic Cells Inhibit CD4 T Cell Proliferation: IL-4 Switches off OX40 Signals Enabling Rapid Proliferation of Th2 Effectors. J Immunol. 2005;174:1433–7. doi: 10.4049/jimmunol.174.3.1433. [DOI] [PubMed] [Google Scholar]
- 25.Kurup VP, Grunig G. Animal models of allergic bronchopulmonary aspergillosis. Mycopathologia. 2002;153:165–77. doi: 10.1023/a:1014963600314. [DOI] [PubMed] [Google Scholar]
- 26.Kurup VP, Mauze S, Choi H, Seymour BW, Coffman RL. A murine model of allergic bronchopulmonary aspergillosis with elevated eosinophils and IgE. J Immunol. 1992;148:3783–8. [PubMed] [Google Scholar]
- 27.Kurup VP, Xia JQ, Crameri R, et al. Purified recombinant A. fumigatus allergens induce different responses in mice. Clin Immunol. 2001;98:327–36. doi: 10.1006/clim.2000.4993. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee B, Greenberger PA, Fink JN, Kurup VP. Immunological characterization of Asp f 2, a major allergen from Aspergillus fumigatus associated with allergic bronchopulmonary aspergillosis. Infect Immunity. 1998;66:5175–82. doi: 10.1128/iai.66.11.5175-5182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurup VP, Banerjee B, Hemmann S, Greenberger PA, Blaser K, Crameri R. Selected recombinant Aspergillus fumigatus allergens bind specifically to IgE in ABPA. Clin Exp Allergy. 2000;30:988–93. doi: 10.1046/j.1365-2222.2000.00837.x. [DOI] [PubMed] [Google Scholar]
- 30.Kurup VP, Xia JQ, Rickaby DA, Dawson CA, Choi H, Fink JN. Aspergillus fumigatus antigen exposure in pulmonary airway resistance in wild-type but not in IL- 4 knockout mice. Clin Immunol. 1999;90:404–10. doi: 10.1006/clim.1998.4656. [DOI] [PubMed] [Google Scholar]
- 31.Barrios C, Brawand P, Berney M, Brandt C, Lambert PH, Siegrist CA. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur J Immunol. 1996;26:1489–96. doi: 10.1002/eji.1830260713. [DOI] [PubMed] [Google Scholar]
- 32.Rathore VB, Johnson B, Fink JN, Kelly KJ, Greenberger PA, Kurup VP. T cell proliferation and cytokine secretion to T cell epitopes of Asp f 2 in ABPA patients. Clin Immunol. 2001;100:228–35. doi: 10.1006/clim.2001.5056. [DOI] [PubMed] [Google Scholar]
- 33.Siegrist CA, Córdova M, Brandt C, Barrios C, Berney M, Tougne C, Kovarik J, Lambert P-H. Determinants of infant responses to vaccines in presence of maternal antibodies. Vaccine. 1998;16:1409–14. doi: 10.1016/s0264-410x(98)00100-5. [DOI] [PubMed] [Google Scholar]
- 34.Kurup VP, Choi HY, Murali PS, Xia JQ, Coffman RL, Fink JN. Immune responses to Aspergillus antigen in IL-4-/- mice and the effect of eosinophils ablation. Allergy. 1999;54:420–7. doi: 10.1034/j.1398-9995.1999.00944.x. [DOI] [PubMed] [Google Scholar]
- 35.Johnson BD, Yan X, Schauer DW, Orentas RJ. Dual expression of CD80 and CD86 produce a tumor vaccine superior to single expression of either molecule. Cellular Immunol. 2003;222:15–26. doi: 10.1016/s0008-8749(03)00079-0. [DOI] [PubMed] [Google Scholar]
- 36.Khan S, McClellan JS, Knutsen AP. Increased sensitivity to IL-4 in patients with Allergic Bronchopulmonary Aspergillosis. Int Arch Allergy Immunol. 2000;123:319–26. doi: 10.1159/000053644. [DOI] [PubMed] [Google Scholar]
- 37.Knutsen AP, Hutchinson PS, Albers GM, Consolino J, Smick J, Kurup VP. Increased sensitivity to IL-4 in cystic fibrosis patients with allergic bronchopulmonary aspergillosis. Allergy. 2004;59:81–7. doi: 10.1046/j.1398-9995.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 38.Knutsen AP. Lymphocytes in allergic bronchopulmonary aspergillosis. Front Biosci. 2003;8:589–602. doi: 10.2741/994. [DOI] [PubMed] [Google Scholar]
- 39.Skov M, Poulsen LK, Koch C. Increased antigen-specific Th-2 response in allergic bronchopulmonary aspergillosis (ABPA) in patients with cystic fibrosis. Pediatr Pulmonol. 1999;27:74–9. doi: 10.1002/(sici)1099-0496(199902)27:2<74::aid-ppul2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40.Cerutti A, Zan H, Schaffer A, Bergsagel L, Harindranath N, Max EE, Casali P. CD40 ligand and appropriate cytokines induce switching to IgG, IgA and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+IgD+ B cell line. J Immunol. 1998;160:2145–57. [PMC free article] [PubMed] [Google Scholar]
- 41.Jeannin P, Delneste Y, Lecoanet-Henchoz S, Gacuchat JF, Ellis J, Bonefoy JY. CD86 (B7-2) on human B cells. A functional role in proliferation and selective differentiation into IgE- and IgG4-producing cells. J Biol Chem. 1997;272:15613–9. doi: 10.1074/jbc.272.25.15613. [DOI] [PubMed] [Google Scholar]
- 42.Nakajima A, Watanabe N, Yoshino S, Yagita H, Okumura K, Azuma M. Requirement of CD28/CD86 co-stimulation in the interaction between antigen-primed T helper type 2 and B cells. Int Immunol. 1997;9:637–44. doi: 10.1093/intimm/9.5.637. [DOI] [PubMed] [Google Scholar]
- 43.Tsuyuki S, Tsuyuki J, Einsle K, Kopf M, Coyle AJ. Costimulation through B7–2 (CD86) is required for the induction of a lung mucosal T helper cell 2 (TH2) immune response and altered airway responsiveness. J Exp Med. 1997;185:1671–9. doi: 10.1084/jem.185.9.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jirapongsananuruk O, Hofer MF, Trumble AE, Norris DA, Leung DYM. Enhanced expression of B7.2 (CD86) in patients with atopic dermatitis: a potential role in the modulation of IgE synthesis. J Immunol. 1999;160:4622–7. [PubMed] [Google Scholar]