Abstract
Glomerulonephritis (GN), the major worldwide cause of chronic renal disease and renal failure, shows a wide spectrum of histological patterns, severity of injury and clinical outcomes that may be related to the nature of the nephritogenic immune response. In the majority of cases, there is evidence of a central role for cognate immunity in the initiation of human GN and contributions of both humoral and cellular effector mechanisms have been demonstrated in both humans and in animal models. T helper cell subsets are known to activate different immune effector mechanisms which influence disease outcomes in infectious and autoimmune diseases and evidence is now accumulating that Th1 and Th2 subsets direct diverging effector pathways that lead to different patterns and severity of glomerular injury in GN. Th1-predominant responses appear to be associated strongly with proliferative and crescentic forms of GN that result in severe renal injury, while Th2 responses are associated with membranous patterns of injury. The challenge remains to understand fully the relevance of T helper cell subset responses to the spectrum of human GN and to apply this new knowledge to the development of more potent and selective therapeutic strategies.
Keywords: crescent, cytokine, delayed type hypersensitivity, T cell
Introduction
Despite the emerging burden of diabetic renal disease, glomerulonephritis (GN) remains a major cause of chronic renal disease and end-stage renal failure requiring dialysis and renal transplantation. There is good evidence for the involvement of cognate immune mechanisms in the majority of cases of human GN. However, apart from antigenic peptides of α3(IV) collagen in glomerular basement membrane (GBM), which are the target for classical (but uncommon) autoimmunity in Goodpasture's disease (Fig. 1), the identities of the nephritogenic antigens are mostly unknown. GN shows a wide spectrum of histological patterns and clinical outcomes, indicating that a variety of immunopathogenic mechanisms of injury is involved.
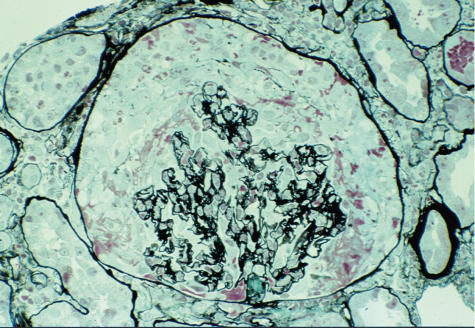
Fig. 1.
The typical appearance of a crescent surrounding the glomerular tuft in a patient with anti-glomerular basement membrane (GBM) glomeronephritis (stain, silver methenamine, magnification ×200).
A central role for T cells, not only in providing help for production of immunoglobulins involved in humoral effector mechanisms, but also in directing cellular immune mechanisms, is now recognized in GN. Differential activation of T helper cell subsets has been proposed as a potential explanation for the variety of patterns of injury in GN [1], and the ability of T helper cells subsets to influence immune effector pathways has been demonstrated in experimental models using inbred rodents. More recent insights into T helper cell differentiation and their involvement in animal models and to human GN will be reviewed. A significant challenge remains to define clearly the relevance of the Th1/Th2 paradigm to understanding the diverse types of human GN and its relevance to therapeutic intervention.
Identifying Th1 and Th2 cells (Table 1)
Table 1.
Characteristics of T helper subsets.
| Th1 cells | Th2 cells | |
|---|---|---|
| Inducing stimuli | IL-12, IFN-γ, IL-18, IL-27, PRR signalling | IL-4 |
| Transcription factors | STAT4, STAT1, T-bet, NFκB | STAT6, GATA3 |
| Cytokines produced | IFN-γ, IL-2, TNF, lymphotoxin α | IL-4, IL-5, IL-13 (IL-10 in mice) |
| Chemokine receptor expression | CXCR3, CCR5, CCR1 | CCR3, CCR4, CCR8 |
| Antibody isotypes | Human: IgG1, IgG3, IgG2 | Human: IgG4, IgE |
| Mouse: IgG2a, IgG3, IgG2b | Mouse: IgG1, IgE | |
| Effector response | Cell-mediated immunity, macrophage activation, antibody-mediated cellular cytotoxicity | Activation of eosinophils, allergy |
Subsets of T helper cells were identified initially in cloned T cells [2] and in mice by their different cytokine profiles and effector functions [3,4], and it is now apparent that the Th1/Th2 paradigm has relevance in human biology and disease [5]. Th1 and Th2 cells are subsets of primed CD4+ T helper cells that can be distinguished functionally by their cytokine profile and their ability to generate different types of immune effector responses. Th1 cells produce interferon (IFN)-γ), interleukin (IL)-2, tumour necrosis factor (TNF) and lymphotoxin-α. They promote macrophage activation and delayed-type hypersensitivity (DTH) effector responses, as well as providing help to B cells to produce complement-fixing antibody isotypes that mediate opsonization and phagocytosis. Th1 cells are critical for elimination of intracellular pathogens such as Listeria monocytogenes and Leishmania major. Th2 cells produce IL-4, IL-5, IL-10 (in mice) and IL-13 that promote production of non-complement-fixing IgG isotypes and IgE and are important for elimination of helminthic and nematode infections and mediation of allergic responses.
Development of Th1/Th2 polarization
Dendritic cells and TCR affinity
Th1 and Th2 cells differentiate from naive precursors (Th0) following specific antigen stimulation via their α/β T cell receptor (TCR). Dendritic cells, because of their ability to migrate from peripheral sites to secondary lymphoid organs, play a dominant role in activation of naive T cells. Subsets of dendritic cells have been identified which may influence Th1/Th2 development [6]. In humans, subsets of dendritic cells derived from either monocytes or plasmacytoid cells with a bias towards stimulating either IFN-γ or IL-4, IL-5 and IL-10 production (respectively) by naive T cells have been demonstrated in vitro. In mice, CD8α+ dendritic cells produce IFN-γ and IL-12 following antigen exposure, whereas CD8α- dendritic cells produce IL-4 and IL-10, leading to Th1 and Th2 polarized responses. CD40 ligation may be important for IL-12 production by dendritic cells [7].
Effects of antigen dose and antigen affinity on Th subset development are complex and findings vary in different experimental systems. Attenuation of the TCR signal, e.g. by low antigen/MHCII binding affinity or low MHCII/TCR affinity, favours Th2 differentiation in vitro when soluble peptide antigens are studied [8], although TCR-independent mechanisms have been proposed for antigen/dose effects [9]. Moderate antigen doses favour Th1 development, but very high and very low doses favour Th2 development of α/β TCR transgenic CD4+ T cells in vitro[10]. However, in vivo responses of intact organisms such as L. major to low levels of antigen challenge appear to be Th1 biased [11].
Cytokine milieu
The cytokine milieu is a key factor in directing Th cell polarization. IFN-γ and IL-12 are the major cytokines that promote Th1 development. Recently, the role of IL-18, IL-27 and potentially IL-23 as cytokines that promote Th1 differentiation has been recognized, suggesting multiple and overlapping mechanisms of induction of Th1 responses [12,13]. IL-4 is the major cytokine responsible for driving Th2 responses. IFN-γ but not IL-12 may play a role in inhibiting Th2 responses, whereas IL-10 appears to be more active in inhibiting Th1 differentiation than promoting Th2 responses [14].
Co-stimulatory signals
In addition to the key role of the cytokine milieu, co-stimulatory signals to T helper cells also influence Th1/Th2 differentiation. CD80 and CD86 signal via CD28, which is expressed constitutively on T cells. Inhibition of CD28 blocks Th2 responses to a number of parasites while leaving Th1 responses intact. Other co-stimulatory molecules, such as OX40 and ICOS, are induced after T cell activation and may be preferentially involved in sustaining Th2 responses [15]. Co-stimulatory signals provided by CD40/CD154 augment IFN-γ production by Th1 cells [16].
Experimental evidence suggests that Th2 differentiation may be the default response of T cells following antigenic stimulation and that Th1 responses need specific additional signals. Innate immune responses to conserved molecular components of pathogens − so-called ‘pathogen associated molecular patterns’ by a limited repertoire of ‘pattern recognition receptors’ (which include Toll-like receptors) expressed by macrophages and dendritic cells may play a role in Th1 differentiation [13]. These receptors augment Th1 differentiation by activating signalling pathways inducting IL-12 production by dendritic cells and IFN-γ by sensitized T cells [17].
Intracellular signalling and transcription factors
Differences between Th1 and Th2 cells in the surface membrane clustering of the TCR complex in lipid rafts may contribute to differences in subsequent intracellular signalling events. Th1 cells show more efficient recruitment and clustering of TCR components in the presence of CD4 [18], which may contribute to more efficient intracellular signalling in Th1 cells. Differential involvement of members of the Src kinase family [19], the Tec kinase family (e.g. Rlk and Itk) and MAP kinases [20] (e.g. JNK [21,22], p38MAPK and GADD45) have been reported in Th1/Th2 differentiation. In Th1 responses, signalling via IFN-γ receptors and STAT1 induces the transcription factor T-bet which optimizes IFN-γ expression and up-regulates expression of the β2 chain of the IL-12 receptor. T-bet also represses Th2 responses by interfering with GATA-3 binding toDNA [23]. IL-12 receptor activation can further enhance Th1 responses via STAT4. IL-4 activates STAT6 which induces GATA-3 expression, which is a potent inducer of Th2 responses. GATA-3 induces its own expression as well as enhancing IL-4 production and inhibiting IFN-γ and IL-12 receptor expression [12].
Chemokine receptor expression
Chemokines may influence antigen recognition and effector and memory functions of T cells by effects on interactions with dendritic cells, homing of T cells to lymph nodes and migration through peripheral tissues [24]. Th1 and Th2 cells appear to express different patterns of chemokine receptors, which may facilitate differential activation responses to chemokines. CXCR3, the receptor for interferon-γ inducible chemokines (IP-10, Mig and I-TAC) is expressed at high levels on IFN-γ-producing Th1 cells and low levels on Th2 cells [25]. CCR3 and CCR4 expression is associated with Th2 cells that express IL-4 [26].
Different patterns of injury and outcome in GN suggest preferential involvement of Th1 or Th2 subsets
Demonstration of the ability of Th1 and Th2 subsets to influence the outcome of infectious diseases in mice [27] provided an impetus to studies of their contribution to GN. Initial evidence for a selective role for Th1 and Th2 subsets in GN came from experimental models using inbred strains of mice and rats and cytokine-deficient mice. Studies of human GN have now begun to demonstrate the value of the Th1/Th2 paradigm to understanding the pathogenic mechanisms and therapeutic potentials in an out-bred human population.
Th1/Th2 subsets in experimental models of GN
Comparison of immunological effector responses, histological features of inflammation and functional injury in Th1 (C57BL/6) or Th2 (BALB/c) dominant mice provided the first evidence that severe crescentic injury induced by an adaptive immune responses to heterologous anti-GBM antibody (nephrotoxic nephritis − NTN) was the result of a Th1 polarized response [28]. Studies of NTN in Lewis and Brown Norway rats also demonstrated that features of cell-mediated glomerular injury (extra-capillary proliferation and accumulation of monocytes and T cells) were associated with a Th1 profile of cytokine production [29]. Conversely, in mercuric chloride-induced autoimmune GN (which results in a non-crescentic, ‘membranous-like’ form of GN) studies using congenic strains of Brown Norway rats demonstrated that susceptibility to GN was associated with preferential activation of Th2 responses [30].
Contribution of cytokines
Studies in murine models have confirmed the dependence of crescentic GN on Th1 cytokines and the ability of interventions which alter the balance of Th1/Th2 cytokines to modulate crescentic injury. Glomerular T cell and macrophage accumulation and crescentic injury is attenuated in mice with genetic deletion of Th1 cytokines, e.g. IL-12 [31], IFN-γ[32] or TNF [33] and by blocking Th1 cytokines with inhibitory antibodies, anti-IL-12 [34] and anti-IFN-γ[28]. Administration of IL-12 augments Th1 responses and crescentic GN [34].
IL-18 is one of several cytokines, including IL-23 and IL-27 that have been identified more recently, that contribute to development of Th1 responses [12]. IL-18 is produced by dendritic cells and macrophages and acts with IL-12 as a co-factor for induction of Th1 development and IFN-γ production [35]. In NTN, administration of IL-18 augmented cutaneous DTH responses and exacerbated crescentic GN in mice [31]. However, the ability of IL-18 to enhance cutaneous DTH and crescentic glomerular injury was also observed in IL-12-deficient mice, indicating that IL-18 may play a local role in recruiting T cells and macrophages via induction of ICAM-1.
In murine lupus, inhibition of IL-18 by cDNA vaccination effectively reduced lymphoproliferation, IFN-γ production and GN and improved survival [36], whereas IL-12-deficient lupus-prone MRL/Fas-lpr mice showed only a modest effect on survival despite marked reductions of renal IFN-γ production, inflammatory cell recruitment and GN [37]. Over-expression of IL-12p40 prevented age-related increases in serum IFN-γ and induced the shift from Th1 to Th2 anti-DNA antibody subclasses in lupus-prone mice, but produced minimal changes in development of GN and survival [38].
In a murine model of experimental autoimmune crescentic GN (EAG) induced by immunization with α3(IV)NC1 collagen, disease susceptibility is associated with local production of Th1 cytokines [39]. In a similar model induced with α3-α5(IV)NC heterodimers, histological injury was reduced in IL-12p40-deficient mice but IFN-γ-deficient mice showed increased CD4+ cell reactivity to the antigen and enhanced dermal DTH and crescentic GN [40], indicating diverging roles for IFN-γ in this autoimmune disease compared with NTN, which does not involve autoimmunity.
Th2 cytokines play a role in attenuating proliferative and crescentic forms of experimental GN and facilitate some humorally mediated models [30]. Mice genetically deficient in Th2 cytokines, IL-4 [41] or IL-10 [42] have more pronounced Th1 responses and develop more severe crescentic GN. Administration of Th2 cytokines (IL-4 and/or IL-10) inhibited the development of crescentic NTN in mice [43] and combined treatment was effective in attenuating established disease [44]. Over-expression of IL-10 by using viral vector–IL-10 gene transfer attenuated crescentic GN in Wistar Kyoto rats [45]. Pharmacological approaches to designed to inhibit Th2 responses have been demonstrated recently to be effective in attenuating heavy metal-induced autoimmune (membranous) GN in Brown Norway rats [46]. This approach involved inhibition of dihydropyridine receptors, which are expressed exclusively on Th2 cells and are involved in TCR-dependent production of IL-4, IL-5 and IL-10.
IL-13 is a product of Th2 cells that promotes Th2 responses. Recent studies in murine NTN demonstrated dissociation between IL-13 effects of cell-mediated and humoral effector mechanisms of glomerular injury [47]. IL-13-deficient mice showed higher levels of circulating nephritogenic antibodies with enhanced Th1 associated IgG2a subclass without effects on glomerular T cell and macrophage recruitment, crescent formation or functional renal injury.
Co-stimulatory signals and Th polarization in experimental GN
In recent years there has been considerable interest in the role of co-stimulatory molecules in GN and their potential to differentially modulate Th1/Th2. Studies in murine NTN showed augmentation of crescentic GN following antibody inhibition of CD86 and reduction of crescent formation following CD80 inhibition, but these effects were not associated with detectable changes in Th1/Th2 polarization [48]. In this study, combined inhibition of CD80 and CD86 signalling using CTLA4-Ig did not affect the development of glomerular injury. Studies with genetically deficient mice produced similar results, with reduced crescentic GN associated with CD80 deficiency and more severe disease with enhanced Th1 cytokines in the setting of CD86 deficiency [49]. Combined CD80 and CD86 deficiency did not affect crescentic GN. In a recent study, monoclonal antibody inhibition of ICOS signalling and administration CTLA4-Ig to block CD28 was reported to be effective in ameliorating murine NTN, without evidence of Th1/Th2 shift in antibody isotype [50]. CD28-deficient mice also showed marked attenuation of NTN [51]. Thus, although a variety of effects of CD80/CD86 and CD28 have been demonstrated in experimental models, evidence is lacking for clear effects on Th cell polarization.
Another member of the B7-CD28 family, ICOS-B7 homologous protein, regulates T cell-dependent humoral responses. Monoclonal antibody inhibition of this signalling pathway in murine lupus effectively prolonged survival and inhibited all IgG subclasses and both Th1 and Th2 cells [52].
CD40/CD154 co-stimulation is important for production of both Th1 and Th2 cytokines by T cells [53]. Inhibition of CD154 with a monoclonal antibody given either prior to disease initiation or to established disease was effective in reducing the severity of crescentic EAG in Wistar Kyoto rats [54]. NTN could not be induced in CD40-deficient mice because they fail to develop effective immune responses to (nephrotoxic) sheep globulin [55]. However, antibody inhibition of CD154 in the effector phase of induction of NTN in mice resulted in reduced glomerular recruitment of Th1 effect cells and injury. Administration of IL-12 to CD40-deficient mice restored Th cell IFN-γ production, renal chemokine expression and glomerular T cell and macrophage recruitment but did not restore renal injury due to ineffective macrophage activation in the absence of CD40 [56]. Expression of CD40 by intrinsic renal cells was demonstrated to play a key role in renal chemokine induction, T cell and macrophage recruitment and injury [55], indicating that CD40 expression by non-immune cells plays an important role in this Th1 effector response.
Signals provided by the innate immune system following exposure to microorganisms may also influence the development of GN. Administration of bacterial cpG-DNA increased Th1 responses and aggravated immune complex induced GN in mice, suggesting signalling via of TLR9 [57]. Infection of lupus-prone NZB/NZW mice with Plasmodium chabaudi increased levels of Th2 cytokines and improved survival [58], whereas Bacille Calmette–Guérin (BCG) vaccination precipitated a lupus-like syndrome in NOD mice [59].
Intracellular signalling pathways and transcription factors
Although a great deal of work has been conducted to define the intracellular signalling pathways activated during T helper subset differentiation, little is known about the involvement of these in GN. STAT4 and STAT6 show a differential role in Th1/Th2 signalling and may be a potential therapeutic target to influence the outcome of GN. In murine lupus, STAT6 deficiency reduced splenocyte IL-4 and increased IFN-γ production and glomerulosclerosis without effects on anti-DNA IgG. This effect was similar to that achieved using anti-IL-4 antibody to inhibit Th2 responses. STAT4-deficient mice showed low splenocyte IFN-γ, consistent with inhibition of Th1 responses. This was associated with accelerated development of proteinuria and an increase in crescent formation, despite lower total anti-DNA IgG [60].
GATA-3 plays a critical role in Th2 development and over-expression of GATA-3 in BXSB/MpJ-Yaa mice (that develop lupus disease) resulted in improved survival and amelioration of GN associated with a Th2 switch in IgG isotypes and decreased serum IFN-γ levels [61]. These two studies provide the first evidence that targeting of Th1/Th2 signalling pathways can alter outcomes of experimental GN.
Evidence in human glomerulonephritis
While Th1/Th2 subsets clearly influence patterns of injury in experimental GN, the dissection of their role in human GN has proved more difficult due to the diverse range of underlying immunopathogenic mechanisms in human disease (e.g. organ-specific or systemic autoimmunity, innocent bystander injury in the context of antigen immune responses to ‘planted’ antigens, probable ‘immune dysregulation’ in IgA nephropathy), our incomplete knowledge of the effector pathways involved and the variable, poorly defined genetic influences on immune responses in human populations. Despite these obstacles, evidence is accumulating to indicate that differential activation of Th1 and Th2 mechanisms is useful in explaining some of the histological patterns of human GN (Fig. 2).
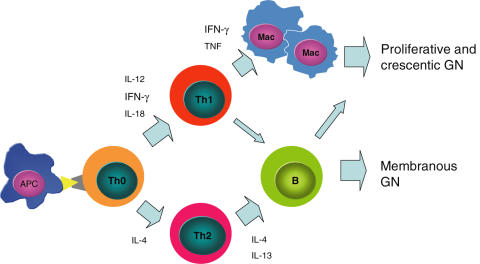
Fig. 2.
A simplified scheme of Th1 and Th2 subset and cytokine involvement in glomerulonephritis. APC: antigen-presenting cell; Mac: macrophage.
In severe and rapidly progressive forms of GN, there is increasing evidence that, independent of their underlying cause, glomerular crescent formation results from an underlying or superimposed Th1 response. Previous studies noting the presence of effector CD4+ cells and macrophages in crescentic lesions, including the uncommon presentation of crescentic lesions in membranous GN [62], imply that glomerular crescent formation is a manifestation of a Th1-mediated DTH-like lesion. Analysis of cytokine profiles from biopsies of a variety of forms of proliferative GN shows higher levels of IL-2 and IFN-γ compared with non-proliferative forms [63] and glomerular CCR5 expression associated with crescentic GN [64]. Studies in human anti-GBM GN support a role for Th1 in injury by demonstrating IFN-γ-predominant antigen-specific effector cell responses in active disease and IL-10 predominance in remission [65]. The potential role for CD25+ regulatory cells in suppressing pathogenic IFN-γ production has also been demonstrated in human anti-GBM GN [66]. These studies [67,68] reveal roles for effector T cells, in addition to the contribution of Th1-associated autoantibody subclasses in both human Goodpasture's disease [69] and animal models [70].
Anti-neutrophil cytoplasmic antibody (ANCA)-associated GN
In ANCA-associated GN, recent studies in animal models have demonstrated the importance of ANCA in triggering disease [71]. Th1 and Th2 cells determine both the IgG subclass and titre of autoantibody by providing B cell help and also have a role as effector cells in vasculitic lesions. Evidence is now accumulating for Th1 predominance of the underlying T cell response. T cells and macrophages are present in glomerular lesions [72] and glomerular biopsies show high IFN-γ and low IL-4 glomerular mRNA expression, indicating a Th1-predominant effector response of patients with ANCA-associated GN [73]. Peripheral blood T cells showed a high IFN-γ : IL-4 cytokine ratio in patients with ANCA-associated GN compared to non-proliferative forms of GN and IgA disease. Effective corticosteroid treatment of the disease resulted in a decrease in the IFN-γ : IL-4 ratio [73].
In patients with active Wegener's granulomatosis, peripheral blood T cells produced high levels of IFN-γ that were suppressible by IL-10, and blood monocytes produced high IL-12 regardless of disease activity [74]. In untreated patients, when the disease was in complete remission, peripheral blood mononuclear cells produced high levels of IL-10 in response to stimulation with one of the putative autoantigens, PR-3 [75]. Although ANCAs of the IgG1 and IgG3 (Th1) subclasses are potent in activating neutrophil FcR, evidence for a unexpected role for IgG4 has emerged recently [76]. Analyses of cytokine profiles in lesions from the nasal mucosa of patients with Wegener's granulomatosis have yielded conflicting results. One study has reported high IFN-γ expression, suggesting Th1 dominance [77]. In another study, increased levels of IL-4 and CCR3 expression were reported in nasal tissues, suggesting Th2 bias; however, both IL-2 and CCR5+ (Th1) and IL-4 and CCR3+ (Th2) cells were present in renal tissues, with no Th2 bias [78].
Lupus nephritis
Human lupus nephritis displays heterogeneity of Th1 and Th2 responses and heterogeneity of glomerular lesions, ranging from severe diffuse proliferative crescentic disease (WHO Class IV), to the less proliferative membranous lupus (WHO Class V), to minimal or no glomerular changes. Evidence for both Th1 and Th2 patterns in serum IgG subclasses and cytokines were found in a group of 31 children with lupus nephritis [79]. Supporting the hypothesis that severe proliferative GN results from a Th1-predominant response, patients with WHO class IV lupus nephritis exhibited increased peripheral blood T cell IFN-γ : IL-4 ratios, increased renal CD3+ cells and macrophages and increased IFN-γ positive cells compared with patients with either WHO Class V or mild glomerular lesions [80]. A predominance of Th1 cytokines (IL-2 and IFN-γ) has been demonstrated in the urinary sediment of patients with active lupus nephritis [81].
Non-proliferative nephritis
Several non-proliferative forms of GN, such as membranous GN and minimal change GN, are associated with an incomplete Th2 response, lack of substantial leukocytic infiltrates and/or the deposition of IgG4 in glomeruli [82–85] and (in minimal change GN) an association with atopy and the production of IL-13 [86,87]. The percentage of IL-4+ peripheral blood T cells was increased significantly in patients with idiopathic membranous nephritis (compared with controls and other forms of GN) and correlated with the amount of proteinuria [88]. In another study, IL-10+/CD4+ cells but not IL-4+/CD4+ cells or IFN-γ/CD4+ cells were increased in patients with idiopathic membranous nephritis [89]. There is evidence for heterogeneity of Th responses in two other important and common forms of GN, IgA nephropathy and lupus nephritis. In both these diseases, severe proliferative disease is associated with a Th1-predominant response, but in IgA nephropathy the onset of the disease may be related to factors including a Th2-predominant environment that promotes dysregulated IgA production.
The ‘hygiene hypothesis’
It has been hypothesized that the incidence of different forms of GN throughout the world is related not only to genetic factors and the relevant aetiological agent or antigen, but also to the nature and extent of exposure to pathogens in early life [90]. This links the ‘hygiene hypothesis’, that proposes that an individual's predisposition to developing Th1 or Th2 predominant responses is determined in part by the context of his or her early encounters with antigens [91–94], to outcomes in GN. This hypothesis may help to explain discordance in GN both between countries and between different groups within countries [90,95]. In particular, this theory may explain the relatively high incidence of proliferative GN (including membranoproliferative GN, associated with Th1 responses [1] in developing countries and the relatively higher incidence of minimal change disease and IgA nephropathy in developed countries.
Implications for future therapeutic interventions
Current therapies targeting Th1/Th2 responses that may be applicable to the treatment of GN include immune deviation by oral tolerance, cytokine-based immunotherapies, for example anti-TNF antibodies, anti-IL-12p40 antibodies that neutralize both IL-12 and IL-23, recombinant IL-10, anti-adhesion molecule therapies and chemokine receptor blockade. These approaches are currently being trialled in other immune diseases, including multiple sclerosis [96] and psoriasis. In the case of anti-TNF therapies, these are entering standard care in rheumatoid arthritis and Crohn's disease.
There are several challenges to overcome before these therapies become a reality for immune-mediated glomerular injury. These include the wide variety of different clinical and histological patterns of GN and relatively low numbers of patients in each group, the particular vulnerability of the glomerulus to inflammatory injury contributing to frequent late presentation of patients with established and perhaps irreversible disease, and our difficulties in defining pathogenic mechanisms of GN rapidly and clearly in individuals in their clinical context.
There are promising early clinical trials suggesting efficacy of immunomodulatory therapies, such as monoclonal anti-TNF antibodies in ANCA-associated vasculitis and GN [97]. The complexities of these therapies are highlighted by a report of development of cutaneous vasculitis and/or lupus with anti-TNF therapies [98]. Further work into disease pathogenesis, as well as the formation of large cooperative clinical trial centres, is needed to move the treatment of immune glomerular injury into the 21st century.
References
- 1.Holdsworth SR, Kitching AR, Tipping PG. Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 1999;55:1198–216. doi: 10.1046/j.1523-1755.1999.00369.x. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–47. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 3.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 4.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 5.Del Prete G. The concept of type-1 and type-2 helper T cells and their cytokines in humans. Int Rev Immunol. 1998;16:427–55. doi: 10.3109/08830189809043004. [DOI] [PubMed] [Google Scholar]
- 6.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 7.Koch F, Stanzl U, Jennewein P, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V, Bhardwaj V, Soares L, Alexander J, Sette A, Sercarz E. Major histocompatibility complex binding affinity of an antigenic determinant is crucial for the differential secretion of interleukin 4/5 or interferon gamma by T cells. Proc Natl Acad Sci USA. 1995;92:9510–4. doi: 10.1073/pnas.92.21.9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grakoui A, Donermeyer DL, Kanagawa O, Murphy KM, Allen PM. TCR-independent pathways mediate the effects of antigen dose and altered peptide ligands on Th cell polarization. J Immunol. 1999;162:1923–30. [PubMed] [Google Scholar]
- 10.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182:1579–84. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon JN, Bretscher PA. Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route and strain of host or parasite. Eur J Immunol. 1998;28:4020–8. doi: 10.1002/(SICI)1521-4141(199812)28:12<4020::AID-IMMU4020>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–58. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 13.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 14.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 15.Jankovic D, Liu Z, Gause WC. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 2001;22:450–7. doi: 10.1016/s1471-4906(01)01975-5. [DOI] [PubMed] [Google Scholar]
- 16.Gorbachev AV, Fairchild RL. CD40 engagement enhances antigen-presenting Langerhans cell priming of IFN-gamma-producing CD4+ and CD8+ T cells independently of IL-12. J Immunol. 2004;173:2443–52. doi: 10.4049/jimmunol.173.4.2443. [DOI] [PubMed] [Google Scholar]
- 17.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 18.Balamuth F, Leitenberg D, Unternaehrer J, Mellman I, Bottomly K. Distinct patterns of membrane microdomain partitioning in Th1 and th2 cells. Immunity. 2001;15:729–38. doi: 10.1016/s1074-7613(01)00223-0. [DOI] [PubMed] [Google Scholar]
- 19.Gimsa U, Mitchison A, Allen R. Inhibitors of Src-family tyrosine kinases favour Th2 differentiation. Cytokine. 1999;11:208–15. doi: 10.1006/cyto.1998.0416. [DOI] [PubMed] [Google Scholar]
- 20.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 21.Yang DD, Conze D, Whitmarsh AJ, et al. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity. 1998;9:575–85. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- 22.Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–5. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 23.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–3. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–74. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim CH, Rott L, Kunkel EJ, et al. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–9. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 28.Huang XR, Tipping PG, Shuo L, Holdsworth SR. Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int. 1997;51:94–103. doi: 10.1038/ki.1997.12. [DOI] [PubMed] [Google Scholar]
- 29.Coelho SN, Saleem S, Konieczny BT, Parekh KR, Baddoura FK, Lakkis FG. Immunologic determinants of susceptibility to experimental glomerulonephritis: role of cellular immunity. Kidney Int. 1997;51:646–52. doi: 10.1038/ki.1997.94. [DOI] [PubMed] [Google Scholar]
- 30.van Vliet E, Uhrberg M, Stein C, Gleichmann E. MHC control of IL-4-dependent enhancement of B cell Ia expression and Ig class switching in mice treated with mercuric chloride. Int Arch Allergy Immunol. 1993;101:392–401. doi: 10.1159/000236482. [DOI] [PubMed] [Google Scholar]
- 31.Kitching AR, Tipping PG, Kurimoto M, Holdsworth SR. IL-18 has IL-12-independent effects in delayed-type hypersensitivity: studies in cell-mediated crescentic glomerulonephritis. J Immunol. 2000;165:4649–57. doi: 10.4049/jimmunol.165.8.4649. [DOI] [PubMed] [Google Scholar]
- 32.Kitching AR, Holdsworth SR, Tipping PG. IFN-gamma mediates crescent formation and cell-mediated immune injury in murine glomerulonephritis. J Am Soc Nephrol. 1999;10:752–9. doi: 10.1681/ASN.V104752. [DOI] [PubMed] [Google Scholar]
- 33.Timoshanko JR, Sedgwick JD, Holdsworth SR, Tipping PG. Intrinsic renal cells are the major source of tumor necrosis factor contributing to renal injury in murine crescentic glomerulonephritis. J Am Soc Nephrol. 2003;14:1785–93. doi: 10.1097/01.asn.0000073902.38428.33. [DOI] [PubMed] [Google Scholar]
- 34.Kitching AR, Tipping PG, Holdsworth SR. IL-12 directs severe renal injury, crescent formation and Th1 responses in murine glomerulonephritis. Eur J Immunol. 1999;29:1–10. doi: 10.1002/(SICI)1521-4141(199901)29:01<1::AID-IMMU1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 35.Kohno K, Kataoka J, Ohtsuki T, et al. IFN-gamma-inducing factor (IGIF) is a co-stimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–50. [PubMed] [Google Scholar]
- 36.Bossu P, Neumann D, Del Giudice E, et al. IL-18 cDNA vaccination protects mice from spontaneous lupus-like autoimmune disease. Proc Natl Acad Sci USA. 2003;100:14181–6. doi: 10.1073/pnas.2336094100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kikawada E, Lenda DM, Kelley VR. IL-12 deficiency in MRL-Fas (lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol. 2003;170:3915–25. doi: 10.4049/jimmunol.170.7.3915. [DOI] [PubMed] [Google Scholar]
- 38.Yasuda T, Yoshimoto T, Tsubura A, Matsuzawa A. Clear suppression of Th1 responses but marginal amelioration of autoimmune manifestations by IL-12p40 transgene in MRL-FAS (lprcg) /FAS (lprcg) mice. Cell Immunol. 2001;210:77–86. doi: 10.1006/cimm.2001.1818. [DOI] [PubMed] [Google Scholar]
- 39.Kalluri R, Danoff TM, Okada H, Neilson EG. Susceptibility to anti-glomerular basement membrane disease and Goodpasture syndrome is linked to MHC class II genes and the emergence of T cell-mediated immunity in mice. J Clin Invest. 1997;100:2263–75. doi: 10.1172/JCI119764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitching AR, Turner AL, Semple T Li, et al. Experimental autoimmune anti-glomerular basement membrane glomerulonephritis: a protective role for IFN-gamma. J Am Soc Nephrol. 2004;15:1764–74. doi: 10.1097/01.asn.0000128968.27705.5e. [DOI] [PubMed] [Google Scholar]
- 41.Kitching AR, Tipping PG, Mutch DA, Huang XR, Holdsworth SR. Interleukin-4 deficiency enhances Th1 responses and crescentic glomerulonephritis in mice. Kidney Int. 1998;53:112–8. doi: 10.1046/j.1523-1755.1998.00733.x. [DOI] [PubMed] [Google Scholar]
- 42.Kitching AR, Tipping PG, Timoshanko JR, Holdsworth SR. Endogenous interleukin-10 regulates Th1 responses that induce crescentic glomerulonephritis. Kidney Int. 2000;57:518–25. doi: 10.1046/j.1523-1755.2000.00872.x. [DOI] [PubMed] [Google Scholar]
- 43.Tipping PG, Kitching AR, Huang XR, Mutch DA, Holdsworth SR. Immune modulation with interleukin-4 and interleukin-10 prevents crescent formation and glomerular injury in experimental glomerulonephritis. Eur J Immunol. 1997;27:530–7. doi: 10.1002/eji.1830270226. [DOI] [PubMed] [Google Scholar]
- 44.Kitching AR, Tipping PG, Huang XR, Mutch DA, Holdsworth SR. Interleukin-4 and interleukin-10 attenuate established crescentic glomerulonephritis in mice. Kidney Int. 1997;52:52–9. doi: 10.1038/ki.1997.303. [DOI] [PubMed] [Google Scholar]
- 45.Higuchi N, Maruyama H, Kuroda T, et al. Hydrodynamics-based delivery of the viral interleukin-10 gene suppresses experimental crescentic glomerulonephritis in Wistar-Kyoto rats. Gene Ther. 2003;10:1297–310. doi: 10.1038/sj.gt.3301988. [DOI] [PubMed] [Google Scholar]
- 46.Savignac M, Gomes B, Gallard A, et al. Dihydropyridine receptors are selective markers of Th2 cells and can be targeted to prevent Th2-dependent immunopathological disorders. J Immunol. 2004;172:5206–12. doi: 10.4049/jimmunol.172.9.5206. [DOI] [PubMed] [Google Scholar]
- 47.Kitching AR, Turner AL, Wilson GR, Edgtton KL, Tipping PG, Holdsworth SR. Endogenous IL-13 limits humoral responses and injury in experimental glomerulonephritis but does not regulate Th1 cell-mediated crescentic glomerulonephritis. J Am Soc Nephrol. 2004;15:2373–82. doi: 10.1097/01.ASN.0000138545.89960.3A. [DOI] [PubMed] [Google Scholar]
- 48.Li S, Holdsworth SR, Tipping PG. B7.1 and B7.2 co-stimulatory molecules regulate crescentic glomerulonephritis. Eur J Immunol. 2000;30:1394–401. doi: 10.1002/(SICI)1521-4141(200005)30:5<1394::AID-IMMU1394>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 49.Odobasic D, Kitching AR, Tipping PG, Holdsworth SR. CD80 and CD86 co-stimulatory molecules regulate crescentic glomerulonephritis by different mechanisms. Kidney Int. 2005;68:584–94. doi: 10.1111/j.1523-1755.2005.00436.x. [DOI] [PubMed] [Google Scholar]
- 50.Okano K, Nitta K, Ogawa S, et al. Effects of double blockade of CD28 and inducible-co-stimulator signaling on anti-glomerular basement membrane glomerulonephritis. J Lab Clin Med. 2004;144:183–92. doi: 10.1016/j.lab.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Nitta K, Horita S, Ogawa S, et al. Resistance of CD28-deficient mice to autologous phase of anti-glomerular basement membrane glomerulonephritis. Clin Exp Nephrol. 2003;7:104–12. doi: 10.1007/s10157-003-0225-3. [DOI] [PubMed] [Google Scholar]
- 52.Iwai H, Abe M, Hirose S, et al. Involvement of inducible co-stimulator-B7 homologous protein co-stimulatory pathway in murine lupus nephritis. J Immunol. 2003;171:2848–54. doi: 10.4049/jimmunol.171.6.2848. [DOI] [PubMed] [Google Scholar]
- 53.Peng X, Kasran A, Warmerdam PA, de Boer M, Ceuppens JL. Accessory signaling by CD40 for T cell activation. induction of Th1 and Th2 cytokines and synergy with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26:1621–7. doi: 10.1002/eji.1830260732. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds J, Khan SB, Allen AR, Benjamin CD, Pusey CD. Blockade of the CD154-CD40 co-stimulatory pathway prevents the development of experimental autoimmune glomerulonephritis. Kidney Int. 2004;66:1444–52. doi: 10.1111/j.1523-1755.2004.00907.x. [DOI] [PubMed] [Google Scholar]
- 55.Ruth AJ, Kitching AR, Semple TJ, Tipping PG, Holdsworth SR. Intrinsic renal cell expression of CD40 directs Th1 effectors inducing experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2003;14:2813–22. doi: 10.1097/01.asn.0000091381.60059.fb. [DOI] [PubMed] [Google Scholar]
- 56.Ruth AJ, Kitching AR, Li M, et al. An IL-12-independent role for CD40-CD154 in mediating effector responses: studies in cell-mediated glomerulonephritis and dermal delayed-type hypersensitivity. J Immunol. 2004;173:136–44. doi: 10.4049/jimmunol.173.1.136. [DOI] [PubMed] [Google Scholar]
- 57.Anders HJ, Banas B, Linde Y, et al. Bacterial CpG-DNA aggravates immune complex glomerulonephritis: role of TLR9-mediated expression of chemokines and chemokine receptors. J Am Soc Nephrol. 2003;14:317–26. doi: 10.1097/01.asn.0000042169.23931.73. [DOI] [PubMed] [Google Scholar]
- 58.Sato MN, Minoprio P, Avrameas S, Ternynck T. Changes in the cytokine profile of lupus-prone mice (NZB/NZW) F1 induced by Plasmodium chabaudi and their implications in the reversal of clinical symptoms. Clin Exp Immunol. 2000;119:333–9. doi: 10.1046/j.1365-2249.2000.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baxter AG, Horsfall AC, Healey D, Ozegbe P, Day S, Williams DG, Cooke A. Mycobacteria precipitate an SLE-like syndrome in diabetes-prone NOD mice. Immunology. 1994;83:227–31. [PMC free article] [PubMed] [Google Scholar]
- 60.Singh RR, Saxena V, Zang S, et al. Differential contribution of IL-4 and STAT6 vs STAT4 to the development of lupus nephritis. J Immunol. 2003;170:4818–25. doi: 10.4049/jimmunol.170.9.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoh K, Shibuya K, Morito N, et al. Transgenic overexpression of GATA-3 in T lymphocytes improves autoimmune glomerulonephritis in mice with a BXSB/MpJ-Yaa genetic background. J Am Soc Nephrol. 2003;14:2494–502. doi: 10.1097/01.asn.0000086473.23379.25. [DOI] [PubMed] [Google Scholar]
- 62.Arrizabalaga P, Sans Boix A, Torras Rabassa A, Darnell Tey A, Revert Torrellas L. Monoclonal antibody analysis of crescentic membranous glomerulonephropathy. Am J Nephrol. 1998;18:77–82. doi: 10.1159/000013310. [DOI] [PubMed] [Google Scholar]
- 63.Kim YS, Zheng S, Yang SH, et al. Differential expression of various cytokine and chemokine genes between proliferative and non-proliferative glomerulonephritides. Clin Nephrol. 2001;56:199–206. [PubMed] [Google Scholar]
- 64.Furuichi K, Wada T, Sakai N, et al. Distinct expression of CCR1 and CCR5 in glomerular and interstitial lesions of human glomerular diseases. Am J Nephrol. 2000;20:291–9. doi: 10.1159/000013603. [DOI] [PubMed] [Google Scholar]
- 65.Cairns LS, Phelps RG, Bowie L, et al. The fine specificity and cytokine profile of T-helper cells responsive to the alpha3 chain of type IV collagen in Goodpasture's disease. J Am Soc Nephrol. 2003;14:2801–12. doi: 10.1097/01.asn.0000091588.80007.0e. [DOI] [PubMed] [Google Scholar]
- 66.Salama AD, Chaudhry AN, Holthaus KA, et al. Regulation by CD25+ lymphocytes of autoantigen-specific T-cell responses in Goodpasture's (anti-GBM) disease. Kidney Int. 2003;64:1685–94. doi: 10.1046/j.1523-1755.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- 67.Wu J, Hicks J, Borillo J, Glass WF, II, Lou YH. CD4(+) T cells specific to a glomerular basement membrane antigen mediate glomerulonephritis. J Clin Invest. 2002;109:517–24. doi: 10.1172/JCI13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dean EG, Wilson GR, Li M, et al. Experimental autoimmune Goodpasture's disease: a pathogenetic role for both effector cells and antibody in injury. Kidney Int. 2005;67:566–75. doi: 10.1111/j.1523-1755.2005.67113.x. [DOI] [PubMed] [Google Scholar]
- 69.Weber M, Lohse AW, Manns M, Meyer zum Buschenfelde KH, Kohler H. IgG subclass distribution of autoantibodies to glomerular basement membrane in Goodpasture's syndrome compared to other autoantibodies. Nephron. 1988;49:54–7. doi: 10.1159/000184986. [DOI] [PubMed] [Google Scholar]
- 70.Kohda T, Okada S, Hayashi A, et al. High nephritogenicity of monoclonal antibodies belonging to IgG2a and IgG2b subclasses in rat anti-GBM nephritis. Kidney Int. 2004;66:177–86. doi: 10.1111/j.1523-1755.2004.00719.x. [DOI] [PubMed] [Google Scholar]
- 71.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–63. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cunningham MA, Huang XR, Dowling JP, Tipping PG, Holdsworth SR. Prominence of cell-mediated immunity effectors in ‘pauci-immune’ glomerulonephritis. J Am Soc Nephrol. 1999;10:499–506. doi: 10.1681/ASN.V103499. [DOI] [PubMed] [Google Scholar]
- 73.Masutani K, Tokumoto M, Nakashima H, et al. Strong polarization toward Th1 immune response in ANCA-associated glomerulonephritis. Clin Nephrol. 2003;59:395–405. doi: 10.5414/cnp59395. [DOI] [PubMed] [Google Scholar]
- 74.Ludviksson BR, Sneller MC, Chua KS, et al. Active Wegener's granulomatosis is associated with HLA-DR+ CD4+ T cells exhibiting an unbalanced Th1-type T cell cytokine pattern: reversal with IL-10. J Immunol. 1998;160:3602–9. [PubMed] [Google Scholar]
- 75.Popa ER, Franssen CF, Limburg PC, Huitema MG, Kallenberg CG, Tervaert JW. In vitro cytokine production and proliferation of T cells from patients with anti-proteinase 3- and antimyeloperoxidase-associated vasculitis, in response to proteinase 3 and myeloperoxidase. Arthritis Rheum. 2002;46:1894–904. doi: 10.1002/art.10384. [DOI] [PubMed] [Google Scholar]
- 76.Holland M, Hewins P, Goodall M, Adu D, Jefferis R, Savage CO. Anti-neutrophil cytoplasm antibody IgG subclasses in Wegener's granulomatosis: a possible pathogenic role for the IgG4 subclass. Clin Exp Immunol. 2004;138:183–92. doi: 10.1111/j.1365-2249.2004.02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muller A, Trabandt A, Gloeckner-Hofmann K, et al. Localized Wegener's granulomatosis. predominance of CD26 and IFN-gamma expression. J Pathol. 2000;192:113–20. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH656>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 78.Balding CE, Howie AJ, Drake-Lee AB, Savage CO. Th2 dominance in nasal mucosa in patients with Wegener's granulomatosis. Clin Exp Immunol. 2001;125:332–9. doi: 10.1046/j.1365-2249.2001.125002332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawasaki Y, Suzuki J, Sakai N, et al. Evaluation of T helper-1/-2 balance on the basis of IgG subclasses and serum cytokines in children with glomerulonephritis. Am J Kidney Dis. 2004;44:42–9. doi: 10.1053/j.ajkd.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 80.Masutani K, Akahoshi M, Tsuruya K, et al. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 2001;44:2097–106. doi: 10.1002/1529-0131(200109)44:9<2097::AID-ART360>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 81.Chan RW, Tam LS, Li EK, et al. Inflammatory cytokine gene expression in the urinary sediment of patients with lupus nephritis. Arthritis Rheum. 2003;48:1326–31. doi: 10.1002/art.11062. [DOI] [PubMed] [Google Scholar]
- 82.Imai H, Hamai K, Komatsuda A, Ohtani H, Miura AB. IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int. 1997;51:270–6. doi: 10.1038/ki.1997.32. [DOI] [PubMed] [Google Scholar]
- 83.Doi T, Mayumi M, Kanatsu K, Suehiro F, Hamashima Y. Distribution of IgG subclasses in membranous nephropathy. Clin Exp Immunol. 1984;58:57–62. [PMC free article] [PubMed] [Google Scholar]
- 84.Haas M. IgG subclass deposits in glomeruli of lupus and nonlupus membranous nephropathies. Am J Kidney Dis. 1994;23:358–64. doi: 10.1016/s0272-6386(12)80997-8. [DOI] [PubMed] [Google Scholar]
- 85.Roberts JL, Wyatt RJ, Schwartz MM, Lewis EJ. Differential characteristics of immune-bound antibodies in diffuse proliferative and membranous forms of lupus glomerulonephritis. Clin Immunol Immunopathol. 1983;29:223–41. doi: 10.1016/0090-1229(83)90026-0. [DOI] [PubMed] [Google Scholar]
- 86.Yap HK, Cheung W, Murugasu B, Sim SK, Seah CC, Jordan SC. Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: evidence for increased IL-13 mRNA expression in relapse. J Am Soc Nephrol. 1999;10:529–37. doi: 10.1681/ASN.V103529. [DOI] [PubMed] [Google Scholar]
- 87.Thomson PD, Stokes CR, Barratt TM, Turner MW, Soothill JF. HLA antigens and atopic features in steroid-responsive nephrotic syndrome of childhood. Lancet. 1976;2:765–8. doi: 10.1016/s0140-6736(76)90600-0. [DOI] [PubMed] [Google Scholar]
- 88.Masutani K, Taniguchi M, Nakashima H, et al. Up-regulated interleukin-4 production by peripheral T-helper cells in idiopathic membranous nephropathy. Nephrol Dial Transplant. 2004;19:580–6. doi: 10.1093/ndt/gfg572. [DOI] [PubMed] [Google Scholar]
- 89.Hirayama K, Ebihara I, Yamamoto S, et al. Predominance of type-2 immune response in idiopathic membranous nephropathy: cytoplasmic cytokine analysis. Nephron. 2002;91:255–61. doi: 10.1159/000058401. [DOI] [PubMed] [Google Scholar]
- 90.Johnson RJ, Hurtado A, Merszei J, Rodriguez-Iturbe B, Feng L. Hypothesis: dysregulation of immunologic balance resulting from hygiene and socioeconomic factors may influence the epidemiology and cause of glomerulonephritis worldwide. Am J Kidney Dis. 2003;42:575–81. doi: 10.1016/s0272-6386(03)00801-1. [DOI] [PubMed] [Google Scholar]
- 91.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–9. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 92.Jones PD, Gibson PG, Henry RL. The prevalence of asthma appears to be inversely related to the incidence of typhoid and tuberculosis: hypothesis to explain the variation in asthma prevalence around the world. Med Hypotheses. 2000;55:40–2. doi: 10.1054/mehy.1999.0997. [DOI] [PubMed] [Google Scholar]
- 93.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matricardi PM, Ronchetti R. Are infections protecting from atopy? Curr Opin Allergy Clin Immunol. 2001;1:413–9. doi: 10.1097/01.all.0000011054.18314.67. [DOI] [PubMed] [Google Scholar]
- 95.The New Zealand Glomerulonephritis Study. Introductory report. Clin Nephrol. 1989;31:239–46. [PubMed] [Google Scholar]
- 96.Fox RJ, Ransohoff RM. New directions in MS therapeutics: vehicles of hope. Trends Immunol. 2004;25:632–6. doi: 10.1016/j.it.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 97.Booth A, Harper L, Hammad T, et al. Prospective study of TNFalpha blockade with infliximab in anti-neutrophil cytoplasmic antibody-associated systemic vasculitis. J Am Soc Nephrol. 2004;15:717–21. doi: 10.1097/01.asn.0000114554.67106.28. [DOI] [PubMed] [Google Scholar]
- 98.Hyrich KL, Silman AJ, Watson KD, Symmons DP. Anti-tumour necrosis factor alpha therapy in rheumatoid arthritis: an update on safety. Ann Rheum Dis. 2004;63:1538–43. doi: 10.1136/ard.2004.024737. [DOI] [PMC free article] [PubMed] [Google Scholar]