Abstract
Autoimmune thyroiditis in humans has been linked to excess iodine intake. A causative relationship between dietary iodine and thyroiditis has been clearly established in animal models of thyroiditis, including the NOD.H2h4 mouse strain, which develops enhanced thyroiditis spontaneously after supplementation of drinking water with sodium iodide. To assess the mechanisms by which iodine may contribute to disease pathogenesis, we have purified hypoiodinated thyroglobulin (Lo-I Tg) from the thyroids of mice fed methimazole and potassium perchlorate. This preparation contained only a trace of iodine and was poorly reactive to monoclonal antibody 42C3, which has been shown previously to distinguish hypoiodinated from normal Tg. A cloned T cell line 2D11 from a diseased NOD.H2h4 mouse proliferated in response to normal Tg, but not to Lo-I Tg. Serum antibodies from NOD.H2h4 mice with thyroiditis were poorly reactive to Lo-I Tg. To determine that these changes were due specifically to iodine content, Lo-I Tg was reiodinated in vitro. Reiodination of Lo-I Tg partially re-established the reactivity of NOD.H2h4 serum antibodies. The data demonstrate that the reactivity of thyroglobulin-specific antibodies and certain T cells are dependent on the iodine content of thyroglobulin. These findings suggest that iodine contributes to autoimmune thyroiditis in the NOD.H2h4 mouse by directly enhancing the antigenicity of thyroglobulin.
Keywords: antigens/epitopes, autoantibodies, autoimmunity, rodent, T lymphocytes
Introduction
Environmental factors such as infection or diet may trigger autoimmunity in genetically predisposed hosts; however, little is understood about how these factors may interact with self-antigens to promote an autoimmune response. One such antigen, thyroglobulin (Tg), is well defined as a major autoantigen in chronic lymphocytic (Hashimoto's) thyroiditis (HT) in humans and its animal model counterpart, experimental autoimmune thyroiditis (EAT). Tg is a large (∼660 kDa), highly glycosylated homodimeric molecule integral to thyroid hormonogenesis. The iodination of selected tyrosyl residues as precursors for the two iodothyronine thyroid hormones thyroxine (T4) and triiodothyronine (T3) is essential to its endocrine function.
Circumstantial evidence suggests a causal link between increased iodine intake and enhanced risk for HT in some patients [1,2]. In studies on human patients with HT, antibody and T cell reactivity to Tg were reduced in relation to reduced iodine content of the Tg molecule [3–6]. The susceptibility of rodent models, such as the diabetes-prone Bio-Breeding rat, to autoimmune thyroiditis can be attenuated by manipulation of host iodine metabolism [7,8].
The NOD.H2h4 strain is a recently developed model useful for dissecting the relationship between iodine and thyroiditis. Derived from the autoimmunity-prone non-obese diabetic (NOD) mouse strain, NOD.H2h4 mice spontaneously develop autoimmune thyroiditis over time, a process significantly accelerated and enhanced by supplementation of dietary iodine [9,10]. Because these experiments exclusively used younger mice, between 16 and 18 weeks of age at the time of killing, we further wanted to determine if iodine supplementation has the same effect on older mice.
The NOD.H2h4 strain presents an attractive opportunity by which to explore the mechanism of iodine in enhancing autoimmunity. One possible mechanism by which iodine may contribute to thyroiditiogenesis is by direct modification of the Tg molecule; increased iodination of Tg may generate novel epitopes that are recognized with greater affinity than antigenic determinants that lack iodine. If the antigenicity of mouse thyroglobulin (mTg) is enhanced by iodination, then mTg deficient in iodine content should be poorly immunogenic. As a corollary, re-establishing the iodine content of hypoiodinated mTg should restore its antigenicity.
The goal of these studies was to assess the reactivity of the autoimmune response in the NOD.H2h4 mouse model to preparations of Tg differing in iodine content. Antibody reactivity and T cell proliferation to poorly iodinated (Lo-I) Tg were markedly reduced. Antibody reactivity could be restored partially by in vitro reiodination (Re-I) of Tg. These data support the conclusion that humoral and cellular immune responses against Tg in the NOD.H2h4 mouse are dependent on the presence of iodine on the Tg molecule.
Materials and methods
Reagents and antibodies
Monoclonal antibodies (mAbs) 42C3, 121B2 and 156A6 were supernatants of murine hybridomas raised against human Tg (huTg) but shown to be cross-reactive to mouse Tg (mTg) [11]. mAb 42C3 reactivity is iodine-dependent, as it does not recognize huTg from an endemic thyroid goitre with an undetectable iodine content [12]. Furthermore, the binding to huTg could be inhibited competitively by T4 or T3, but not non-iodothyroxine (T0) [12]. 121B2 and 156A6 binding to Tg is not I-dependent [3].
The antigenic specificity of mAb 42C3 has been described previously [3,12]. The reactivity of mAb 42C3 crosses species specificity, indicating that it recognizes a conserved epitope on the Tg molecule. Furthermore, it does not react to other iodinated control proteins such as bovine serum albumin iodinated in vitro, indicating that iodination is insufficient by itself to cause reactivity to this antibody [12].
NOD.H2h4 mice
Animals were housed in the JHU Animal Services conventional facilities operated under the guidelines of the American Association for the Accreditation of Laboratory Animal Care (AAALAC). Founder mice were the gift of Dr Linda Wicker (Merck Laboratories, Rahway, NJ, USA). Thyroiditis was assessed by resection of thyroid after euthanasia and histological grading as described previously [9]. Briefly, 5 µm haemotoxylin and eosin-stained thyroid sections were graded by the following scale according to the approximate area of histological infiltration: 0 = no disease; 1 = 1–20%; 2 = 20–30%; 3 = 30–50%; 4 > 50%.
Purification of mTg
Mouse Tg was purified as described previously [5]. For the Lo-I protein, thyroids were collected from mice given drinking water supplemented with 0·05% (w/v) methimazole (Sigma, St Louis, MO, USA) and 1·0% (w/v) potassium perchlorate (Sigma) for 5 weeks. A total of 120 Lo-I and 300 normal (Norm-I) thyroid glands were resected and homogenized into phosphate buffered saline (PBS) containing protease inhibitors. Debris was removed by centrifugation and supernatant was applied to a 1·6 × 88 cm2 Sephacryl S300 column equilibrated to PBS at a flow rate of 0·3 ml/min.
Iodine content assay and in vitro iodination
The iodine content of each preparation was assessed as previously described [5]. Briefly, 5 µg of protein were hydrolysed in concentrated H2SO4. Organified iodine was displaced by 1 mM bromide and the change in OD405 in a ceric ammonium sulphate/sodium arsenate redox reaction plotted against a standard curve. For in vitro reiodination of Lo-I Tg, four iodobeads (Pierce, Rockford, IL, USA) were primed in 1·5 mM potassium iodide (Sigma) and added to 600 µg of Lo-I or Norm-I Tg for 30 min at room temperature with continuous gentle agitation. Supernatants were dialysed against four changes of PBS [3], and the product was designated Re-I Tg.
SDS-PAGE and Western blot
Protein, 17 µg, was loaded into a 4–15% Tris-HCl gradient gel (Bio-Rad, Hercules, CA, USA) in denaturing, non-reducing loading buffer and run at 50 V for 3·5 h. Gels were either silver-stained (Pierce) or transferred onto Hybond-ECl nitrocellulose (Amersham Biosciences, Piscataway, NJ, USA) at 300 mA for 1 h at 4°C, blocked overnight in PBS +5% non-fat dry milk at 4°C, washed in PBS +0·05% Tween-20 and incubated with either 1 : 1000 gt α-mTg or 1 : 500 with 42C3 hybridoma supernatant. Goat anti-mTg was identified with 1 : 500 rabbit anti-goat IgG-biotin (KPL, Gaithersburg, MD, USA) and 42C3 identified by goat anti-mouse IgG-biotin (Sigma). Both were revealed with 1 : 1000 extravidin–peroxidase (Sigma) and developed in 3,3′-diaminobenzidine (Sigma).
Enzyme-linked immunosorbent assay (ELISA)
To evaluate the quantity of Tg in each preparation, microtitre plates were coated with each mTg at a concentration of 1 µg/ml and probed using a goat antibody against mouse Tg, followed by washing and secondary incubation with polyclonal rabbit anti-goat Ig (KPL) secondary antibody conjugated to biotin followed by extravidin–peroxidase (Sigma). 3,3′,5,5 Tetramethyl benzidine dihydrochloride (TMB) (KPL) was added as substrate and the plate was incubated for 5 min. The reaction was stopped with 0·18 M H2SO4 and the OD was read at 450 nm in a 96-well plate reader (Dynex, Chantilly, VA, USA).
To determine the reactivity of monoclonal antibodies to the Tg preparations, microtitre plates were coated with Lo-I, Re-I and Norm-I as above and probed by mAbs 42C3, 121B2 and 156A6 supernatants diluted 1 : 100 in PBS followed by biotinylated goat α-mouse Ig (Sigma) secondary, then extravidin–phosphatase (Sigma). Substrate p-nitrophenyl phosphate (pNPP) (Sigma) was added, incubated for 20 min and stopped with 3 M NaOH.
To determine Tg antibody levels in serum, maxiSorp 96-well plates (Nalge Nunc, Rochester, NY, USA) were coated with 1 µg/ml of Lo-I, Re-I and Norm-I, then blocked with 1% bovine serum albumin (BSA) (Sigma). Sera diluted in PBS were incubated with each preparation, washed and incubated with either 1 : 750 rabbit anti-mouse IgG1 (ICN, Irvine, CA, USA) or 1 : 1000 rabbit anti-mouse IgG2b (ICN). OD405 was read after development with pNPP incubated for 30 min and stopped with 3 M NaOH.
T cell generation and proliferation
T cell lines were generated in our laboratory from splenocytes following multiple rounds of stimulation with mTg. A CD4+ line, 2D11, was selected as an mTg-specific representative clone [13]; 1 × 104 cells in 200 µl of complete RPMI-1640 were stimulated with 450 ng of mTg in triplicate. After 48 h at 37°C in 5% CO2, cells were pulsed with 1 µCi of [3H]-methyl thymidine (Amersham) for 18 h. Incorporated specific radioactivity was measured by a,-scintillation counter (Wallac, Finland).
Statistics
Statistical tests are used as noted, as examined by Microsoft Excel (Microsoft, Redmond, WA, USA) for Students’t-test or Sigma Stat (Jandel Scientific, San Rafael, CA, USA) for Mann–Whitney U-test or Fisher's exact test.
Results
Iodine accelerates and enhances thyroid disease of NOD.H2h4 mice
In order to determine whether iodine accelerates the course or exacerbates the severity of thyroid disease in older animals, thyroid disease was compared in iodine-treated mice and age-matched untreated mice over a span of age ranges. As seen in Table 1, iodine-fed NOD.H2h4 mice developed thyroid disease with earlier onset, greater prevalence and increased severity compared to age-matched untreated NOD.H2h4 animals.
Table 1. Prevalence and severity of thyroiditis in NOD.H2h4 in mice with increasing age.
| Affected | Thyroid histopathology score (n) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (weeks) | Treatment | Total n | n | % | 0 | 1 | 2 | 3 | 4 | Severity U-test† |
| < 16 | 8 weeks 0·15% NaI | 15 | 12* | 80·0 | 3 | 12 | 0 | 0 | 0 | |
| 16–23 | 21 | 18* | 85·7 | 3 | 11 | 4 | 3 | 0 | <2 × 10−6 | |
| 24–27 | 35 | 29* | 82·9 | 6 | 12 | 9 | 7 | 1 | <2 × 10−6 | |
| 28–35 | 8 | 8* | 100·0 | 0 | 0 | 2 | 3 | 3 | 0·0037 | |
| > 36 | 30 weeks 0·15% NaI | 21 | 21 | 100·0 | 0 | 4 | 5 | 2 | 10 | |
| < 16 | Untreated | 2 | 0 | 0·0 | 2 | 0 | 0 | 0 | 0 | |
| 16–23 | 19 | 1 | 5·3 | 18 | 1 | 0 | 0 | 0 | ||
| 24–27 | 18 | 3 | 16·7 | 15 | 3 | 0 | 0 | 0 | ||
| 28–35 | 9 | 4 | 44·4 | 5 | 2 | 1 | 0 | 1 | ||
| 36–47 | 44 | 17 | 38·6 | 27 | 12 | 3 | 0 | 2 | ||
| 48–55 | 49 | 18 | 36·7 | 31 | 8 | 1 | 3 | 6 | ||
| > 55 | 23 | 13 | 56·5 | 10 | 6 | 2 | 0 | 5 | ||
NOD.H2h4 mice were either untreated or given drinking water supplemented with NaI for the dose and treatment regimens given prior to killing. Untreated mice represent spontaneous disease development in NOD.H2h4 animals. Mice were killed at the ages indicated and thyroid histopathology was assessed. Statistical analysis of disease prevalence was performed by Fisher's exact test;
Statistical analysis of disease severity was performed by Mann–Whitney U-test rank sum comparison of iodine-treated mice against untreated mice of comparable age.
significant difference (P < 0·05).
Untreated mice developed thyroid infiltrates over time with steadily increasing prevalence and severity. However, the prevalence of thyroid histopathology failed to become completely penetrant in untreated NOD.H2h4 animals, even in mice older than 1 year. The untreated mice that did progress to disease developed thyroid infiltrates with histopathological characteristics similar to the disease of iodine-fed NOD.H2h4 mice, including extensive mononuclear cell infiltration and thyroid follicle disruption (data not shown).
In contrast, 8 weeks of dietary supplementation with 0·15% (w/v) sodium iodide in drinking water was sufficient to induce 100% penetrant thyroid infiltration in mice as young as 28 weeks of age at the time of killing (Table 1). Furthermore, the prevalence of thyroid infiltration was increased significantly in the animals fed with 0·15% NaI at all ages studied, compared to untreated mice (P < 0·03). Treatment with lower doses of iodine, 0·05% (w/v) for 16 weeks also resulted in a significantly more prevalent disease (P = 0·042). Together, these data indicate that dietary iodine supplementation induces thyroid disease in NOD.H2h4 mice by accelerating the onset of thyroid infiltration.
Furthermore, iodine supplementation enhanced the severity of disease in NOD.H2h4 mice. By comparing the area of thyroid infiltration between iodine-treated animals and age-matched untreated mice, disease severity was increased in the iodine-treated animals at all ages studied. Severe thyroid histopathology of Grades 3 or 4 was seen in NOD.H2h4 mice less than 35 weeks of age treated with sodium iodide. This disease appears to advance in severity with age. In contrast, only a single untreated control mouse of comparable age progressed to severe Grade 4 disease (Table 1). Mann–Whitney U-test comparison of the thyroid histopathology scores shown in Table 1 demonstrate a statistically significant increase in the severity of thyroid lesions in the iodine-treated mice, compared to untreated (P < 0·005). Taken together, these findings indicate that iodine induces autoimmune thyroid disease in NOD.H2h4 mice by accelerating the onset of disease and enhancing the prevalence and severity of disease.
Preparation of Lo-I and Re-I Tg
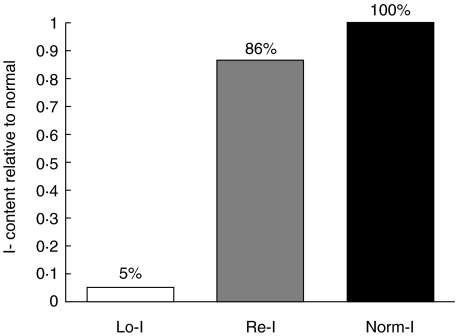
Poorly iodinated (Lo-I) Tg was purified from the thyroids of mice given drinking water supplemented with methimazole and potassium perchlorate to block the uptake and organification of intrathyroidal iodine. Controls were given normal water and normal (Norm-I) Tg purified from their thyroids. The iodine content of each preparation is presented in Fig. 1. The iodine content of Lo-I Tg was 5% that of Norm-I Tg.
Fig. 1.
Normal (Norm-I) and poorly iodinated (Lo-I) mTg were purified from mouse thyroids. Lo-I Tg was reiodinated in vitro (Re-I). The change in OD405 over 1 min was plotted against a standard curve to attain values of atoms of iodide per molecule of mTg. Data are representative of three independent experiments.
Lo-I Tg was reiodinated in vitro as described in Materials and methods. Re-I Tg had a markedly increased iodine content relative to Lo-I Tg, approaching 85% of Norm-I Tg (Fig. 1). Free iodide was not detected in any of the three Tg preparations (data not shown).
Analysis of Lo-I, Re-I and Norm-I Tg
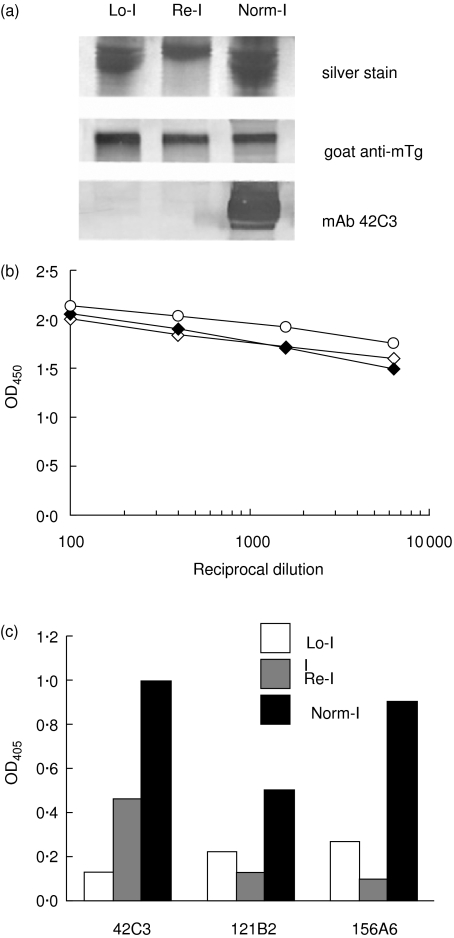
To investigate the properties of mTg with different iodine contents, each sample was analysed by reducing sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot. A major band corresponding to >300 kDa was visible in all three preparations by silver stain and blotting with polyclonal goat anti-mTg (Fig. 2a, top and middle), showing that the mTg was not fundamentally altered by the treatments. Probing mTg with mAb 42C3 demonstrated an iodine-dependent reactivity; Norm-I Tg displayed a major band of > 300 kDa whereas Lo-I Tg did not (Fig. 2a, bottom). Notably, Re-I Tg could not be visualized by immunoblotting with mAb 42C3, possibly because the limit of detection by 42C3 is not sufficiently sensitive to visualize the reiodinated Tg.
Fig. 2.
Analysis of poorly iodinated (Lo-I), poorly iodinated Tg reiodinated in vitro (Re-I) and normal (Norm-I) mTg. (a) Silver stain (top panel) and immunoblots of the proteins probed with a goat antibody against mouse Tg (middle panel) or a monoclonal antibody 42C3 (bottom panel). (b) Relative evaluation by enzyme-linked immunosorbent assay (ELISA) of the quantity of Tg in each preparation using a goat antibody against mouse Tg. (c) Tg preparations probed by monoclonal antibodies 42C3, 121B2 and 156A6. The epitope binding of 42C3 is iodine dependent, while those of 121B2 and 156A6 are not.
We verified that the Re-I Tg partially recovered its immunoreactivity by an ELISA technique described below. To explore further the immunoreactivity of mTg in each preparation, all three preparations were immobilized to microtitre plate wells at equivalent concentrations. Goat anti-mTg antibodies confirmed comparable concentrations of mTg in each preparation by ELISA. At multiple dilutions of antibody, Lo-I, Re-I and Norm-I Tg were equally reactive to the goat anti-mTg antibodies (Fig. 2b). The mTg-coated microtitre plates were then probed with 42C3 as well as two other mAbs (121B2 and 156A6) developed in a manner similar to 42C3, and also cross-reactive to mTg. In contrast to 42C3, 121B2 and 156A6 do not recognize T3 or T4 and do not require iodine for binding. All three mAbs demonstrated decreased binding to poorly iodinated (Lo-I) mTg relative to normal Tg (Fig. 2c). This decrease in reactivity is as great as 10-fold in the case of 42C3 (Fig. 2c). Upon in vitro iodination of Lo-I mTg (Re-I), 42C3 reactivity was partially restored; 42C3 reactivity to Re-I Tg was fourfold greater than to Lo-I Tg. (Fig. 2c). The iodine-dependence of 42C3 reactivity appears to be similar for mouse Tg as for human Tg.
In contrast, 121B2 and 156A6 showed decreased reactivity to Re-I Tg. The decreased reactivities of 121B2 and 156A6 for mTg may be explained in terms of epitope topography. It has been reported that poorly iodinated forms of Tg are conformationally altered [14]. The relatively poor reactivity of 121B2 and 156A6 with Lo-I mTg supports this conclusion. In vitro reiodination may not have been sufficient to restore the conformation of the Lo-I mTg molecule to its native form. As the 121B2 and 156A6 epitopes are not normally iodinated, in vitro iodination may also have further altered epitopes by iodination of tyrosyl residues, shown by Saboori et al. [12]. Taken together, these data support the hypothesis that reiodination of Tg re-establishes some epitopes, such as those recognized by 42C3, but eliminates others, such as those seen by 121B2 and 156A6 [3,12].
Reactivity of NOD.H2h4 antibodies to Lo-I, Re-I & Norm-I Tg
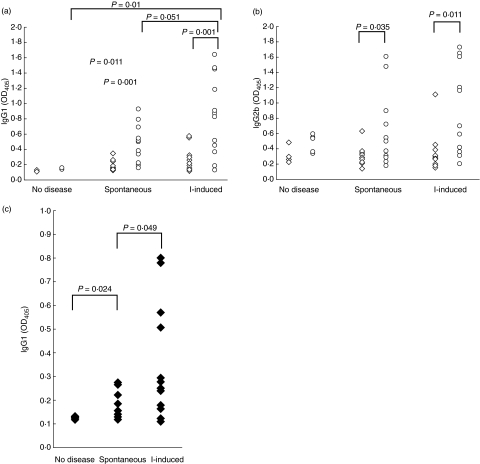
We investigated whether antibodies from mice with iodine-induced thyroiditis would preferentially recognize iodinated forms of thyroglobulin. The reactivity of serum autoantibodies from diseased NOD.H2h4 mice to each Tg preparation was determined. Lo-I, Re-I and Norm-I Tg were immobilized to microtitre plate wells, and incubated with sera from animals with thyroiditis. We speculated further that antibodies from mice with iodine-induced disease might be more dependent on the iodine content of Tg than antibodies from mice with spontaneous disease. Therefore, we included two groups of NOD.H2h4 mice with thyroiditis. One group of animals had developed autoimmune thyroiditis spontaneously by 1 year of age. A second group of younger animals developed the accelerated, enhanced thyroiditis induced by excess iodine intake. Both groups of animals were selected for severe disease, and were comparable in histopathological severity (data not shown). The control group consisted of mice that had failed to develop disease, despite advanced age or iodine supplementation. Isotype-specific secondary antibodies were used to examine the subclass specificity of the antibody response against mTg.
In all diseased animals, shown in Fig. 3, IgG1 and IgG2b antibodies preferentially recognized Norm-I Tg relative to Lo-I (P < 0·035). Animals with either spontaneous or iodine-induced thyroiditis had significantly greater antibody levels than non-diseased animals, as would be expected (P < 0·015). However, animals with iodine-induced thyroiditis appeared to have significantly greater antibody responses to normally iodinated Tg than animals with spontaneous thyroiditis (P = 0·05) (Fig. 3a,b). IgG1 antibodies to reiodinated Tg demonstrated a similar reactivity pattern: sera from animals with either form of disease were significantly more reactive to Re-I Tg than were sera of non-diseased animals (P < 0·05). Notably, sera from animals with iodine-induced thyroiditis had significantly greater reactivity to Re-I Tg than sera from animals with spontaneous thyroiditis (P < 0·05) (Fig. 3c), again suggesting that the development of antibody responses in NOD.H2h4 thyroid disease is directed to newly iodinated epitopes of Tg.
Fig. 3.
Reactivity of sera from NOD.H2h4 against poorly iodinated (Lo-I), poorly iodinated Tg reiodinated in vitro (Re-I) or normal (Norm-I) mTg. Microtitre plates were coated with Lo-I (◊) or Norm-I (○) mTg at a concentration of 1 µg/ml. The reactivity of sera from NOD.H2h4 mice (at 1 : 100 dilution) with iodine-induced (n = 11), spontaneous (n = 12) or no (n = 5) thyroiditis to each Tg was exposed by isotype-specific secondary antibody conjugates for (a) IgG1 and (b) IgG2b. (c) Reactivity of NOD.H2h4 sera with Re-I Tg-coated microtitre plates probed by IgG1-specific secondary antibody conjugate. Animals with iodine-induced disease in this experiment began iodine-supplementation at 8–12 weeks of age, and were continued for 10 weeks. Animals with spontaneous disease were aged 52 ± 10 weeks and fed diets not supplemented with excess iodine. Data represent the mean of duplicate wells. Statistics are by two-tailed Student's t-test.
Proliferation of cloned cell line 2D11 in response to mTg
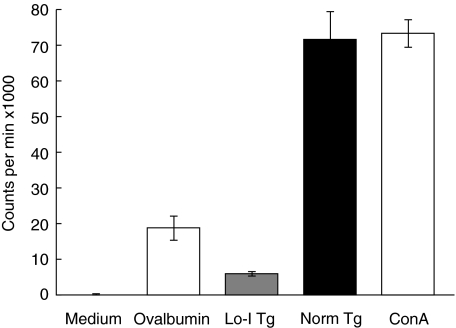
Several cloned CD4+ T cell lines were generated from NOD.H2h4 mice with iodine-induced disease [13]. One of these cloned lines, 2D11, was shown to proliferate with high specificity for mTg. This line was selected to see if it could distinguish Lo-I Tg from Norm-I Tg. Line 2D11 demonstrated a specific proliferative response to Norm-I Tg, relative to medium and ovalbumin. However, 2D11 failed to proliferate in response to equivalent concentrations of Lo-I Tg (Fig. 4). Thus, 2D11 is not only specific for mTg, but discriminates according to the iodine content of the Tg.
Fig. 4.
Proliferative response of T cell-cloned line 2D11 to Lo-I and Norm-I mTg. 1 × 104 cells in 200 µl of complete RPMI were stimulated with 450 ng of mTg for 48 h. The values represent means of triplicate wells. Concanavalin A (ConA) was used as a positive control.
Discussion
The hormonogenic role of thyroglobulin is well described; its autoantigenic properties less so. Evidence in human disease and animal models suggest that the autoantigenicity of thyroglobulin in predisposed individuals may be modulated, even triggered, by its state of iodination. Epidemiological evidence in human disease suggests an association between increased dietary iodine intake and autoimmune thyroiditis in Argentine, Icelandic and Chinese populations [15–18]. Such an association may be significant from a public health perspective, due to the popularity of salt iodization programmes throughout the world. Increased iodine intake through iodinated medication may also have a causative relationship with autoimmune thyroiditis. Iodized oil treatment of non-toxic goitre has been shown to correlate with increased prevalence of autoimmune thyroid disease, as has amiodarone, a common iodine-containing anti-arrhythmic drug [19,20].
In vitro studies of human Hashimoto's thyroiditis (HT) have suggested that the role of iodine in autoimmune thyroid disease may involve its influence on the antigenicity of thyroglobulin. Our laboratory has demonstrated previously that peripheral blood lymphocytes from HT patients, typically responsive to Tg, do not proliferate in response to hypoiodinated human Tg from non-toxic goitrous thyroid. Reiodination of this hypoiodinated huTg partially re-established proliferation [6]. Similarly, the reactivity of serum autoantibodies from HT patients to hypoiodinated huTg was diminished, relative to normal huTg [3–5].
A causal relationship between environmental iodine and autoimmune thyroid disease has been explored further in a variety of animal models. In particular, several disease models have shown that manipulations of iodine metabolism influence susceptibility to thyroiditis. Disease has been shown to be enhanced upon iodine supplementation in the BUF rat [21,22], the BB/W rat [7,8], the Cornell Strain chicken [23,24] and, more recently, the NOD.H2h4 mouse [9,10]. Conversely, thyroiditis is ameliorated by suppression of iodine metabolism in the BUF and BB/W rats [8,22] and the Obese strain chicken [25], and in experimentally induced thyroiditis in mice [26].
The goal of this investigation has been to understand the mechanisms underlying the contribution of iodine to thyroid autoimmunity. Iodine may induce changes in thyrocytes that induce thyroid autoimmunity, as has been postulated for the mechanism of iodine-enhanced thyroiditis in the Obese strain chicken [27]. The findings presented here are not inconsistent with this model. Alternatively, iodine may contribute directly to the immunogenicity of thyroglobulin. Iodination of Tg may generate novel epitopes that initiate thyroiditogenesis.
Iodine treatment of other strains of mice induces little or no thyroiditis. Thus, iodine is a prototype of an environmental risk factor that enhances disease in a genetically predisposed host. The data presented here article strongly support the view that one action of iodine is to increase the autoantigenic properties of thyroglobulin in NOD.H2h4 mice. These findings do not exclude other activities of iodine that may also contribute to disease, such as an increase in intrathyroidal reactive oxygen intermediates [28] or increased adhesion molecules [29].
Unfortunately, the primary immunodominant epitopes of Tg remain unidentified. Several lines of study, primarily with human Tg peptides in animal models, have identified pathogenic subdominant peptides, some of which encompass highly conserved hormonogenic tyrosyl residues. Studies using peptides derived from pro-hormonogenic residues from human Tg as immunogens in murine EAT have found that iodination of several of these peptides enhanced EAT. Champion et al. cloned two mouse I-Ak-restricted T cell lines whose proliferation and IL2 release in response to mTg was dependent on the iodination of antigen; these two cell lines did not proliferate in response to poorly iodinated whole mTg, very similar to our 2D11 line [30]. Later this group identified the iodine-dependent cognate peptide of both lines as a highly conserved 9-mer inclusive of the pro-hormonogenic 2553 tyrosyl residue of huTg [31]. Kong et al. found that lymph node cells from mice immunized with a 31-mer peptide (a sequence including Champion's 9-mer) transferred disease to naive recipients [32]. This transfer was enhanced if the immunizing huTg2549–2560 peptide was specifically iodinated at the pro-hormonogenic 2553l-thyroxine residue.
Cross-recognition between iodinated and non-iodinated peptide-stimulated cells showed a similar enhancement of proliferation in response to peptide iodination [32]. There is a lack of consensus in the literature as to whether highly conserved pro-hormonogenic regions of the Tg molecule are more important to thyroid pathogenesis than species-specific structural regions of the molecule [33–37].
It remains unclear whether the pathogenic mechanisms underlying iodine-induced thyroiditis in the NOD.H2h4 mice are the same as the truly spontaneous disease these animals develop. Our finding that iodine supplementation of NOD.H2h4 mice enhances disease well into maturity suggest that iodine possesses immunomodulatory properties independent of the underlying predisposition to autoimmunity conferred by the NOD background of these mice. If iodine were simply accelerating heritable autoimmune mechanisms one might expect that, at later time-points, disease in untreated mice would be indistinguishable from iodine-treated animals. Our antibody and lesion severity data demonstrate that iodine treatment enhances disease regardless of the age of the animal.
By using hypoiodinated native Tg, we are able to assess immunological responses not restricted to individual epitopes. These data demonstrate that antibody and T cell responses to Tg in the NOD.H2h4 mouse are dependent on the iodination of Tg. These findings are consistent with the conclusion that thyroiditis in the NOD.H2h4 mouse is directed in part against iodinated epitopes of thyroglobulin and suggest a direct role for iodine in enhancing the antigenicity of Tg. This hypothesis is supported further by the finding that reiodination of hypoiodinated Tg partially re-establishes its recognition by serum autoantibodies in both spontaneous and iodine-induced models.
We sought to determine whether the epitopes recognized by NOD.H2h4 mice were different depending on whether the animals developed disease spontaneously with age or induced by supplemented iodine intake. We hypothesized that animals with iodine-induced disease preferentially recognize iodinated mTg. We also considered the possibility that untreated animals with spontaneous thyroid disease recognize iodinated epitopes of thyroglobulin. To this end, we produced several forms of mTg with differing iodine contents. By comparing NOD.H2h4 mice with iodine-induced disease to those with spontaneous disease we found that animals with iodine-induced disease direct their antibody response against iodinated thyroglobulin more than animals with purely spontaneous disease. Furthermore, reiodinating hypoiodinated thyroglobulin reestablished antibody reactivity in the iodine-induced disease better than in the spontaneous disease. In addition, the finding that older NOD.H2h4 mice fed iodine have more severe disease than age-matched controls shows that iodine treatment has lasting effect on the course of disease.
Future directions of study intend to identify the epitopes of thyroglobulin relevant to the thyroiditis of NOD.H2h4 mice. In particular, the iodine-dependent epitope of the 2D11 cloned line may prove to be of great interest. If 2D11 distinguishes iodinated from non-iodinated Tg, the possibility that this particular epitope includes a pro-hormonogenic region of the molecule may yield information on the nature of autoreactive specificity at a clonal level.
Acknowledgments
The authors would like to express their gratitude to Elizabeth A. Stafford and Marina Afanasyeva for critical readings of this manuscript and helpful discussions. This work has been supported in part by NIH grant no. DK42174.
References
- 1.Foley TP., Jr The relationship between autoimmune thyroid disease and iodine intake: a review. Endokrynol Pol. 1992;43(Suppl. 1):53–69. [PubMed] [Google Scholar]
- 2.Rose NR, Rasooly L, Saboori AM, Burek CL. Linking iodine with autoimmune thyroiditis. Environ Health Perspect. 1999;107(Suppl. 5):749–52. doi: 10.1289/ehp.99107s5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saboori AM, Rose NR, Bresler HS, Vladut-Talor M, Burek CL. Iodination of human thyroglobulin (Tg) alters its immunoreactivity. I. Iodination alters multiple epitopes of human Tg. Clin Exp Immunol. 1998;113:297–302. doi: 10.1046/j.1365-2249.1998.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saboori AM, Rose NR, Kuppers RC, Butscher WG, Bresler HS, Burek CL. Immunoreactivity of multiple molecular forms of human thyroglobulin. Clin Immunol Immunopathol. 1994;72:121–8. doi: 10.1006/clin.1994.1115. [DOI] [PubMed] [Google Scholar]
- 5.Saboori AM, Rose NR, Butscher WG, Burek CL. Modification of a nonincinerative method for determination of iodine in iodoproteins. Anal Biochem. 1993;214:335–8. doi: 10.1006/abio.1993.1500. [DOI] [PubMed] [Google Scholar]
- 6.Rasooly L, Rose NR, Saboori AM, Ladenson PW, Burek CL. Iodine is essential for human T cell recognition of human thyroglobulin. Autoimmunity. 1998;27:213–19. doi: 10.3109/08916939808993833. [DOI] [PubMed] [Google Scholar]
- 7.Allen EM, Appel MC, Braverman LE. The effect of iodide ingestion on the development of spontaneous lymphocytic thyroiditis in the diabetes-prone BB/W rat. Endocrinology. 1986;118:1977–81. doi: 10.1210/endo-118-5-1977. [DOI] [PubMed] [Google Scholar]
- 8.Braverman LE, Paul T, Reinhardt W, Appel MC, Allen EM. Effect of iodine intake and methimazole on lymphocytic thyroiditis in the BB/W rat. Acta Endocrinol (Copenh) 1987;281(Suppl.):70–6. doi: 10.1530/acta.0.114s070. [DOI] [PubMed] [Google Scholar]
- 9.Rasooly L, Burek CL, Rose NR. Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clin Immunol Immunopathol. 1996;81:287–92. doi: 10.1006/clin.1996.0191. [DOI] [PubMed] [Google Scholar]
- 10.Braley-Mullen H, Sharp GC, Medling B, Tang H. Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Autoimmun. 1999;12:157–65. doi: 10.1006/jaut.1999.0272. [DOI] [PubMed] [Google Scholar]
- 11.Bresler HS, Burek CL, Rose NR. Autoantigenic determinants on human thyroglobulin. I. Determinant specificities of murine monoclonal antibodies. Clin Immunol Immunopathol. 1990;54:64–75. doi: 10.1016/0090-1229(90)90006-c. [DOI] [PubMed] [Google Scholar]
- 12.Saboori AM, Caturegli P, Rose NR, Mariotti S, Pinchera A, Burek CL. Tryptic peptides of human thyroglobulin. II. Immunoreactivity with sera from patients with thyroid diseases. Clin Exp Immunol. 1994;98:459–63. doi: 10.1111/j.1365-2249.1994.tb05513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma R, Barin JG, Talor M, Rose NR, Burek CL. Phenotypic and functional characterization of NKT cells in autoimmune thyroiditis in the NOD.H2h4 mouse. Faseb J. 2002;A:326. [Google Scholar]
- 14.Berg G, Ekholm R. Electron microscopy of low iodinated thyroglobulin molecules. Biochim Biophys Acta. 1975;386:422–31. doi: 10.1016/0005-2795(75)90285-8. [DOI] [PubMed] [Google Scholar]
- 15.Harach HR, Escalante DA, Onativia A, Lederer OJ, Saravia DE, Williams ED. Thyroid carcinoma and thyroiditis in an endemic goitre region before and after iodine prophylaxis. Acta Endocrinol (Copenh) 1985;108:55–60. doi: 10.1530/acta.0.1080055. [DOI] [PubMed] [Google Scholar]
- 16.Laurberg P, Pedersen KM, Hreidarsson A, Sigfusson N, Iversen E, Knudsen PR. Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J Clin Endocrinol Metab. 1998;83:765–9. doi: 10.1210/jcem.83.3.4624. [DOI] [PubMed] [Google Scholar]
- 17.Guan H, Teng W, Cui B. [An epidemiological survey of thyroid disorders in an area with high iodine content in water supply] Zhonghua Nei Ke Za Zhi. 2001;40:597–601. [PubMed] [Google Scholar]
- 18.Huang Q, Jin R, Zou D. [Study on the effects of increased iodized salt intake on the incidence of thyroid diseases] Zhonghua Liu Xing Bing Xue Za Zhi. 2001;22:455–8. [PubMed] [Google Scholar]
- 19.Papanastasiou L, Alevizaki M, Piperingos G, Mantzos E, Tseleni-Balafouta S, Koutras DA. The effect of iodine administration on the development of thyroid autoimmunity in patients with nontoxic goiter. Thyroid. 2000;10:493–7. doi: 10.1089/thy.2000.10.493. [DOI] [PubMed] [Google Scholar]
- 20.Martino E, Aghini-Lombardi F, Bartalena L, et al. Enhanced susceptibility to amiodarone-induced hypothyroidism in patients with thyroid autoimmune disease. Arch Intern Med. 1994;154:2722–6. doi: 10.1001/archinte.1994.00420230115013. [DOI] [PubMed] [Google Scholar]
- 21.Allen EM, Braverman LE. The effect of iodine on lymphocytic thyroiditis in the thymectomized buffalo rat. Endocrinology. 1990;127:1613–16. doi: 10.1210/endo-127-4-1613. [DOI] [PubMed] [Google Scholar]
- 22.Cohen SB, Weetman AP. The effect of iodide depletion and supplementation in the buffalo strain rat. J Endocrinol Invest. 1988;11:625–7. doi: 10.1007/BF03350197. [DOI] [PubMed] [Google Scholar]
- 23.Sundick RS, Herdegen DM, Brown TR, Bagchi N. The incorporation of dietary iodine into thyroglobulin increases its immunogenicity. Endocrinology. 1987;120:2078–84. doi: 10.1210/endo-120-5-2078. [DOI] [PubMed] [Google Scholar]
- 24.Bagchi N, Brown TR, Urdanivia E, Sundick RS. Induction of autoimmune thyroiditis in chickens by dietary iodine. Science. 1985;230:325–7. doi: 10.1126/science.4048936. [DOI] [PubMed] [Google Scholar]
- 25.Brown TR, Sundick RS, Dhar A, Sheth D, Bagchi N. Uptake and metabolism of iodine is crucial for the development of thyroiditis in obese strain chickens. J Clin Invest. 1991;88:106–11. doi: 10.1172/JCI115265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Champion BR, Rayner DC, Byfield PG, Page KR, Chan CT, Roitt IM. Critical role of iodination for T cell recognition of thyroglobulin in experimental murine thyroid autoimmunity. J Immunol. 1987;139:3665–70. [PubMed] [Google Scholar]
- 27.Bagchi N, Brown TR, Sundick RS. Thyroid cell injury is an initial event in the induction of autoimmune thyroiditis by iodine in obese strain chickens. Endocrinology. 1995;136:5054–60. doi: 10.1210/endo.136.11.7588241. [DOI] [PubMed] [Google Scholar]
- 28.Bagchi N, Brown TR, Herdegen DM, Dhar A, Sundick RS. Antioxidants delay the onset of thyroiditis in obese strain chickens. Endocrinology. 1990;127:1590–5. doi: 10.1210/endo-127-4-1590. [DOI] [PubMed] [Google Scholar]
- 29.Bonita RE, Rose NR, Rasooly L, Caturegli P, Burek CL. Adhesion molecules as susceptibility factors in spontaneous autoimmune thyroiditis in the NOD-H2h4 mouse. Exp Mol Pathol. 2002;73:155–63. doi: 10.1006/exmp.2002.2470. [DOI] [PubMed] [Google Scholar]
- 30.Champion BR, Varey AM, Katz D, Cooke A, Roitt IM. Autoreactive T-cell lines specific for mouse thyroglobulin. Immunology. 1985;54:513–19. [PMC free article] [PubMed] [Google Scholar]
- 31.Champion BR, Page KR, Parish N, et al. Identification of a thyroxine-containing self-epitope of thyroglobulin which triggers thyroid autoreactive T cells. J Exp Med. 1991;174:363–70. doi: 10.1084/jem.174.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong YC, McCormick DJ, Wan Q, et al. Primary hormonogenic sites as conserved autoepitopes on thyroglobulin in murine autoimmune thyroiditis. Secondary role of iodination. J Immunol. 1995;155:5847–54. [PubMed] [Google Scholar]
- 33.Bresler HS, Burek CL, Hoffman WH, Rose NR. Autoantigenic determinants on human thyroglobulin. II. Determinants recognized by autoantibodies from patients with chronic autoimmune thyroiditis compared to autoantibodies from healthy subjects. Clin Immunol Immunopathol. 1990;54:76–86. doi: 10.1016/0090-1229(90)90007-d. [DOI] [PubMed] [Google Scholar]
- 34.Henry M, Zanelli E, Piechaczyk M, Pau B, Malthiery Y. A major human thyroglobulin epitope defined with monoclonal antibodies is mainly recognized by human autoantibodies. Eur J Immunol. 1992;22:315–19. doi: 10.1002/eji.1830220205. [DOI] [PubMed] [Google Scholar]
- 35.Henry M, Malthiery Y, Zanelli E, Charvet B. Epitope mapping of human thyroglobulin. Heterogeneous recognition by thyroid pathologic sera. J Immunol. 1990;145:3692–8. [PubMed] [Google Scholar]
- 36.Shimojo N, Saito K, Kohno Y, Sasaki N, Tarutani O, Nakajima H. Antigenic determinants on thyroglobulin: comparison of the reactivities of different thyroglobulin preparations with serum antibodies and T cells of patients with chronic thyroiditis. J Clin Endocrinol Metab. 1988;66:689–95. doi: 10.1210/jcem-66-4-689. [DOI] [PubMed] [Google Scholar]
- 37.Kohno Y, Nakajima H, Tarutani O. Interspecies cross-reactive determinants of thyroglobulin recognized by autoantibodies. Clin Exp Immunol. 1985;61:44–8. [PMC free article] [PubMed] [Google Scholar]