Abstract
The intestinal barrier function is often impaired in a variety of diseases including chronic inflammatory bowel disease. Increased intestinal permeability during episodes of active disease correlates with destruction or rearrangement of the tight junction protein complex. IFN-γ has been widely studied for its effect on barrier function and tight junction structures but its mode of action remains unclear. Since the claudin family of tight junction proteins is proposed to be involved in barrier maintenance we studied the effect of IFN-γ on claudin expression in relation to epithelial barrier function. Cycloheximide and protease inhibitors were used to study mechanisms of IFN-γ mediated barrier disruption. Intestinal epithelial cells were exposed to IFN-γ and permeability was evaluated by horse radish peroxidase (HRP) and 4 kD FITC-dextran fluxes. Occludin and claudin-1, -2, -3, and -4 tight junction protein expression was determined by Western blotting. Occludin and claudin-2 protein expression was dramatically reduced after IFN-γ exposure, which correlated with increased permeability for HRP and FITC-dextran. Interestingly, cleavage of claudin-2 was observed after incubation with IFN-γ. Serine protease inhibitor AEBSF completely abrogated IFN-γ mediated barrier disruption which was associated with preservation of claudin-2 expression. Moreover, IFN-γ induced loss of barrier integrity was found to affect claudin-2 and occludin expression through different mechanisms. Since inhibition of serine protease activity abrogates IFN-γ mediated barrier disruption this may be an important target for therapeutic intervention.
Keywords: epithelial permeability, IFN-γ, claudin, tight junction, serine protease activity
Introduction
Intestinal barrier integrity is often found to be impaired in diseases such as food allergy, coeliac disease, asthma, arthritis, and inflammatory bowel disease (IBD) [1–5]. Impaired barrier integrity involves increased permeability of the gut and interestingly in Crohn's patients increased intestinal permeability was found to precede episodes of relapse [6–10]. In addition, even in mildly inflamed IBD tissue, epithelial tight junctional strand formations were dramatically affected [11–13]. Tight junction complexes actively control paracellular permeability allowing passage of water and small solutes. These tight junctions comprise a network of proteins that are coupled to cytoskeletal filaments [14–17]. Mediators of intestinal inflammation, e.g. pro-inflammatory cytokines, can affect intestinal barrier integrity. IFN-γ in this respect was found to disrupt epithelial barrier function by affecting the tight junction proteins ZO-1 and occludin as well as peri-junctional actin filament formation [18–21]. Recently, the claudin family of tight junction proteins has been identified and implicated in maintenance of tight junction complex integrity [22–24]. However, the functional role of the various claudins in maintenance of intestinal barrier integrity is poorly understood. Both claudins and occludin are coupled either directly or indirectly to actin filaments of the cytoskeleton, through association with ZO-1 or other proteins [25–27]. Patterns of claudin expression vary along the gut and epithelial cells express different types of claudins connecting cells through formation of zipper like structures [28–31]. Amongst others claudin-1, -2, -3 and -4 are expressed in the intestinal epithelium [28,29]. Claudin-1 and -4 have been found to increase epithelial barrier properties whereas claudin-2 is associated with leaky tight junctions [32–35].
We studied the mechanisms by which IFN-γ affects intestinal barrier integrity with special reference to tight junction protein expression. Differential sensitivity of claudins to immune mediators will further elucidate their role in barrier disruption during processes of inflammation and may reveal innovative targets for therapeutical intervention.
Materials and methods
Cells and antibodies
The human intestinal cell line T84 was purchased from the ATCC (Manassas, VA, USA) and used at passages 57–64. Antibodies against claudin-1 (JAY.8), claudin-2 (MH44), claudin-3 (Z23.JM), claudin-4 (3E2C1) and occludin (Z-T22) were obtained from Zymed (San Francisco, CA, USA).
Cell culture
T84 cells were cultured on 12-mm transwell inserts (0·4 µm, Corning Costar, Rochester, NY, USA) in DMEM/F12 glutamax I with penicillin (100 IU/ml), streptomycin (100 µg/ml) (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 5% heat inactivated fetal bovine serum (Greiner Bio-One Inc., Longwood, FL, USA). Medium was refreshed every 2 days during cultivation and every 24 h in an experimental setting. T84 cells were used 14 days after confluence.
Incubation with IFN-γ
Dose–response and kinetics
Cells were incubated for 24, 48 or 72 h with 0, 50, 100 or 500 U/ml human recombinant IFN-γ (HyCult Biotechnology B.V., Uden, the Netherlands) added to the serosal compartment. Every 24 h the culture medium was changed and fresh IFN-γ added.
IFN-γ and cycloheximide
In order to investigate IFN-γ induced barrier disruption and tight junction protein expression, cycloheximide was used to block de novo protein synthesis. T84 monolayers were incubated for 72 h with 0, 0·002, 0·02 or 0·2 µg/ml cycloheximide in both apical and serosal compartments (Sigma-Aldrich B.V., St. Louis, MO, USA) in presence or absence of 100 U/ml IFN-γ.
IFN-γ and protease inhibitors
The involvement of proteases in the IFN-γ (100 U/ml) induced barrier disruption was studied using protease inhibitors. Serine protease inhibitor AEBSF (4-(2-aminoethyl)-benzenesulphonyl fluoride; 0·1 mM), aspartic protease inhibitor pepstatin A (10−5 M), and cysteine protease inhibitors E-64, the cell permeable E-64d (100 µM) (Sigma-Aldrich B.V), and calpeptin (5 ng/ml; cell permeable calpain inhibitor) (Calbiochem, Darmstadt, Germany) were used.
Measurement of resistance
Epithelial barrier integrity was assessed by measuring transepithelial resistance (TER; Ω.cm2) with the epithelial volt-ohm meter (EVOM; World Precision Instruments, Berlin, Germany). TER measurements were performed prior to medium refreshment at 24, 48 and 72 h of incubation.
Macromolecular permeability
Functional permeability studies were performed by measurement of macromolecular transport of 4 kD FITC-dextran [36] or 44 kD horseradish peroxidase (HRP) [37] over the T84 monolayers at 24, 48 and 72 h. One hour prior to HRP measurements the culture medium was refreshed. HRP, 5 µl of 1 mM solution (type VI-A, Sigma-Aldrich B.V) was added to the luminal compartment. After 30 min, HRP concentrations in the serosal compartment were determined enzymatically using tetramethylbenzidin (TMB; VWR International, Darmstadt, Germany) substrate and detected with a spectrophotometer (Ultramark, Bio-Rad, Hercules, CA, USA) at 450 nm. HRP fluxes (pmol HRP/cm2/h) were calculated. Permeability for 4 kD FITC-dextran was determined in cultures incubated with IFN-γ or during AEBSF incubations. Prior to dextran fluxes the medium was refreshed with culture medium without phenol red, after one hour 5 µl (stock 100 mg/ml) 4 kD FITC-dextran was added to the luminal compartment. After 30 min 100 µl sample was collected from the serosal compartment and the fluorescent signal measured at excitation wave length 485 nm and emission 520 nm (FLUOstar Galaxy®, BMG Labtechnologies, Durham, NC, USA). FITC-dextran fluxes were calculated as pmol FITC-dextran/cm2/h.
Gel electrophoresis and immunoblotting
Cell samples were collected in Laemmli buffer (2% SDS, 25% glycerol, 62·5 mM tris HCl pH 6·8), except when used for cell fractionation (see below); and kept on ice. Protein determinations were performed using the DC-protein assay (Bio-Rad) according to the manufactures protocol with minor modifications. After protein determination 2·5%β-mercapto-ethanol was added, samples were boiled for 5 min, centrifuged (5 min, 10000 ×g) and stored at −20 °C. Electrophoresis (20 µg protein per slot) was performed in 10% or 12% SDS-page gels and samples were transferred to PVDF membranes (Roche Diagnostics, Indianapolis, IN, USA). Blots were blocked in TBST/5% Protivar (Nutricia, Zoetermeer, the Netherlands) followed by 1 h incubation with primary antibodies to claudin-1 (1 : 100), claudin-2 (1 : 800), claudin-3 (1 : 1000), claudin-4 (1 : 1000) (23 kD), and occludin (1 : 10000) (65 kD). After washing, blots were incubated with goat anti-rabbit or -mouse IgG-HRP (Santacruz Biotechnology, Santa Cruz, CA, USA) and Lumi-Light plus (Roche Diagnostics) for substrate detection. Tight junction protein expression was quantified by densitometry (BLU) using the Lumi-Imager (Roche Diagnostics). Equal protein loading and transfer was verified by Coomassie Brilliant Blue staining of the blots. The BLU values of test conditions were calculated relative to controls within each time group (%BLU).
Cell fractionation
IFN-γ incubations resulted in fragmentation of the claudin-2. In order to elucidate the site of cleavage we determined whether the fragment was retrieved in the cytosol, the Triton-X soluble membrane fraction or Triton-X insoluble cytoskeletal fraction. Cell fractionation was performed according to a method by Clarke et al. [38], with some modifications. In short, T84 cells were incubated 72 h with 100 U/ml IFN-γ, washed in PBS and collected in lysis buffer A (5 mM Tris-HCl, 0·05 M sucrose, 1 mM EGTA and protease inhibitors (mini protease inhibitor cocktail tablet EDTA free, Roche Diagnostics) and sonicated on ice. Samples were centrifuged in an Airfuge (Beckman Coulter, Mijdrecht, the Netherlands) for 5 min at 70000 ×g. Supernatants containing the cytosolic fraction were collected, and pellets resuspended in lysis buffer A in the presence of 1% Triton-X. The pellet was disrupted and the sample put on ice for 30 min, and centrifuged. Supernatants containing the Triton-X soluble membrane fraction were collected, and pellets resuspended in lysis buffer B (150 mM NaCl, 50 mM tris-HCl, 2 mM EGTA, 1% NP-40, 0·1% sodium deoxycholate, 0·1% SDS, protease inhibitors). The pellet was disrupted, put on ice for 30 min, and centrifuged. Supernatants containing the Triton-X insoluble cytoskeletal fraction were collected; equal volumes of Laemmli buffer were added to all three fractions. Protein determinations were performed and samples prepared for immunoblot analyses.
Data analyses
All data are presented as mean ± SEM. Data were analysed with the univariate anova using SPSS Version 10 software.
Results
Barrier disruption of T84 monolayers during IFN-γ exposure
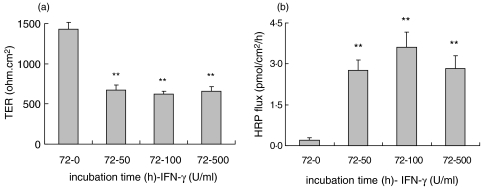
T84 cells were grown on filters forming monolayers with constitutively high TER and low permeability for HRP or 4 kD FITC-dextran. Incubations with IFN-γ resulted in pronounced time–dependent effects on barrier integrity. As compared to controls TER decreased after 48 h of incubation in all dosage groups (P < 0·001). IFN-γ affected barrier disruption was found to be more pronounced at 72 h when a more than 60% decrease in TER was observed in all IFN-γ incubations (1426 ± 89 versus 621 ± 34 Ω.cm2; TER control versus 100 U/ml IFN-γ; P < 0·001) (Fig. 1a). Decrease in TER was associated with increased HRP fluxes at 48 h (P < 0·01) and 72 h (P < 0·001) (Fig. 1b, 72 h). Although IFN-γ induced barrier disruption was prominent after 48 h, the effects on permeability were even more pronounced after 72 h. Therefore 72 h incubation was chosen for further mechanistic studies on functional permeability. Fluxes in control cultures were consistently low (< 0·5 pmol/cm2/h).
Fig. 1.
Effects of IFN-γ on T84 epithelial barrier integrity. (a) IFN-γ (0–500 U/ml) incubation decreased TER after 72 h of incubation in all dosage groups (**P < 0·001, 72 h, n = 5). (b) Decrease in TER is associated with increased permeability for macromolecules (HRP fluxes; **P < 0·001, 72 h, n = 3).
HRP flux may reflect contribution of the transcellular route of macromolecular transport. Therefore we also examined 4 kD FITC-dextran fluxes to verify the involvement of the paracellular route reflecting tight junction complex integrity. T84 cells were incubated for 72 h with 100 U/ml IFN-γ resulting in reduced TER (1571 ± 41 versus 947 ± 31 Ω.cm2; P < 0·001, n = 5) and similar to HRP permeability we found 4 kD FITC-dextran fluxes to be increased. Control fluxes were consistently low while IFN-γ incubation increased fluxes more than 12 fold (35 ± 15 versus 431 ± 87 pmol/cm2/h; P < 0·01, n = 5).
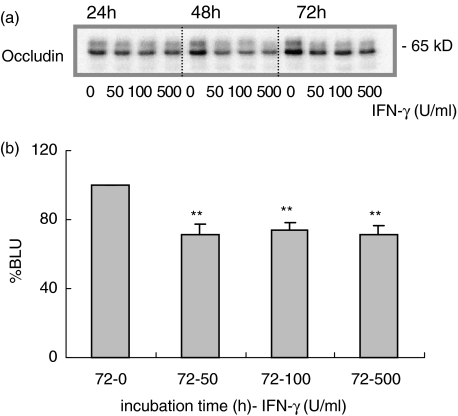
IFN-γ affects occludin expression
Expression of transmembrane tight junction proteins was determined in whole cell lysates by Western blotting. As published earlier IFN-γ induced barrier disruption is associated with decreased occludin expression. At 24 h the 500 U/ml dosage of IFN-γ only slightly reduced occludin expression (P < 0·01) while at 48 h occludin expression dropped further in all IFN-γ dosage groups (data not shown). This effect was sustained at 72 h of incubation (Fig. 2a,b; P < 0·001).
Fig. 2.
IFN-γ treatment and occludin expression. (a) IFN-γ (0–500 U/ml) decreased occludin protein expression at 48 and 72 h (representative Western blot). (b) Densitometric analysis of 6 independent experiments, presented relative to controls after 72 h (% BLU) (**P < 0·001, 72 h).
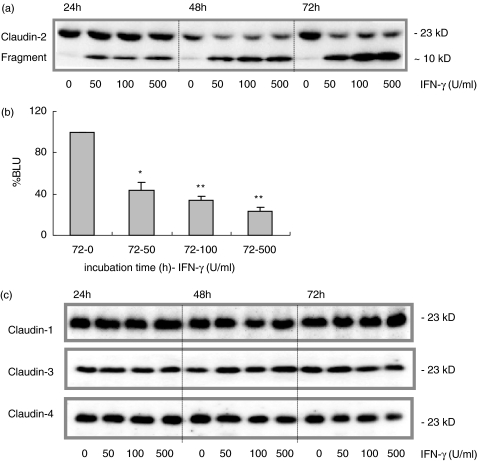
Effects of IFN-γ on claudin expression
IFN-γ incubation decreased claudin-2 expression which correlated with decreased TER and increased HRP-flux. After 48 and 72 h claudin-2 expression was dramatically reduced compared to controls in all dosage groups (Fig. 3a,b, 72 h; P < 0·01). IFN-γ not only decreased claudin-2 expression but also resulted in the formation of a distinct fragment of approximately 10 kD, which already appeared after 24 h of incubation in all dosage groups (P < 0·001).
Fig. 3.
IFN-γ and claudin-2 protein expression. (a) IFN-γ (0–500 U/ml) decreased claudin-2 protein expression at 48 and 72 h. A degradation product of approximately 10 kD occurred as early as 24 h of incubation and was most prominent after 72 h of IFN-γ exposure (shown in a representative Western blot). (b) Densitometry analysis of 5 different experiments revealed dramatic decrease in claudin-2 expression after 72 h (*P < 0·01, **P < 0·001), presented relative to controls (% BLU). (c) Claudin-1, -3, and -4 were only marginally affected (representative Western blots are shown).
In contrast, claudin-1 (n = 5) expression levels appeared to increase at 24 h in all IFN-γ incubation groups (P < 0·001), whereas minor reduction was observed at 48 h (100 U/ml, P < 0·001) and 72 h (50 U/ml, P < 0·005). Claudin-3 (n = 4) expression was not affected by IFN-γ and claudin-4 (n = 6) was marginally decreased after 72 h, and only at the highest dose group (500 U/ml, P < 0·005). Figure 3c shows representative Western blots for these claudins at 24–72 h of IFN-γ incubation. The minor changes of claudin-1 and -4 in protein expression did not correlate with the permeability changes after 48–72 h of IFN-γ exposure. Since minor effects on claudin-1, -3 and -4 were not related to the effects observed on claudin-2 and occludin during IFN-γ incubation, these claudins could serve as internal controls for the protein lysates used.
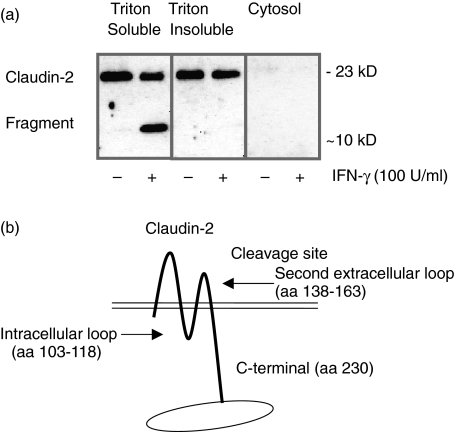
Cellular localization of claudin-2 and its fragment
Cell fractionation of IFN-γ exposed cells revealed claudin-2 to be located in the Triton-X soluble membrane and Triton-X insoluble cytoskeletal fraction (Fig. 4a). The fragment occurring after claudin-2 degradation by IFN-γ, was only present in the Triton-X soluble membrane fraction. The Triton-X extractability shows that the cleaved fragment is no longer affiliated with the cytoskeleton and probably is no longer bound to the tight junction complex. Since the antibody recognizes the C-terminal part of the claudin-2 protein, and the fragment (∼10 kD) was identified in the Triton-X soluble membrane fraction, detached from the cytoskeleton, this suggests specific cleavage sites (Fig. 4b).
Fig. 4.
Western blot of cellular localization of claudin-2 and its fragment after 72 h incubation with IFN-γ (100 U/ml) (n = 2). (a) Claudin-2 was located in the Triton-X soluble membrane and Triton-X insoluble cytoskeletal fraction, while the claudin-2 fragment could only be retrieved from the membrane fraction. (b) Since the antibody recognizes the C-terminal part of claudin-2 and the ∼10 kD fragment is detected in the membrane fraction, detached from the cytoskeleton, it suggests that claudin-2 is cleaved either in the intracellular or second extracellular loop.
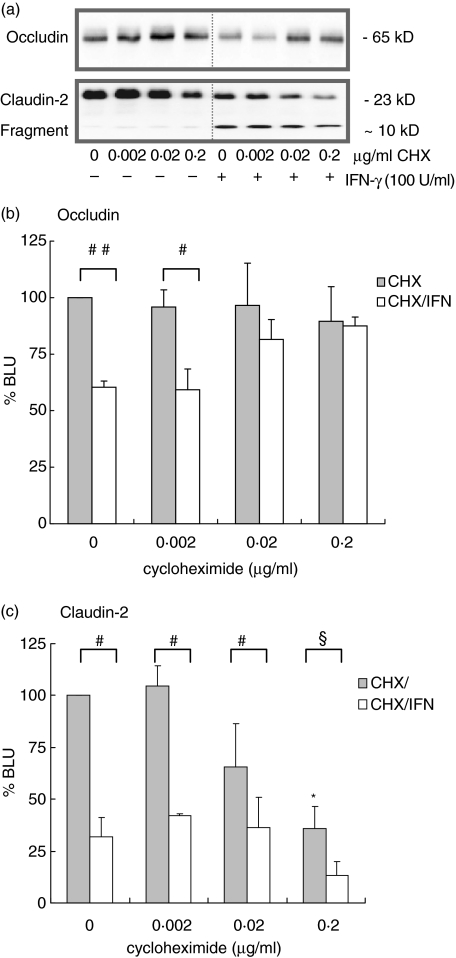
Cycloheximide ameliorates IFN-γ mediated barrier disruption
The IFN-γ mediated reduction in TER (P < 0·005) and increase of HRP fluxes (P < 0·005) was dose-dependently inhibited by cycloheximide (0·02 and 0·2 µg/ml, Fig. 5). Cycloheximide did not affect basal HRP flux or TER although there was a trend towards increase of TER (data not shown). IFN-γ decreased occludin expression after 72 h (Fig. 6a,b; P < 0·001) this could be prevented by coincubation with 0·02 and 0·2 µg/ml cycloheximide. Cycloheximide treatment (0·2 µg/ml) decreased basal expression of claudin-2 (Fig. 6a.c; P < 0·005) indicating that claudin-2 protein homeostasis involves de novo protein synthesis. In addition, cycloheximide did not prevent IFN-γ induced fragmentation of claudin-2.
Fig. 5.
Involvement of de novo protein synthesis in IFN-γ induced barrier disruption. (a) IFN-γ (100 U/ml) reduced TER (**P < 0·005, n = 7) which could be prevented by cycloheximide (0–0·2 µg/ml) in a dose-dependent fashion. (b) The IFN-γ (72 h, 100 U/ml) induced increase of HRP fluxes (*P < 0·01, **P < 0·005, n = 5) was also prevented by cycloheximide.
Fig. 6.
Effects of cycloheximide (0–0·2 µg/ml) on occludin and claudin-2 expression. (a) Representative Western blot of occludin and claudin-2. (b) Cycloheximide (0·02–0·2 µg/ml) prevented IFN-γ (72 h, 100 U/ml) mediated reduction of occludin expression (#P < 0·05, ##P < 0·001, n = 4), whereas it did not affect basal occludin expression. (c) Cycloheximide decreased basal claudin-2 expression indicating that claudin-2 protein homeostasis involves de novo protein synthesis (*P < 0·005, n = 3). IFN-γ induced claudin-2 fragmentation was not prevented by cycloheximide (#P < 0·05, §P = 0·057).
Protease inhibitors and IFN-γ mediated barrier disruption
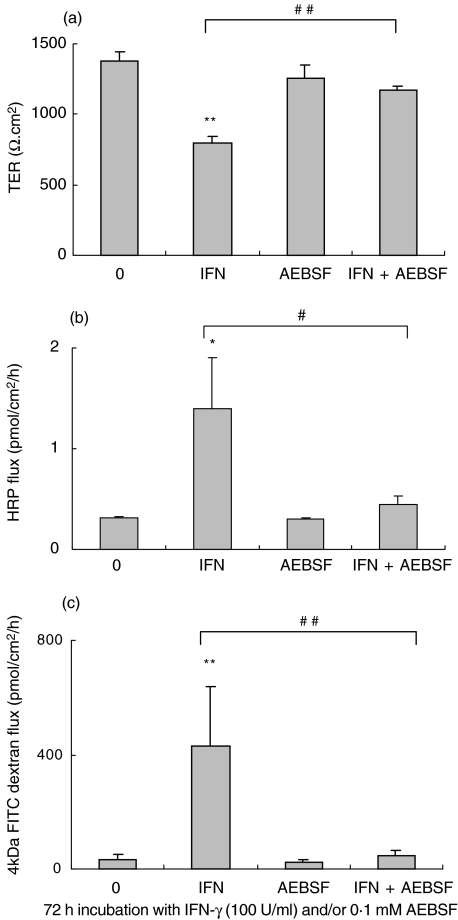
IFN-γ mediated decrease in claudin-2 and occludin expression may involve specific protease activity. Therefore we coincubated T84 cells with IFN-γ and various protease inhibitors. Cysteine protease inhibitors E64 and E64d, calpeptin and aspartic protease inhibitor pepstatin A were not effective in abrogating the IFN-γ effect. In contrast, serine protease inhibitor AEBSF totally abrogated the IFN-γ effect on TER at 48 h and 72 h (Fig. 7a; control versus IFN-γversus IFN-γ/AEBSF, 1380 ± 74 versus 795 ± 48 versus 1175 ± 25 Ω.cm2; 72 h, P < 0·005). AEBSF not only prevented drop in TER but also reduced IFN-γ induced HRP and 4 kD FITC-dextran flux (Fig. 7b,c; P < 0·02 or P < 0·005).
Fig. 7.
Effect of proteases on barrier disruption. (a) Serine protease inhibitor AEBSF (0·1 mM) totally abrogated the IFN-γ (72 h, 100 U/ml) mediated decrease in TER (**P < 0·005, ##P < 0·005, n = 5). (b,c) AEBSF also abrogated the IFN-γ induced increase in HRP and 4 kD FITC-dextran flux (#P < 0·02, ##P < 0·005, *P < 0·001, **P < 0·005, n = 3).
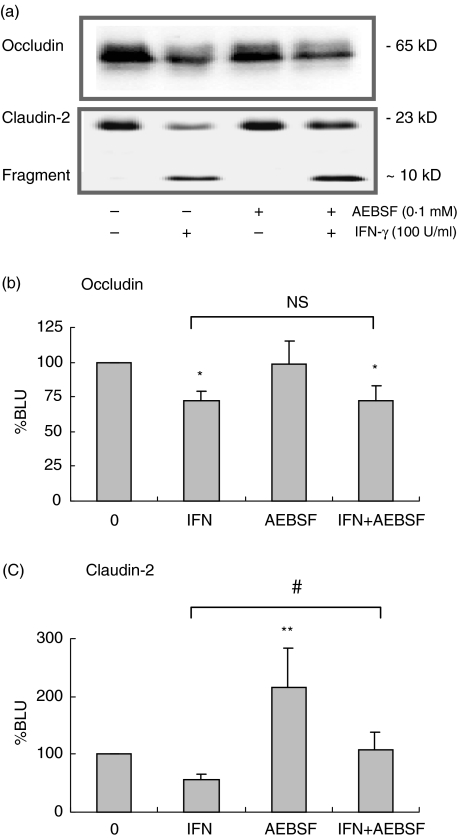
Effect of AEBSF on claudin-2 and occludin expression levels
As AEBSF was able to prevent IFN-γ mediated barrier disruption we determined the effects of AEBSF on occludin and claudin-2 expression. AEBSF completely prevented the IFN-γ mediated decrease in claudin-2 expression after 72 h (Fig. 8a,c; P < 0·01). The AEBSF treatment appeared to interfere with basal claudin-2 expression levels as it enhanced claudin-2 expression (P < 0·001). Reduction in occludin expression by IFN-γ could not be prevented by AEBSF (Fig. 8a,b; P < 0·01). In addition, AEBSF did not affect basal occludin expression.
Fig. 8.
Effects of AEBSF on occludin and claudin-2 expression. (a) Representative Western blot of occludin and claudin-2. (b) Reduced occludin expression (*P < 0·01, n = 6) could not be prevented by AEBSF. (c) Densitometry revealed increased claudin-2 expression (**P < 0·001), while it prevented IFN-γ (72 h, 100 U/ml) mediated decrease in claudin-2 expression (#P < 0·01, n = 6).
Discussion
We evaluated the effect of IFN-γ on claudin and occludin expression in relation to intestinal epithelial permeability. In Crohn's patients mucosal T-cell reactivity is shifted towards a Th1-type dominance and IFN-γ is released by these cells upon activation. IFN-γ is a central mediator in the initiation and perpetuation of mucosal inflammation and intestinal barrier disruption may be part of its effect. IFN-γ is known to enhance epithelial permeability via disruption of tight junction complexes. Enhanced intestinal permeability is associated with the pathology of Crohn's patients and in mucosal biopsies of these patients the tight junction structures were found to be disrupted. Ferrier et al. [39] provided further evidence for the role of IFN-γ in enhancing intestinal permeability. They showed that stress induced intestinal permeability was associated with increased mucosal IFN-γ expression. Furthermore, they reported permeability not to be enhanced in SCID and IFN-γ deficient mice under similar conditions.
We studied the mechanisms of barrier disruption by IFN-γin vitro and as reported by various other groups epithelial cells exposed to IFN-γ show dramatic reduction in TER [18–20,40]. Typical dose-related effects may imply full IFN-γ receptor occupancy to occur at 50 U/ml. Indeed other studies found IFN-γ to be effective at lower dosages (1–10 U/ml) and dose–response effects at high dosages were only marginal [18–21,36,40]. The IFN-γ mediated reduction in TER in our experiments was associated with increased HRP and 4 kD FITC-dextran fluxes, the latter confirming the involvement of the paracellular permeability route. Increased FITC-dextran fluxes have been reported before [36,40], and in conjunction with our data, it becomes clear that even at low concentrations IFN-γ can increase paracellular permeability for potentially antigenic macromolecules.
Occludin expression was found to be diminished in mucosal biopsies of IBD patients [13,41]. By analysing total cell lysates of epithelial cell cultures, we found IFN-γ to decrease the expression of both 65 kD occludin and a larger molecular weight band probably representing the phosphorylated form of occludin [42,43]. Decreased phosphorylation may reflect altered cellular distribution [20]. Decrease in occludin expression can be the result of IFN-γ induced reduction of the occludin promotor activity [20,44]. However, since inhibition of protein synthesis prevented the IFN-γ effect on occludin expression other pathways are likely to be involved. From our observations and those by others it can be concluded that IFN-γ mediated decrease in occludin expression requires de novo protein synthesis and that occludin is functionally involved in maintenance of epithelial barrier integrity [23,24,45–47].
Interestingly, the effects of IFN-γ on claudin-2 expression were more pronounced. A distinct claudin-2 fragment appeared during IFN-γ incubation suggesting cleavage of the claudin-2 protein. This phenomenon was specific for claudin-2 since no cleavage was observed in the other claudin family members claudin-1, -3 and -4. Evidently, the effects of IFN-γ on claudin-2 expression are related to barrier disruption whereas claudin-1, -3 and -4 expression levels were not. However, this does not exclude these claudins from being affected, and recently it was reported that IFN-γ treatment resulted in internalization of claudin-1 and -4 [48]. The claudin-2 antibody we used recognizes a C-terminal peptide sequence, with the fragment being ∼10 kD in size, this further supported cleavage of claudin-2 (230 aa) in the intracellular loop (aa 103–116) or in the C-terminal extracellular loop (aa 138–163). Cell fractionation experiments confirmed the fragment to be localized in the Triton-X soluble membrane fraction, detached from the cytoskeleton. Since cycloheximide decreases constitutive claudin-2 expression there seems to be continuous de novo synthesis of this tight junction protein. Nevertheless, IFN-γ enhances claudin-2 turnover rendering de novo protein synthesis insufficient in compensating the degradation. The observed claudin-2 fragmentation may be of particular relevance during processes of inflammation since it may facilitate the migration of dendritic cells and neutrophils through the paracellular gateway [41,45,49].
As cycloheximide could not prevent claudin-2 fragmentation we investigated whether IFN-γ could induce protease activity. Calpain can be found in rat intestinal cells and IFN-γ strongly enhances calpain activity in immune cells [50–52]. However, both calpeptin as well as generic aspartic and cysteine protease inhibitors could not prevent the IFN-γ mediated barrier disruption. In contrast, the serine protease inhibitor AEBSF totally abrogated IFN-γ mediated decrease in TER and increase in flux. This finding is in line with observations by Megyeri et al. [53] who reported AEBSF to inhibit cytokine-induced permeability in the blood–brain barrier.
Protease activity as observed in IFN-γ mediated barrier disruption may involve proteases that are released as consequence of IFN-γ induced apoptosis. Recently Bojarski et al. [54] found certain proteases to be involved in cleavage of occludin and claudin-2 during induction of apoptosis with staurosporine. However, Bruewer et al. [48] could not reduce IFN-γ mediated barrier disruption in T84 cells by inhibition of the apoptotic pathway. Hence, further studies are required to determine whether IFN-γ activates proteases, independent from the apoptotic pathway, that contribute to barrier disruption.
AEBSF treatment did not prevent occludin changes in our experiments where other studies suggested a role for serine proteases in epithelial permeability and occludin degradation [55,56]. We did not find the multiple low molecular weight bands as reported in these studies, indicating that IFN-γ reduces occludin expression through a different mechanism. By combined incubations of AEBSF and IFN-γ the claudin-2 expression levels were brought back to baseline levels which correlated with abrogation of barrier disruption by AEBSF. Whether AEBSF protects from IFN-γ mediated barrier disruption by preservation of claudin-2 expression or by means of other mechanisms remains to be elucidated. Incubation with AEBSF increased basal expression levels of claudin-2 and IFN-γ still reduced claudin-2 expression as compared to AEBSF alone. Hence, AEBSF might increase claudin-2 half-life by inhibiting certain serine proteases. This mechanism may affect both basal claudin-2 expression as well as IFN-γ induced protease activity. Furthermore, Swiss-Prot virtual analysis revealed that indeed claudin-2 contains serine protease cleavage sites, which are not found in claudin-1, -3 and -4. It is important to note that increased claudin-2 expression is correlated with improved barrier properties in T84 cells incubated with IL-17 [57]. On the other hand tight junctions became more leaky when claudin-2 was transfected into MDCK cells, however, no change in permeability for dextran was observed [35]. In conjunction with our data this implies that besides enhancing epithelial conductance claudin-2 may be involved in preventing macromolecular permeability.
In conclusion, both claudin-2 and occludin are important in maintaining intestinal barrier function and mucosal inflammatory mediators such as IFN-γ differentially regulate their expression. Since the IFN-γ effect on barrier disruption may be mediated through serine protease activity these enzymes could be important targets for therapeutical intervention in diseases with underlying intestinal barrier defects.
Acknowledgments
We appreciate Dr Ig. Jurgen M. Karczewski and Dr Jack Groot for helpfull discussions and support with cell fractionation. We also thank Tessa Wijnhoven, Marleen Koetsier and Henri Braat for practical assistance and Dr Rob Verdooren for his support with the statistical evaluation.
References
- 1.DeMeo MT, Mutlu EA, Keshavarzian A, Tobin MC. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34:385–96. doi: 10.1097/00004836-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Benard A, Desreumeaux P, Huglo D, Hoorelbeke A, Tonnel AB, Wallaert B. Increased intestinal permeability in bronchial asthma. J Allergy Clin Immunol. 1996;97:1173–8. doi: 10.1016/s0091-6749(96)70181-1. [DOI] [PubMed] [Google Scholar]
- 3.Munkholm P, Langholz E, Hollander D, et al. Intestinal permeability in patients with Crohn's disease and ulcerative colitis and their first degree relatives. Gut. 1994;35:68–72. doi: 10.1136/gut.35.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–81. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 5.Sartor RB. Microbial factors in the pathogenesis of Crohn's disease, ulcerative colitis and experimental intestinal inflammation. In: Kirsner JB, editor. Inflammatory Bowel Diseases. 5. Philadelphia: W.B. Saunders Company; 1999. pp. 1753–81. [Google Scholar]
- 6.D'Inca R, Di Leo V, Corrao G, et al. Intestinal permeability test as a predictor of clinical course in Crohn's disease. Am J Gastroenterol. 1999;94:2956–60. doi: 10.1111/j.1572-0241.1999.01444.x. [DOI] [PubMed] [Google Scholar]
- 7.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 8.Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437–9. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 9.Olaison G, Sjodahl R, Tagesson C. Abnormal intestinal permeability in Crohn's disease. A possible pathogenic factor. Scand J Gastroenterol. 1990;25:321–8. doi: 10.3109/00365529009095493. [DOI] [PubMed] [Google Scholar]
- 10.Soderholm JD, Peterson KH, Olaison G, et al. Epithelial permeability to proteins in the noninflamed ileum of Crohn's disease? Gastroenterology. 1999;117:65–72. doi: 10.1016/s0016-5085(99)70551-2. [DOI] [PubMed] [Google Scholar]
- 11.Marin ML, Greenstein AJ, Geller SA, Gordon RE, Aufses AH. A freeze fracture study of Crohn's disease of the terminal ileum: changes in epithelial tight junction organization. Am J Gastroenterol. 1983;78:537–47. [PubMed] [Google Scholar]
- 12.Schmitz H, Barmeyer C, Fromm M, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–9. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 13.Gassler N, Rohr C, Schneider A, et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–28. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- 14.Madara JL. Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am J Physiol. 1987;253:C171–5. doi: 10.1152/ajpcell.1987.253.1.C171. [DOI] [PubMed] [Google Scholar]
- 15.Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 16.Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851–7. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 17.Turner JR, Angle JM, Black ED, Joyal JL, Sacks DB, Madara JL. PKC-dependent regulation of transepithelial resistance: roles of MLC and MLC Kinase. Am J Physiol. 1999;277:C554–62. doi: 10.1152/ajpcell.1999.277.3.C554. [DOI] [PubMed] [Google Scholar]
- 18.Madara JL, Stafford J. Interferon–gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–7. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams RB, Planchon SM, Roche JK. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993;150:2356–63. [PubMed] [Google Scholar]
- 20.Youakim A, Ahdieh M. Interferon–gamma decreases barrier function in T84 cells by reducing ZO- 1 levels and disrupting apical actin. Am J Physiol. 1999;276:G1279–88. doi: 10.1152/ajpgi.1999.276.5.G1279. [DOI] [PubMed] [Google Scholar]
- 21.Sugi K, Musch MW, Field M, Chang EB. Inhibition of Na(+),K(+)-ATPase by Interferon gamma Down-regulates Intestinal Epithelial Transport and Barrier Function. Gastroenterology. 2001;120:1393–03. doi: 10.1053/gast.2001.24045. [DOI] [PubMed] [Google Scholar]
- 22.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–50. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–73. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- 24.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–93. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 25.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–26. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–63. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and zo-1 forms independent complexes with zo-2 and zo-3. J Biol Chem. 1999;274:35179–85. doi: 10.1074/jbc.274.49.35179. [DOI] [PubMed] [Google Scholar]
- 28.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4 and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–22. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 29.Heiskala M, Peterson PA, Yang Y. The roles of Claudin superfamily proteins in paracellular transport. Traffic. 2001;2:93–8. doi: 10.1034/j.1600-0854.2001.020203.x. [DOI] [PubMed] [Google Scholar]
- 30.Tsukita S, Furuse M. Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol. 2000;149:13–6. doi: 10.1083/jcb.149.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–03. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonoda N, Furuse M, Sasaki H, et al. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147:195–04. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inai T, Kobayashi J, Shibata Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur J Cell Biol. 1999;78:849–55. doi: 10.1016/S0171-9335(99)80086-7. [DOI] [PubMed] [Google Scholar]
- 34.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–27. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of Zonulae Occludentes from Tight to Leaky Strand Type by Introducing Claudin-2 into Madin-Darby Canine Kidney I Cells. J Cell Biol. 2001;153:263–72. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders SE, Madara JL, McGuirk DK, Gelman DS, Colgan SP. Assessment of inflammatory events in epithelial permeability: a rapid screening method using fluorescein dextrans. Epithelial Cell Biol. 1995;4:25–34. [PubMed] [Google Scholar]
- 37.Berin MC, Kiliaan AJ, Yang PC, Groot JA, Kitamura Y, Perdue MH. The influence of mast cells on pathways of transepithelial antigen transport in rat intestine. J Immunol. 1998;161:2561–6. [PubMed] [Google Scholar]
- 38.Clarke H, Soler AP, Mullin JM. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J Cell Sci. 2000;113:3187–96. doi: 10.1242/jcs.113.18.3187. [DOI] [PubMed] [Google Scholar]
- 39.Ferrier L, Mazelin L, Cenac N, et al. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795–04. doi: 10.1016/s0016-5085(03)01057-6. [DOI] [PubMed] [Google Scholar]
- 40.Fish SM, Proujansky R, Reenstra WW. Synergistic effects of interferon gamma and tumour necrosis factor alpha on T84 cell function. Gut. 1999;45:191–8. doi: 10.1136/gut.45.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001–9. doi: 10.1016/S0002-9440(10)63051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol. 1997;137:1393–01. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakaguchi T, Kohler H, Gu X, McCormick BA, Reinecker HC. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell Microbiol. 2002;4:367–81. doi: 10.1046/j.1462-5822.2002.00197.x. [DOI] [PubMed] [Google Scholar]
- 44.Mankertz J, Tavalali S, Schmitz H, et al. Expression from the human occludin promoter is affected by tumor necrosis factor (alpha) and interferon (gamma) J Cell Sci. 2000;113:2085–90. doi: 10.1242/jcs.113.11.2085. [DOI] [PubMed] [Google Scholar]
- 45.Colgan SP, Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across cultured intestinal epithelial monolayers is modulated by epithelial exposure to IFN-gamma in a highly polarized fashion. J Cell Biol. 1993;120:785–98. doi: 10.1083/jcb.120.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor CT, Murphy A, Kelleher D, Baird AW. Changes in barrier function of a model intestinal epithelium by intraepithelial lymphocytes require new protein synthesis by epithelial cells. Gut. 1997;40:634–40. doi: 10.1136/gut.40.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250–4. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 48.Bruewer M, Luegering A, Kucharzik T, et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–72. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 49.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 50.Ibrahim M, Upreti RK, Kidwai AM. Calpain from rat intestinal epithelial cells: age-dependent dynamics during cell differentiation. Mol Cell Biochem. 1994;131:49–59. doi: 10.1007/BF01075724. [DOI] [PubMed] [Google Scholar]
- 51.Smith ME, van der Maesen K, Somera FP. Macrophage and microglial responses to cytokines in vitro: phagocytic activity, proteolytic enzyme release, and free radical production. J Neurosci Res. 1998;54:68–78. doi: 10.1002/(SICI)1097-4547(19981001)54:1<68::AID-JNR8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 52.Deshpande RV, Goust JM, Chakrabarti AK, Barbosa E, Hogan EL, Banik NL. Calpain expression in lymphoid cells. Increased mRNA and protein levels after cell activation. J Biol Chem. 1995;270:2497–05. doi: 10.1074/jbc.270.6.2497. [DOI] [PubMed] [Google Scholar]
- 53.Megyeri P, Nemeth L, Pabst KM, Pabst MJ, Deli MA, Abraham CS. 4-(2-Aminoethyl) benzenesulfonyl fluoride attenuates tumor-necrosis- factor-alpha-induced blood–brain barrier opening. Eur J Pharmacol. 1999;374:207–11. doi: 10.1016/s0014-2999(99)00224-1. [DOI] [PubMed] [Google Scholar]
- 54.Bojarski C, Weiske J, Schoneberg T, et al. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci. 2004;117:2097–07. doi: 10.1242/jcs.01071. [DOI] [PubMed] [Google Scholar]
- 55.Wan H, Winton HL, Soeller C, et al. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy. 2001;31:279–94. doi: 10.1046/j.1365-2222.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- 56.Winton HL, Wan H, Cannell MB, et al. Class specific inhibition of house dust mite proteinases which cleave cell adhesion, induce cell death and which increase the permeability of lung epithelium. Br J Pharmacol. 1998;124:1048–59. doi: 10.1038/sj.bjp.0701905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins Regulate the Intestinal Barrier in Response to Immune Mediators. Gastroenterology. 2000;118:1001–11. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]