Abstract
n-3 Polyunsaturated fatty acids (PUFAs) are recognized as having an anti-inflammatory effect, which is initiated and propagated via a number of mechanisms involving the cells of the immune system. These include: eicosanoid profiles, membrane fluidity and lipid rafts, signal transduction, gene expression and antigen presentation. The wide-range of mechanisms of action of n-3 PUFAs offer a number of potential therapeutic tools with which to treat inflammatory diseases. In this review we discuss the molecular, animal model and clinical evidence for manipulation of the immune profile by n-3 PUFAs with respect to inflammatory bowel disease. In addition to providing a potential therapy for inflammatory bowel disease there is also recent evidence that abnormalities in fatty acid profiles, both in the plasma phospholipid membrane and in perinodal adipose tissue, may be a key component in the multi-factorial aetiology of inflammatory bowel disease. Such abnormalities are likely to be the result of a genetic susceptibility to the changing ratios of n-3 : n-6 fatty acids in the western diet. Evidence that the fatty acid components of perinodal adipose are fuelling the pro- or anti-inflammatory bias of the immune response is also reviewed.
Keywords: immune response, inflammatory bowel disease, polyunsaturated fatty acids
Introduction
The history of fatty acids
Over the years, fat has played a much-maligned role as an unhealthy component of our diet. It is only recently, with the growing realization that there are many different forms of fat, that it has been deemed conceivable that they have a range of differing biological properties and physiological effects. The first advances in the understanding of the differing properties of fats were made in the field of cardiovascular medicine. Evans & Burr, in the United States, had discovered the essential fatty acids as early as 1929. In 1937 the British physiologist Hugh Sinclair, having visited Evans, suggested that a deficiency in some fatty acids might account for the rise in western diseases, such as ischaemic heart disease. In 1944 he visited the Greenland Eskimos and noted their significantly lower incidence of heart disease and other inflammatory diseases. His letter to the Lancet in 1956, entitled ‘Deficiency of essential fatty acids and atherosclerosis etcetera’, challenged the long-held belief that dietary fat of any kind was related to atheroma [1]. Danish scientists Bang & Dyerberg attributed the paucity of ischaemic heart disease in the Greenland Eskimos to their marine diet [2]. Low incidences of heart disease in Japan and in Norway during the German invasion, when fish consumption increased significantly, mirror these findings. Among other inflammatory diseases noted to have a low incidence in populations consuming a marine diet high in omega-3 fatty acids is inflammatory bowel disease [3]. In Japan, the gradual replacement of a traditional diet high in fish-based n-3 PUFAs and increasing dietary consumption of n6 PUFAs in meat has been linked with the increasing incidence of Crohn's disease [4].
Where do polyunsaturated fatty acids come from?
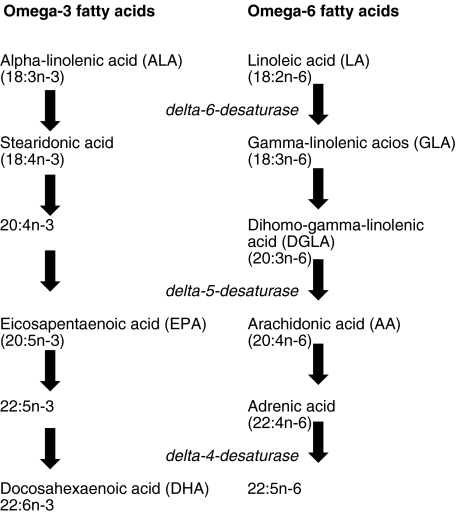
In western man, diet normally supplies adequate amounts of saturated fatty acids but cell membranes also require unsaturated fatty acids (that is fatty acids with carbon double bonds) to maintain their structure, fluidity and function. The ‘n’ or ‘omega’ number of the fatty acid nomenclature refers to the number of double bonds. A mechanism exists for the insertion of double bonds called desaturation. Plants possess enzyme Δ9-desaturase (E.C. 1·14·99·5) which catalyses this reaction and enables them to insert double bonds into oleic acid to produce linoleic acid (LA), from which omega-6 fatty acids are derived, and alpha-linolenic acid (ALA), from which omega-3 fatty acids are derived. Animals lack the enzyme Δ9-desaturase and are therefore unable to synthesize LA and ALA. LA and ALA must therefore be consumed in the diet; hence they are called essential fatty acids. Once consumed LA and ALA are converted by a chain of elongation and desaturation reactions to arachidonic acid (AA) and eicosapentaenoic acid (EPA, 20 : 5n-3), respectively. EPA is then elongated further to form docosahexaenoic acid (DHA, 22 : 6n-3). Many marine plants, especially unicellular algae in phytoplankton, are capable of carrying out the steps that yield the long-chain n-3 polyunsaturated fatty acids EPA and DHA from ALA. It is the formation of EPA and DHA by marine algae and their transfer through the food chain to fish that accounts for the abundance of n-3 PUFAs in some marine fish oils (Fig. 1).
Fig. 1.
The metabolic pathways of omega-3 and omega-6 fatty acids.
What is the significance of polyunsaturated fatty acids in the diet?
Omega-3 PUFAs in particular are essential for normal foetal development and after birth for the biochemical development of the brain and retina. In rhesus monkeys dietary deficiency of omega-3 fatty acids during development results in functional changes in the newborn, including diminished vision, abnormal electroretinographs, impaired visual evoked potentials, polydipsia and disturbed cognition. Similar studies in humans are limited by ethical considerations, but these findings do raise the question of whether infant formulas should be supplemented with omega-3 fatty acids.
It appears that it is not only the amount of essential fatty acids in our diet that is important but also the ratio of omega-3 to omega-6 fatty acids. The Palaeolithic diet on which man evolved and for which our genetic profile is programmed has a ratio of omega-3 : omega-6 of 1, as does the traditional Greek diet used in the Lyon Heart Study, which showed significant prevention of ischaemic heart disease. The scientific basis for this is likely to be related to the inability of mammals to interconvert between the omega-3 and omega-6 pathways. As their metabolisms require the same enzymes this results in competition between the two families. Therefore, an excess of one family can interfere with metabolism of the other, significantly reducing its incorporation into tissue lipids.
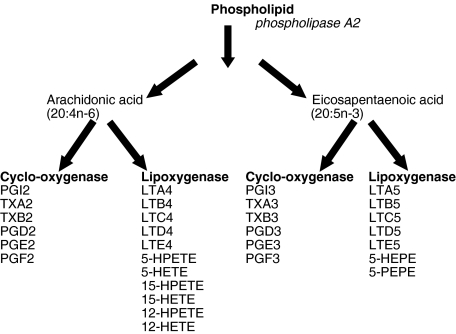
Studies show that if omega-3 fatty acids (DHA and EPA) are incorporated into the phospholipid membrane of cells in higher proportions than omega-6 fatty acids (AA) the resulting eicosanoids released in the inflammatory cascade, including prostaglandins class 3 and PGE3, are less proinflammatory than the prostaglandins classes 1 and 2, thromboxanes A2 and leukotrienes (especially LTB4), released by AA if omega-6 fatty acids predominate (Fig. 2).
Fig. 2.
Eicosanoid synthesis from arachidonic acid and EPA.
Ratios of polyunsaturated fatty acids in the western diet
In industrialized society the ratio of n-6 : n-3 is high due to increased consumption of n-6 rich vegetable oils and decreased consumption of n-3 rich foods such as fish. Epidemiological studies suggest that our intake of n-6 fatty acids had increased from 10 g/day in the late 1970s to 15 g/day by the 1990s. This is an increase from a balanced ratio of 1 to a ratio between 15 and 16·7 : 1. Although vegetable oils such as palm, sunflower, rapeseed and soya-bean have a low n-6 : n-3 ratio the process of partial hydrogenation they undergo for commercial use depletes the n-3 levels. Intensive cereal-based livestock production results in less n-3 in meat and the decreased use of butterfat, lard and tallow also diminishes the amount of n-3 in our diet today. The decreases in the amount of n-3 and increases in levels of n-6 in the western diet are reflected in the levels measured in breast milk [5].
The British Nutrition Foundation Task Force on Unsaturated Fatty Acids recommends a daily intake of 0·5–1·0 g of n-3 long-chain fatty acids [6]. In an attempt to restore a healthier ratio of fatty acids in the western diet the UK Foods Standards Agency held an alpha linolenic workshop in 2002 to look into the possibility of feeding livestock ALA rich oils in order to increase the population's dietary intake. There are concerns about the oxidative stress that can result from increases in intake of n-3 fatty acids if they are not accompanied by adequate amounts of vitamin E, an antioxidant also present in higher levels in more primitive diets.
Studies have looked at the significance of an increased ratio of n-6 : n-3 in a variety of disease processes. In colorectal cancer a ratio of less than 2·5 : 1 reduces rectal cell proliferation [7]. In cardiovascular disease a ratio of less that 4 : 1 produced a decrease in total mortality. A ratio of 2–3 : 1 suppressed inflammation in patients with rheumatoid arthritis and a ratio of 5 : 1 benefited asthma sufferers [8]. Increasing dietary n-3 fatty acids has also been shown to decrease the incidence of malignancy in experimental animal with mammary, pancreatic, prostatic and gastrointestinal neoplasms [9–12].
Links between lipids and immune system
Abnormalities of fat have been implicated in Crohn's disease since Crohn himself described the characteristic pathological fat wrapping and adipose tissue hypertrophy on surgical specimens [13–15]. As early as 1992 it was observed that patients with inflammatory bowel disease had an abnormal plasma phospholipid fatty acid profile [16]. Geerling et al. showed that in patients with active and inactive Crohn's disease there is a specific loss of omega-3 fatty acids in the plasma phospholipids and in adipose tissue compared with controls [17]. Kuroki et al. also showed serum concentrations of total n-3 and total PUFAs to be lower in Crohn's patients than in a matched group of normal controls [18]. Unpublished work in this department has showed a specific loss of n-3 PUFAs in the perinodal adipose tissue in Crohn's patients (work submitted, Westcott et al.). The loss of the omega-3 fatty acids which increases the n-6 : n-3 ratio, would lead to a predominance of proinflammatory eicosanoids. These observations suggest a specific link between PUFAs, the immune system and pathogenesis of this disease. However, a lower than normal n-6 : n-3 fatty acid ratio is seen in peripheral blood mononuclear cells (PBMCs) in patients with Crohn's disease, suggesting that there may also be changes in the cellular uptake and metabolism of fatty acid in cells of the immune system [19].
The relevance of the loss in unsaturated fats being demonstrable in the perinodal adipose tissues is of particular interest as the perinodal fat is usually incorporated into the lymphoid cells of the lymph node (as discussed below). However, the correlation between the n-6 : n-3 ratio inside the lymph node in the perinodal adipocytes is lost in Crohn's disease, with the n-6 : n-3 ratio being higher then normal in the perinodal adipocytes (probably as a result of the loss of n-3) but lower than normal in the cells of the lymph node. (work submitted, Westcott et al.) Changes in fatty acid storage in adipose tissue and uptake and metabolism of fatty acids in immune cells may contribute as much as changes in dietary fatty acids to the immunological changes in Crohn's disease. Recent evidence for this comes from the findings that dietary fish oil supplementation in Crohn's disease patients results in lower production of the key inflammatory mediators, prostaglandin E2 (PGE2) and interferon (IFN)-gamma by blood mononuclear cells [20]. The contribution of multiple factors to the immunological changes in Crohn's disease may be the basis for some of the equivocal results in studies using fatty acids to treat Crohn's disease in which the dietary component alone is changed.
The observation that adipose tissue associated with the heart, pericardium and large blood vessels was metabolically active as opposed to being merely structural, as had been thought previously, led to the investigation of adipose deposits at other sites [21]. In mammals adipose tissue is always split into a few large depots and many scattered smaller ones. Many of the smaller ones contain lymph nodes and all large lymph nodes and their connecting ducts are embedded in adipose tissue. It is notable that even in the leanest mammals there are always adipose deposits around lymph nodes and these deposits seem to be preferentially spared in starvation. These observations led to the hypothesis that perinodal adipose tissue could be participating in the immune response.
In vivo and in vitro studies showed that perinodal adipose tissue had special properties, which enable it to interact locally with lymphoid tissues [22]. Cultured lymphoid cells stimulate lipolysis by up to threefold in explants of perinodal adipose tissue, much more that in similarly treated samples from adipose deposits elsewhere [23]. In addition, adipocytes from around mesenteric, omental and popliteal lymph nodes have lower rates of spontaneous lipolysis but release more glycerol in the presence of combinations of noradrenalin, tumour necrosis factor-α (TNF-α) and interleukins than adipocytes from sites in the same depot more distant from the nodes [24]. Triacylglycerols in all perinodal adipose tissue studied contained more polyunsaturated fats and fewer saturated ones than those in adipose tissue remote from nodes [25].
These observations were consistent with the hypothesis that perinodal adipose provides an energy source for lymphoid cells, and hence plays a role in the relationship between dietary lipids and immune activity by sequestering fatty acids for the exclusive use of the immune system. The dendritic cells, which frequent lymph nodes and ducts and have a major role in antigen presentation, may be mediating this interaction, as they have been noted to acquire large droplets of triacylglycerols [26]. This proposed relationship between adipose tissue and the immune system might also explain the redistribution of adipose tissue that takes place after a long period of chronic inflammation. In Crohn's disease, abnormalities of fat in the mesentery have long been noted on surgical specimens as characteristic features of the disease. The cytokine TNF-α seems to be involved in the unusual arrangements and accumulation of mesenteric adipose tissue [14]. Interestingly, in another immune disease HIV there are also characteristic changes in the morphology of the adipose tissue, with enlargement in those areas associated with lymphoid tissue and shrinkage in those that are not. Retroviral infection is known to make dendritic cells alter their secretion of some of the cytokines to which perinodal adipocytes respond [27,28].
Polyunsaturated fatty acids and inflammatory bowel disease
Animal models of inflammatory bowel disease and PUFAs
Shoda et al. investigated the therapeutic efficacy of n-3 PUFAs in rats with trinitrobenzene sulphonic acid (TNBS)-induced colitis, a recognized model for Crohn's disease. In these models an elemental diet (ED) enriched with 2%n-3 rich perilla oil was compared to an ED enriched with 2%n-6 rich safflower oil. In the n-3 oil group plasma levels of LTB4 and the ulcer index were significantly reduced compared with those in the n-6 group. Moreover, the ulcer index and LTB4 levels were significantly correlated. In this study, when enrichment of the ED with ALA was compared with enrichment with EPA/DHA, the former was therapeutically superior at suppressing levels of LTB4 and hence the ulcer index [29].
Vilaseca et al. also used the TNBS rat model in two groups treated with 4 weeks of a dietary supplement enriched with either sunflower (n-6) or cod liver oil (n-3). Luminal eicosanoid levels increased significantly in both groups after intracolonic administration of TNBS. Generation of PGE2 and LTB4 peaked at day 3 and declined thereafter, whereas TXB2 continued to increase from days 3–20 in the n-6 group. This response was blunted in the n-3 group. Macroscopic and histological levels of inflammatory damage were scored at 20, 30 and 50 days. In the n-3 group the score was markedly reduced after 30 days and inflammation and ulceration were almost absent by 50 days. The study concluded that this was due to the blunting of the TXB2 increase in the n-3 group [30]. Hirata et al. also looked at PGE2 levels in a dextran sulphate sodium (DSS)-induced rat model of UC. They showed a correlation between lower levels of PGE2 and more severe colonic lesions suggesting that PGE2 might act as an inhibitory factor against the development of inflammation in intestinal mucosa [31]. Empey et al. provided further evidence for a protective effect of n-3 PUFAs, judged on a macroscopic and histological basis in rats with 4% acetic acid-induced mild colitis [32].
In the most recent study, rats were fed a diet rich in either n-3 or n-6 fatty acid for 12 days before enteritis was induced using TNBS. Histologically, the mucosal changes were significantly more severe in the rats that had been fed the n-6 diet than those that had been fed the n-3 diet. Serum IL-6 levels were significantly higher in the n-6 group than the n-3 group suggesting that a possible mechanism for suppression of mucosal inflammation could be blockage of mucosal IL-6 secretion. Interestingly, there were no differences in TNF-α levels between the two groups [33].
Despite the seemingly promising therapeutic efficacy of n-3 PUFAs in animal models of induced colitis other authors have queried the wisdom of extrapolating results directly from animals to humans in view of the potential physiological and pathological differences that exist between animal models and the human form of the disease.
Nutritional studies
The deficiency of omega-3 fatty acids in patients with Crohn's disease suggests that there is therapeutic potential to modulate the immune response by increasing the n-3 : n-6 ratio. Further rationale for the use of n-3 fatty acids lies in the anti-inflammatory properties of the eicosanoids that are derived from them. It was the use of elemental feeds as a supportive treatment for Crohn's disease that focused attention on the potential therapeutic significance of fat. Meta-analyses showed a remission rate of 60% with enteral feeds used on an intention to treat basis [34]. More recently there has been controversy regarding the efficacy of enteral therapy for inflammatory bowel disease in adults and the rates quoted in the meta-analysis questioned. In contrast, there is firm evidence to support the use of enteral feeding as primary therapy for Crohn's disease in children [35]. There are problems inherent to enteral therapy such as palatability, compliance, aspiration, non-absorption and limited data on its mode of action. However, even in adults they have a useful, steroid-sparing effect. Currently, enteral therapy is not used routinely as the primary treatment except in paediatric populations and specific adult subgroups. There is currently no evidence to support the use of enteral nutrition in ulcerative colitis.
It has now been established that the type and amount of fat in enteral feed is relevant to their efficacy [36]. Initially it was hypothesized that it was the absence of whole proteins, and therefore the decreased antigenic load, in elemental feeds that was responsible for inducing remission but subsequent studies showed that polymeric feeds in which nitrogen is presented as undigested protein, and in which fat provided 30–35% of the calories were equally effective [34]. Subsequent trials showed that increasing levels of long chain triglycerides (LCTs) had a detrimental effect whereas no obvious deleterious effects were seem with medium chain triglycerides (MCTs). Bamba et al. demonstrated that qualitatively among LCTs, high levels of the n-6 PUFA have a worse effect than high oleic acid levels. This result has, however, not been reproduced consistently [37].
Ikehata et al. looked at the effects of immunonutrition on the cellular mediators of intestinal inflammation. They supplemented the parenteral nutrition in 10 patients with active Crohn's disease with an infusion of the n-3 fatty acid eicosapentaenoic acid (EPA) for 2 weeks. They isolated polymorphonuclear leucocytes (PMNLs), from the patients’ blood, before and after the treatment, and measured the amount of leukotriene B4 (LTB4) and leukotriene B5 (LTB5) they produced. They showed LTB5 levels to be significantly lower in the patients with Crohn's disease than in healthy control subjects but the level of LTB5 and the ratio of LTB5 : LTB4 increased significantly after supplementation with EPA. This trial did not, however, correlate their cellular findings with clinical improvement in the disease [38].
Clinical trials
Epidemiological observations of the incidence of inflammatory bowel disease and the significance of fat in the formulation of elemental feeds seen to induce remission in Crohn's disease led to a number of trials to study the apparently anti-inflammatory effects of n-3 PUFAs as a putative treatment in inflammatory bowel disease. The abnormal plasma phospholipid fatty acid profile changes noted in inflammatory bowel disease strengthened the rationale for using n-3 PUFAs as a treatment. In addition, changes in the eicosanoids derived from plasma phospholipids have been noted in inflammatory bowel disease. For example, one of the most inflammatory arachidonic acid metabolites leukotriene B4 (LTB4) is elevated in inflamed intestinal mucosa [39]. Another AA metabolite, thromboxane A2 (TXA2), is released as an early change even in uninflamed tissue in inflammatory bowel disease [40]. n-3 PUFAs also inhibit interleukin (IL)-1β, TNF production and act as free radical scavengers [41,42].
The conflicting results from the trials have been ascribed to a number of causes, the main ones being patient selection, influence of concomitant therapy, choice of placebo, patient compliance and differences in study design. In general, the increased compliance associated with new formulations of fish oils that have less unpleasant side effects offer a potentially useful therapeutic modality for the management of inflammatory bowel disease. Therapeutic strategies that prevent recurrence would be of particular benefit, as endoscopic follow-up of patients who have had resections of ileal Crohn's disease show a postoperative recurrence rate of 73% within 1 year and 85% within 3 years. Half the patients followed-up in a 10-year postoperative period will require further surgical intervention [43].
Putative mechanisms for the immunomodulatory effects of n-3 PUFAs
Despite some conflicting evidence on the effects of n-3 PUFAs on the immune system there is still a strong body of evidence to suggest an immunomodulatory role for them. To this end a great deal of work has been conducted recently in an attempt to elucidate a mechanism by which they may be exerting their effects. There are six main avenues of current research investigating a potential mechanism of immunomodulatory mechanism.
Modulation by eicosanoids
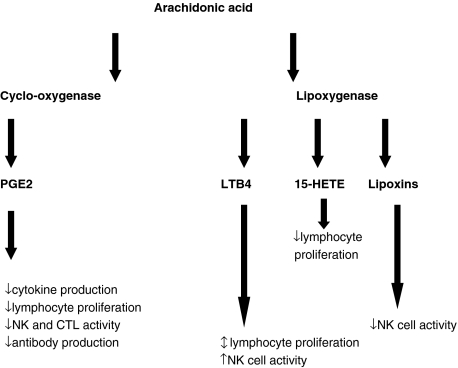
The production of cytokines and nitric oxide is regulated by eicosanoids, therefore it has been suggested that an alteration in the amount or type of eicosanoid could provide some explanation of the effects of n-3 PUFAs (Fig. 3).
Fig. 3.
Immunomodulatory effects of eicosanoids.
The eicosanoids derived from AA are more proinflammatory than those derived from EPA or DHA. In addition, dietary supplementation in human and animal studies with FO results in a decrease in membrane levels of AA [58,59] (as they are replaced with the n-3 PUFA) and an associated decrease in its capacity to produce AA-derived eicosanoids, as a result of competitive inhibition of oxygenation of AA [60,61]. There is a consequent increase in the level of EPA-derived eicosanoids and as a result there are significant effects on processes such as platelet aggregation, vasoconstriction, neutrophil function, inflammation and immunity [62].
The main source of eicosanoids is macrophages, but it appears that their production can be modulated not only by their anatomical site and the nature of their stimulus, but also by the presence of lymphocytes. As well as being the main source of eicosanoids immune cells are also subject to their regulatory effects. The most studied of these is PGE2. T lymphocytes have receptors for PGE1 and PGE2 and these PGs suppress T lymphocyte proliferation, T cell-mediated toxicity, IL-2 production and natural killer (NK) cell activity in vitro[63,64]. B cells also have PGE2 receptors and PGE2 can modulate antibody production. PGs also inhibit production of IL-1 and TNF by macrophages [65]. NK cell activity is enhanced by LTB4, which can also enhance IFN-γ production by lymphocytes [66,67]. There is new evidence that prostaglandins also regulate chemokine receptor expression, migration and apoptosis of dendritic cells [68]. Eicosanoids clearly have a direct effect on the function of immune cells and as they themselves can be modulated by n-3 PUFAs, this provides a putative mechanism for their effects.
n-3 PUFAs, membrane fluidity and lipid rafts
Fatty acids have important structural roles in the membrane. The composition of cell membranes is usually characteristic for the cell type. Stimulation of lymphocytes is accompanied by an almost immediate de novo synthesis and turnover of membrane phospholipids. If there is an increase in the proportion of unsaturated fatty acids in the membrane its fluidity increases. Dietary supplementation with n-3 fatty acids produced a sustained increase in membrane fluidity, even after withdrawal of the supplementation, in human erythrocyte membranes [69]. Other studies have supported this finding [70,71].
There are also fatty acid-dependent effects on the conformation of protein complexes in the membrane. A number of membrane-bound enzymes and receptors are highly sensitive to their fatty acid environment. It has recently been proposed that initiation and propagation of the signalling events taking place in immune cells occur in specialized membrane regions called lipid rafts [72,73]. These detergent-insoluble glycolipid domains are specialized membrane compartments enriched in cholesterol and glycolipids. They also contain signalling molecules, suggesting that they may act as platforms for multi-component transduction complexes. On receptor binding, immune receptors become raft-associated and additional components of the signalling pathways are recruited to rafts in order to form signalling complexes. Entry of immune receptors may be responsible for regulation of cell activation and raft integrity certainly seems to be crucial to the initiation and maintenance of intracellular signals.
In vitro evidence suggests that n-3 PUFAs suppress T cell activation by displacing acylated, signalling proteins from rafts by altering raft lipid composition [74]. Docosahexaenoic acid (DHA) causes partial disorganization of lipid rafts in stimulated human peripheral blood mononuclear cells, resulting in displacement of phospholipases D (PLD). This displacement of PLD seems to be responsible for its activation. Activation of PLD is known to stimulate antiproliferative signals in lymphoid cells, which may account for the immunosuppressive effects of DHA [75]. Dietary n-3 PUFAs have now also been shown to differentially modulate lipid raft composition in vivo[76]. Therefore, the effects of the fatty acid environment on lymphocyte membrane receptors and enzymes, offers an alternative hypothesis for how n-3 PUFAs might exert an effect. Lipid rafts are also known to be sensitive to pharmacological agents, making them potential targets for immune regulation.
n-3 PUFAs and signal transduction
Lipids are involved in many intracellular signalling pathways. Some phospholipids are acted upon by phospholipases to generate secondary messengers, such as phosphatidylinositol 4,5-bisphosphate generating diacylglycerol and inositol-1, 4, 5-triphosphate. Others are involved in activating or stabilizing enzymes in the signalling pathways, such as phosphatidylserine, which is required for the activation of protein kinase C (PKC). As all phospholipids and the secondary messengers that they generate contain fatty acyl chains it is conceivable that altering the type of fatty acid may change the properties of these molecules. There are many signal transduction pathways in immune cells, particularly in T and B-lymphocytes, where they amplify extracellular signals to trigger transcriptional events in the nucleus.
EPA and DHA are capable of inhibiting PKC, and decreasing generation of the relevant secondary messengers, without affecting protein kinase A (PKA) [77–79]. Consistent with the suppression of generation of secondary messengers by n-3 PUFAs is a study showing that dietary supplementation with 14·4 g per day of FO for 10 weeks resulted in a decrease in production of inositol-1, 4, 5-triphosphate by human peripheral blood neutrophils stimulated with platelet activating factor (PAF) and LTB4. Many secondary messengers in immune cells utilize changes in intracellular free calcium as a key step in the pathway and fatty acids can influence these changes. Oleic, but not stearic acid, inhibited the concanavalin A (Con-A) stimulated rise in intracellular free calcium concentration in a CTL cell line [80]. Other studies have suggested that the fatty acids act by blocking calcium entry into the cells by having a direct effect upon receptor operated calcium channels.
A novel mechanism by which fatty acids modulate signalling pathways and target gene expression is the reciprocal modulation of the activation of Toll-like receptor (TLR) 4, and its down-stream signalling pathways, by saturated and unsaturated fatty acids [81,82]. Unsaturated fatty acids suppress the NF [kappa] B activation & cyclo-oxygenase (COX)-2 expression that can be induced by a TLR-2 agonist in macrophages. In contrast, saturated fatty acids potentiate TLR-2 agonist-induced NF [kappa] B activation and COX-2 expression in macrophages [83]. COX-2 expression is undetectable in normal intestine but it is induced in apical epithelial cells of inflamed foci in inflammatory bowel disease [84]. The mucosal inflammation in inflammatory bowel disease is a consequence of the inflammatory response to microbiota in the bowel and TLRs provide the route by which recognition of the bacteria occurs. The differential modulation of TLRs by different types of dietary fatty acid could have profound implications for the role of dietary fats in modulation of the immune and inflammatory responses induced by activation of TLRs in the intestine. Manipulation of the composition of dietary fat could modulate not only TLR activation but also signalling paths downstream of TLRs, target gene expression and the consequent cellular responses.
n-3 PUFAs and gene expression
Fatty acids also affect the expression of genes for proteins involved in hepatic fatty acid and lipoprotein metabolism. They may act by covalent modification, redox state or proteolytic modification or through binding of an activating ligand by the transcription factor in the same fashion as steroid hormones. Alternatively, they may act by altering the levels of other mediators, such as eicosanoids and cytokines, which affect gene expression. Studies are currently working on the theory that fatty acids serve as ligands for transcription factors.
Cells of the immune system contain many transcriptional factors which regulate a whole range of cytokines, adhesion molecules, enzymes involved in mediator generation and acute phase proteins, one of which is NF [kappa] B. There has been speculation that the effects of PUFAs on the expression of cytokines and adhesion molecules are mediated via this transcription factor. NF [kappa] B is activated by phosphorylation, often by PKC, and a recent study by Camandola et al. supported this assertion by showing that AA strongly stimulates the nuclear translocation of NK [kappa] B in the human promonocyte line, probably through the effects of eicosanoids on the activation of PKC [85].
Forman et al. studied the activators and ligands for the peroxisome proliferator-activated receptor family (PPAR): a group of key nuclear receptors involved in the regulation of lipid homeostasis [86–89]. PPARs regulate the expression of target genes by binding to DNA sequence elements, called PPAR response elements. These studies showed that PGs A2, B2, D2 and 15deoxy[DELTA] 12, 14-PGJ2 are activators of PPAR-[alpha], [gamma] and [delta], that LTB4, 8-HETE and 12-HETE are activators of PPAR- [alpha] only and that PGE2, PGF2α and I2 are not activators of PPAR. Later data suggested that most eicosanoids are poor ligands for PPAR. PUFAs themselves are activators of PPAR; in fact, they bind directly to PPAR [alpha], [gamma] and [delta][86,88]. EPA-derived 8-HETE is a potent activator and ligand of PPAR and PPAR isoforms have also been identified in lymphoid tissues in rat spleen and lymph node, suggesting that genes within cells of the immune system could indeed be subject to regulation by PUFAs [89].
Colonic epithelial cells, which express high levels of PPAR-gamma protein, produce inflammatory cytokines that play a role in inflammatory bowel disease [90]. PPAR-gamma ligands significantly interfere with the ability of colonocytes to express the immunomodulatory cytokines implicated in the manifestation of intestinal inflammation by attenuating cytokine gene expression. This is thought to occur via inhibition of the activation of the transcription factor, NF kappaB [91]. Bassaganya-Riera et al. showed a direct correlation between clinical amelioration of dextran sodium sulphate (DSS) and CD4+-induced colitis by CLA in mice and CLA-induced PPAR-gamma and -delta, transcriptionally modulated PPAR-gamma and -delta-responsive gene clusters involved in lipid metabolism and epithelial cell maturation. Loss of the PPAR gamma in the colon, by the use of specific deletions, abrogated the beneficial effects of CLA in DSS-induced colitis in this model. In addition, CLA repressed TNF-alpha expression and NF [kappa] B activation while inducing the immunoregulated cytokine transforming growth factor beta-1 (TGF-beta-1) [92]. This work provides molecular evidence that CLA ameliorates colitis via a PPAR-dependent mechanism, hence supplying a potential therapeutic tool for the manipulation of intestinal inflammation by the use of PPAR ligands.
n-3 PUFA modulation of antigen presenting capacity
The effects of n-3 PUFAs have been noted already. As demonstrated by Hughes et al. in vivo, when healthy human volunteers are given a dietary fish oil supplement for 21 days the intensity of expression of MHC II antigen-presenting molecules on peripheral blood monocytes decreases significantly, although the percentage of monocytes expressing the molecules does not change. However, when the monocytes are stimulated with IFN-γ (with the exception of HLA–DQ) both the intensity of MHC II molecule expression and the percentage of monocytes expressing MHC II decreases [93]. These results were reproduced, in vitro, by the same group when they incubated human monocytes for 48 h at 37 degrees, with and without EPA and DHA. In the samples with EPA there was a lower intensity of expression of HLA-DR and ICAM-1 but in the DHA samples there was a higher intensity of expression of the same molecules. Activation with IFN-γ, however, significantly lowered the expression of HLA-DR, -DP and ICAM-1 with both EPA and DHA. Both the in vitro and in vivo experiments therefore suggest that the mechanism of modulation of the immune response by n-3 PUFAs might be via modulation of antigen-presenting cell function.
The capacity of antigen-presenting cells to function effectively is also supported by the apparent down-regulation of expression of adhesion molecules, specifically ICAM-1, by n-3 PUFAs. The theory for this being that without appropriate adhesion molecule expression the ability of antigen bearing cells in circulation to travel to lymphoid areas, where they present antigen to T lymphocytes and stimulate an immune response, would be extremely limited.
n-3 PUFA modulation of gastrointestinal flora
The role of intestinal bacteria has long been recognized in human colitis. Studies have shown an excess of entero-adhesive and entero-haemorrhagic Escherichia coli in ulcerative colitis and reduced levels of Bifidobacteria in patients with Crohn's disease [94,95]. Any effects exerted by PUFAs on intestinal flora could therefore alter the balance of inflammation in the intestine. Ringo et al. demonstrated that dietary lipids had an effect on gastrointestinal flora, in particular, on the level of lactic acid bacteria [96]. Kankaanpaa et al. characterized this effect, showing that high concentrations of PUFAs inhibited the growth and mucus adhesion of selected Lactobacilli, while growth and mucus adhesion of Lactobacilli casei Shirota was promoted by low concentrations of gamma-linolenic acid and arachidonic acid. They also showed that PUFAs altered bacterial adhesion sites on Caco-2 cells [97]. It is suggested that dietary PUFAs affect mucosal adhesion sites for gastrointestinal bacteria by modifying the composition of the intestinal wall.
As the adhesion of gastrointestinal bacteria to the mucosa is pivotal to the beneficial effects of probiotics, these findings suggest that the action of probiotics in the gut could be modulated by dietary PUFAs. This effect was illustrated in gnotobiotic pigs, in which omega-3 PUFA administration significantly increased the number of Lactobacilli paracasei, adhering to jejunal mucosa [98]. This stimulatory effect of PUFAs on lactobacilli adhesion suggests that they could be used to enhance the effectiveness of probiotics. Amelioration of colonic inflammation by dietary supplementation with conjugated linoleic acid (CLA) was demonstrated by challenging pigs fed either a soybean-supplemented or CLA-supplemented diet with a colitis-inducing bacterial pathogen. The group fed CLA preventatively before onset of enteric disease showed attenuated inflammatory lesion development as evaluated by histology and immunohistochemistry. In addition cytokine profiles and lymphocyte subset distributions in the pigs fed CLA resembled those of non-infected pigs [99].
These studies, together with others showing that human-derived Bifidobacterium species can biosynthesize conjugated linoleic acid [100] and that addition of probiotic bacteria to infant formulae can differentially influence the infant's plasma lipid composition [101], suggest a definite role for the interactions between PUFAs and gastrointestinal bacteria in the balance of inflammation in the intestine.
Conclusions
This review describes the complex interactions between lipids and the immune system and explains how these might provide an insight into the multi-factorial aetiology of inflammatory bowel disease. It illustrates how PUFAs modulate a wide-range of steps on the pathway to inflammation, from their effects on signal transduction via the protein kinase and Toll-like receptor pathways, to their role in modulation of expression of genes for the inflammatory cytokines implicated in colitis via the PPAR family of nuclear receptors. The influence of dietary PUFAs on phospholipids, their eicosanoid derivatives and the transmembrane-signalling lipid rafts into which they are arranged provide multiple mechanisms for dietary modulation of the balance of inflammatory mediators in the gut. In addition, gut mucosal inflammation is now recognized as being determined by the gastrointestinal microflora. We review evidence for the interactions between PUFAs and bacteria and the implications for the enhanced use of probiotics. Recognition of the multiple mechanisms by which PUFAs exert their effects provides potential therapeutic tools for the amelioration of colitis.
Furthermore, based on emerging evidence of abnormalities of PUFA ratios in perinodal adipocytes and immune cells in blood we have suggested a role for PUFAs as a potential factor in the complex aetiology of inflammatory bowel disease.
Table 1. Clinical trials using n-3 fatty acids in inflammatory bowel disease.
| Author | Type of trial | Type and number of patients | Fatty acid dose and duration | Clinical end-point |
|---|---|---|---|---|
| McCall et al. (1989) [44] | Uncontrolled | 6-active UC | 3–4 g/day EPA 12 weeks | Improved symptoms/histology/LTB4 production |
| Salomon et al. (1990) [45] | Uncontrolled | 10-UC refractory to medication | 2·7 g/day EPA 1·8 g/day DHA 8 weeks | 70% patients showed improvement in disease activity measures |
| Lorenz et al. (1989) [46] | Placebo-controlled cross-over | 29-CD | 3·2 g/day n-3 fatty acid | Improvement in disease activity scores in UC not CD |
| 10-UC | 7 months (1-month washout) | |||
| Hawthorne et al. (1992) [47] | Placebo-controlled | 96-UC (differing stages of activity) | 4·5 g/day 1 year | Steroid-sparing effect in active disease/no prevention of relapse/more than 50% reduction in LTB4 |
| Stenson et al. (1992) [48] | Randomized, double-blinded, placebo-controlled cross-over | 24-active UC | 5·4 g/day n-3 versus oliveoil placebo, 4/12 (1/12 washout) | Improvements in weight and histology/60% decrease in LTB4/no steroid-sparing effect |
| Aslan et al. (1992) [49] | Placebo-controlled cross-over | 17-active UC | 4·2 g/day n-3, 3/12 (2/12 washout) versus corn oil placebo | 72% patients showed steroid-sparing effects/50% had improvement in disease, no improvement in histology |
| Mate et al. (1996) [50] | Controlled | 38-CD (in remission) | Fish enriched diet, 2 years | Longer symptomatic remission on diet |
| Loeschke et al. (1996) [51] | Placebo-controlled | 64-UC (in remission, 3/12 ASAs permitted | 5·1 g/day versus corn oil placebo, 2 years | Less relapses in FO group after 3/12. Not sustained at 2 years |
| Lorenz-Meyer (1996) [52] | Placebo-controlled | 204-CD (recovering from relapse, 8/52 steroids permitted | 5·1 g/day versus low-carb diet versus placebo | None prevented relapses |
| Belluzzi et al. (1996) [53] | Double-blinded, placebo-controlled | 78-CD (high risk of relapse) | Novel enteric coated formulation, 2·7 g/day n-3, 1 year | At 1 year 59% of FO group in remission versus 26% placebo group |
| Dichi et al. (2000) [54] | Randomized controlled trial cross-over | 10 mild to moderate UC | 2 g/day sulphasalazine versus 5·4 g/day n-3 PUFA, 2 months | Increased disease activity in n-3 PUFA group |
| Middleton et al. (2002) [55] | Double-blind, randomized controlled trial | 63 UC | Linolenic, EPA and DHA versus placebo, 1 year | No maintenance of remission in UC |
| Gassull et al. (2002) [36] | Multi-centre randomized controlled trial double-blinded | 62 active CD | Polymeric diet (35 g/day lipid) (a) high oleate, low linoleate/(b)low oleate, high linoleate versus oral steroid (1 mg/ kg/day) | Remission rates/polymeric diet/(a) 27%/ (b)63%/steroid 79% |
| Barbosa et al. (2003) [56] | Randomized controlled trial placebo-controlled | Nine mild/moderate UC on 2 g/day sulphasalazine | 4·5 g/day n-3 PUFA versus placebo 2 months (2-month washout) | Significant reduction in oxidative stress in n-3 PUFA group but no change in disease activity scores |
| Bjorkkjaer et al. (2004) [57] | Placebo-controlled | 9-CD 10-UC | 10 ml duodenal administration seal oil versus soy oil, 10 days | Seal oil had significant beneficial effect on joint pain maintained at 6 months |
| Trebble et al. (2004) [19] | Randomized controlled trial, placebo-controlled | 31 CD | 2·7 g EPA/DHA + anti-oxidant versus olive oilplacebo, 24 weeks | Significantly lowered production of PGE2 and IFN-γ by peripheral blood monocytes |
References
- 1.Sinclair HM. Deficiency of essential fatty acids and atherosclerosis, etcetera. Lancet. 1956;270:381–3. [PubMed] [Google Scholar]
- 2.Bang HO. The composition of food consumed by Greenlandic Eskimos. Acta Med Scand. 1976;200:69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- 3.Kromann N, Green A. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950–74. Acta Med. 1980;208:401–6. [PubMed] [Google Scholar]
- 4.Shoda R, Matsueda K, Yamato S, Umeda N. Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am J Clin Nutr. 1996;63:741–5. doi: 10.1093/ajcn/63.5.741. [DOI] [PubMed] [Google Scholar]
- 5.Sanders TA, Reddy S. Infant brain lipids and diet. Lancet. 1992;340:1093–4. [PubMed] [Google Scholar]
- 6.British Nutritional Foundation. Task force on unsaturated fatty acids. New York: Chapman & Hall; 1992. [Google Scholar]
- 7.Chajes V, Bougnoux P. Omega-6/omega-3 polyunsaturated fatty acid ratio and cancer. World Rev Nutr Diet. 2003;92:133–51. [PubMed] [Google Scholar]
- 8.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–79. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Stavro PM, Thompson LU. Dietary flaxseed inhibits human breast cancer growth and metastasis and downregulates expression of insulin-like growth factor and epidermal growth factor receptor. Nutr Cancer. 2002;43:187–92. doi: 10.1207/S15327914NC432_9. [DOI] [PubMed] [Google Scholar]
- 10.Caygill CP, Charlett A, Hill MJ. Fat, fish, fish oil and cancer. Br J Cancer. 1996;74:159–64. doi: 10.1038/bjc.1996.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merendino N, Molinari R, Loppi B, et al. Induction of apoptosis in human pancreatic cancer cells by docosahexaenoic acid. Ann NY Acad Sci. 2003;1010:361–4. doi: 10.1196/annals.1299.143. [DOI] [PubMed] [Google Scholar]
- 12.Leitzmann MF, Stampfer MJ, Michaud DS, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–16. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 13.Crohn BB, Ginsburg L, Oppenheimer GD. Regional ileitis, a clinical and pathological entity. JAMA. 1932;99:1323–9. [Google Scholar]
- 14.Desreumaux P, Ernst O, Geboes K, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology. 1999;117:73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- 15.Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn's disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79:955–8. doi: 10.1002/bjs.1800790934. [DOI] [PubMed] [Google Scholar]
- 16.Geerling BJ, Houwelingen AC, Badart-Smook A, Stockbrugger RW, Brummer RJ. Fat intake and fatty acid profile in plasma phospholipids and adipose tissue in patients with Crohn's disease, compared with controls. Am J Gastroenterol. 1999;94:410–7. doi: 10.1111/j.1572-0241.1999.869_a.x. [DOI] [PubMed] [Google Scholar]
- 17.Geerling BJ, Stockbrugger RW, Brummer RJ. Nutrition and inflammatory bowel disease: an update. Scand J Gastroenterol Suppl. 1999;230:95–105. doi: 10.1080/003655299750025615. [DOI] [PubMed] [Google Scholar]
- 18.Kuroki F, Iida M, Matsumoto T, Aoyagi K, Kanamoto K, Fujishima M. Serum n3 polyunsaturated fatty acids are depleted in Crohn's disease. Dig Dis Sci. 1997;42:1137–41. doi: 10.1023/a:1018873217192. [DOI] [PubMed] [Google Scholar]
- 19.Trebble TM, Arden NK, Wootton SA, et al. Fish oil and antioxidants alter the composition and function of circulating mononuclear cells in Crohn disease. Am J Clin Nutr. 2004;80:1137–44. doi: 10.1093/ajcn/80.5.1137. [DOI] [PubMed] [Google Scholar]
- 20.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–8. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13:277–96. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 22.Pond CM. Physiological specialisation of adipose tissue. Prog Lipid Res. 1999;38:225–48. doi: 10.1016/s0163-7827(99)00003-x. [DOI] [PubMed] [Google Scholar]
- 23.Pond CM, Mattacks CA. Interactions between adipose tissue around lymph nodes and lymphoid cells in vitro. J Lipid Res. 1995;36:2219–31. [PubMed] [Google Scholar]
- 24.Pond CM, Mattacks CA. In vivo evidence for the involvement of the adipose tissue surrounding lymph nodes in immune responses. Immunol Lett. 1998;63:159–67. doi: 10.1016/s0165-2478(98)00074-1. [DOI] [PubMed] [Google Scholar]
- 25.Mattacks CA, Pond CM. The effects of feeding suet-enriched chow on site-specific differences in the composition of triacylglycerol fatty acids in adipose tissue and its interactions in vitro with lymphoid cells. Br J Nutr. 1997;77:621–43. doi: 10.1079/bjn19970061. [DOI] [PubMed] [Google Scholar]
- 26.Maroof A, English NR, Bedford PA, Gabrilovich DI, Knight SC. Developing dendritic cells become ‘lacy’ cells packed with fat and glycogen in press. Immunology. 2005. in press. [DOI] [PMC free article] [PubMed]
- 27.Kelleher P, Williams NJ, Knight SC. Interleukin-12 administration in retroviral infection of mice increases the potential to produce functional dendritic cells from bone marrow stem cells. Immunol Lett. 1999;65:51–4. doi: 10.1016/s0165-2478(98)00124-2. [DOI] [PubMed] [Google Scholar]
- 28.Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA, Atkinson RL. Association of adenovirus infection with human obesity. Obes Res. 1997;5:464–9. doi: 10.1002/j.1550-8528.1997.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 29.Shoda R, Matsueda K, Yamato S, Umeda N. Therapeutic efficacy of N-3 polyunsaturated fatty acid in experimental Crohn's disease. J Gastroenterol. 1995;30(Suppl. 8):98–101. [PubMed] [Google Scholar]
- 30.Vilaseca J, Salas A, Guarner F, Rodriguez R, Martinez M, Malagelada JR. Dietary fish oil reduces progression of chronic inflammatory lesions in a rat model of granulomatous colitis. Gut. 1990;31:539–44. doi: 10.1136/gut.31.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirata I, Murano M, Nitta M, et al. Estimation of mucosal inflammatory mediators in rat DSS-induced colitis. Possible role of PGE (2) in protection against mucosal damage. Digestion. 2001;63(Suppl. 1):73–80. doi: 10.1159/000051915. [DOI] [PubMed] [Google Scholar]
- 32.Empey LR, Jewell LD, Garg ML, Thomson AB, Clandinin MT, Fedorak RN. Fish oil-enriched diet is mucosal protective against acetic acid-induced colitis in rats. Can J Physiol Pharmacol. 1991;69:480–7. doi: 10.1139/y91-072. [DOI] [PubMed] [Google Scholar]
- 33.Andoh A, Tsujikawa T, Ishizuka I, et al. n-3 fatty acid-rich diet prevents early response of interleukin-6 elevation in trinitrobenzene sulfonic acid-induced enteritis. Int J Mol Med. 2003;12:721–5. [PubMed] [Google Scholar]
- 34.Griffiths AM, Ohlsson A, Sherman PM, Sutherland LR. Meta-analysis of enteral nutrition as a primary treatment of active Crohn's disease. Gastroenterology. 1995;108:1056–67. doi: 10.1016/0016-5085(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 35.Kleinman RE, Baldassano RN, Caplan A, et al. Nutrition support for pediatric patients with inflammatory bowel disease: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39:15–27. doi: 10.1097/00005176-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Gassull MA, Fernandez-Banares F, Cabre E, et al. Fat composition may be a clue to explain the primary therapeutic effect of enteral nutrition in Crohn's disease: results of a double blind randomised multicentre European trial. Gut. 2002;51:164–8. doi: 10.1136/gut.51.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bamba T, Shimoyama T, Sasaki M, et al. Dietary fat attenuates the benefits of an elemental diet in active Crohn's disease: a randomized, controlled trial. Eur J Gastroenterol Hepatol. 2003;15:151–7. doi: 10.1097/00042737-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Ikehata A, Hiwatashi N, Kinouchi Y, et al. Effect of intravenously infused eicosapentaenoic acid on the leukotriene generation in patients with active Crohn's disease. Am J Clin Nutr. 1992;56:938–42. doi: 10.1093/ajcn/56.5.938. [DOI] [PubMed] [Google Scholar]
- 39.Sharon P, Stenson WF. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984;86:453–60. [PubMed] [Google Scholar]
- 40.Rampton DS, Collins CE. Thromboxanes in inflammatory bowel disease – pathogenic and therapeutic implications [Review] Aliment Pharmacol Ther. 1993;7:357–67. [PubMed] [Google Scholar]
- 41.DeLuca P, Rossetti RG, Alavian C, Karim P, Zurier RB. Effects of gammalinolenic acid on interleukin-1 beta and tumor necrosis factor-alpha secretion by stimulated human peripheral blood monocytes: studies in vitro and in vivo. J Invest Med. 1999;47:246–50. [PubMed] [Google Scholar]
- 42.Dooper MM, van Riel B, Graus YM, M'Rabet L. Dihomo-gamma-linolenic acid inhibits tumour necrosis factor-alpha production by human leucocytes independently of cyclooxygenase activity. Immunology. 2003;110:348–57. doi: 10.1046/j.1365-2567.2003.01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956–63. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 44.McCall TB, O'Leary D, Bloomfield J, O'Morain CA. Therapeutic potential of fish oil in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 1989;3:415–24. doi: 10.1111/j.1365-2036.1989.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 45.Salomon P, Kornbluth AA, Janowitz HD. Treatment of ulcerative colitis with fish oil n-3-omega-fatty acid: an open trial. J Clin Gastroenterol. 1990;12:157–61. doi: 10.1097/00004836-199004000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Lorenz R, Weber PC, Szimnau P, Heldwein W, Strasser T, Loeschke K. Supplementation with n-3 fatty acids from fish oil in chronic inflammatory bowel disease − a randomized, placebo-controlled, double-blind cross-over trial. J Intern Med. 1989;225:225–32. doi: 10.1111/j.1365-2796.1989.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 47.Hawthorne AB, Daneshmend TK, Hawkey CJ, et al. Treatment of ulcerative colitis with fish oil supplementation: a prospective 12 month randomised controlled trial. Gut. 1992;33:922–8. doi: 10.1136/gut.33.7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stenson WF, Cort D, Rodgers J, et al. Dietary supplementation with fish oil in ulcerative colitis. Ann Intern Med. 1992;116:609–14. doi: 10.7326/0003-4819-116-8-609. [DOI] [PubMed] [Google Scholar]
- 49.Aslan A, Triadafilopoulos G. Fish oil fatty acid supplementation in active ulcerative colitis: a double-blind, placebo-controlled, crossover study. Am J Gastroenterol. 1992;87:432–7. [PubMed] [Google Scholar]
- 50.Mate JCJ, Garcia-Samaniego JPJ. Does dietary fish oil maintain the remission of Crohn's Disease? Gastroenterology. 1993;100:A228. [Google Scholar]
- 51.Loeschke K, Ueberschaer B, Pietsch A, et al. n-3 fatty acids only delay early relapse of ulcerative colitis in remission. Dig Dis Sci. 1996;41:2087–94. doi: 10.1007/BF02093614. [DOI] [PubMed] [Google Scholar]
- 52.Lorenz-Meyer H, Bauer P, Nicolay C, et al. Study Group Members (German Crohn's Disease Study Group) Omega-3 fatty acids and low carbohydrate diet for maintenance of remission in Crohn's disease. A randomized controlled multicenter trial. Scand J Gastroenterol. 1996;31:778–85. doi: 10.3109/00365529609010352. [DOI] [PubMed] [Google Scholar]
- 53.Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn's disease. N Engl J Med. 1996;334:1557–60. doi: 10.1056/NEJM199606133342401. [DOI] [PubMed] [Google Scholar]
- 54.Dichi I, Frenhane P, Dichi JB, et al. Comparison of omega-3 fatty acids and sulfasalazine in ulcerative colitis. Nutrition. 2000;16:87–90. doi: 10.1016/s0899-9007(99)00231-2. [DOI] [PubMed] [Google Scholar]
- 55.Middleton SJ, Naylor S, Woolner J, Hunter JO. A double-blind, randomized, placebo-controlled trial of essential fatty acid supplementation in the maintenance of remission of ulcerative colitis. Aliment Pharmacol Ther. 2002;16:1131–5. doi: 10.1046/j.1365-2036.2002.01286.x. [DOI] [PubMed] [Google Scholar]
- 56.Barbosa DS, Cecchini R, El Kadri MZ, Rodriguez MA, Burini RC, Dichi I. Decreased oxidative stress in patients with ulcerative colitis supplemented with fish oil omega-3 fatty acids. Nutrition. 2003;19:837–42. doi: 10.1016/s0899-9007(03)00162-x. [DOI] [PubMed] [Google Scholar]
- 57.Bjorkkjaer T, Brunborg LA, Arslan G, et al. Reduced joint pain after short-term duodenal administration of seal oil in patients with inflammatory bowel disease: comparison with soy oil. Scand J Gastroenterol. 2004;39:1088–94. doi: 10.1080/00365520410009429. [DOI] [PubMed] [Google Scholar]
- 58.Magrum LJ, Johnston PV. Effect of culture in vitro with eicosatetraenoic (20 : 4 (n-6)) and eicosapentaenoic (20 : 5(n-3)) acids on fatty acid composition, prostaglandin synthesis and chemiluminescence of rat peritoneal macrophages. Biochim Biophys Acta. 1985;836:354–60. doi: 10.1016/0005-2760(85)90139-0. [DOI] [PubMed] [Google Scholar]
- 59.Calder PC, Yaqoob P, Harvey DJ, Watts A, Newsholme EA. Incorporation of fatty acids by concanavalin A-stimulated lymphocytes and the effect on fatty acid composition and membrane fluidity. Biochem J. 1994;300:509–18. doi: 10.1042/bj3000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee TH, Hoover RL, Williams JD, et al. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985;312:1217–24. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- 61.Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–71. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 62.Chapkin RS, Hubbard NE, Erickson KL. 5-series peptido-leukotriene synthesis in mouse peritoneal macrophages: modulation by dietary n-3 fatty acids. Biochem Biophys Res Commun. 1990;171:764–9. doi: 10.1016/0006-291x(90)91212-b. [DOI] [PubMed] [Google Scholar]
- 63.Hwang D. Essential fatty acids and immune response. FASEB J. 1989;3:2052–61. doi: 10.1096/fasebj.3.9.2501132. [DOI] [PubMed] [Google Scholar]
- 64.Goodwin JS, Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983;3:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- 65.Kunkel SL, Remick DG, Strieter RM, Larrick JW. Mechanisms that regulate the production and effects of tumor necrosis factor-alpha. Crit Rev Immunol. 1989;9:93–117. [PubMed] [Google Scholar]
- 66.Rola-Pleszczynski M, Gagnon L, Sirois P. Leukotriene B4 augments human natural cytotoxic cell activity. Biochem Biophys Res Commun. 1983;113:531–7. doi: 10.1016/0006-291x(83)91758-8. [DOI] [PubMed] [Google Scholar]
- 67.Chang KJ, Saito H, Tatsuno I, Tamura Y, Watanabe K, Yoshida S. Comparison of the effect of lipoxygenase metabolites of arachidonic acid and eicosapentaenoic acid on human natural killer cell cytotoxicity. Prostaglandins Leukot Essent Fatty Acids. 1989;38:87–90. doi: 10.1016/0952-3278(89)90090-2. [DOI] [PubMed] [Google Scholar]
- 68.Morelli AE, Thomson AW. Dendritic cells under the spell of prostaglandins. Trends Immunol. 2003;24:108–11. doi: 10.1016/s1471-4906(03)00023-1. [DOI] [PubMed] [Google Scholar]
- 69.Lund EK, Harvey LJ, Ladha S, Clark DC, Johnson IT. Effects of dietary fish oil supplementation on the phospholipid composition and fluidity of cell membranes from human volunteers. Ann Nutr Metab. 1999;43:290–300. doi: 10.1159/000012797. [DOI] [PubMed] [Google Scholar]
- 70.Hagve TA, Woldseth B, Brox J, Narce M, Poisson JP. Membrane fluidity and fatty acid metabolism in kidney cells from rats fed purified eicosapentaenoic acid or purified docosahexaenoic acid. Scand J Clin Laboratory Invest. 1998;58:187–94. doi: 10.1080/00365519850186571. [DOI] [PubMed] [Google Scholar]
- 71.Tappia PS, Ladha S, Clark DC, Grimble RF. The influence of membrane fluidity, TNF receptor binding, cAMP production and GTPase activity on macrophage cytokine production in rats fed a variety of fat diets. Mol Cell Biochem. 1997;166:135–43. doi: 10.1023/a:1006875010120. [DOI] [PubMed] [Google Scholar]
- 72.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–2. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 73.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–32. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 74.Stulnig TM, Huber J, Leitinger N, et al. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem. 2001;276:37335–40. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- 75.Diaz O, Berquand A, Dubois M, et al. The mechanism of docosahexaenoic acid-induced phospholipase D activation in human lymphocytes involves exclusion of the enzyme from lipid rafts. J Biol Chem. 2002;277:39368–78. doi: 10.1074/jbc.M202376200. [DOI] [PubMed] [Google Scholar]
- 76.Fan YY, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr. 2003;133:1913–20. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- 77.Speizer LA, Watson MJ, Brunton LL. Differential effects of omega-3 fish oils on protein kinase activities in vitro. Am J Physiol. 1991;261:E109–14. doi: 10.1152/ajpendo.1991.261.1.E109. [DOI] [PubMed] [Google Scholar]
- 78.May CL, Southworth AJ, Calder PC. Inhibition of lymphocyte protein kinase C by unsaturated fatty acids. Biochem Biophys Res Commun. 1993;195:823–8. doi: 10.1006/bbrc.1993.2119. [DOI] [PubMed] [Google Scholar]
- 79.Tappia PS, Man WJ, Grimble RF. Influence of unsaturated fatty acids on the production of tumour necrosis factor and interleukin-6 by rat peritoneal macrophages. Mol Cell Biochem. 1995;143:89–98. doi: 10.1007/BF01816941. [DOI] [PubMed] [Google Scholar]
- 80.Richieri GV, Kleinfeld AM. Free fatty acid perturbation of transmembrane signaling in cytotoxic T lymphocytes. J Immunol. 1989;143:2302–10. [PubMed] [Google Scholar]
- 81.Lee JY, Ye J, Gao Z, et al. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–51. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 82.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–9. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 83.Lee JY, Plakidas A, Lee WH, et al. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–86. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 84.Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- 85.Camandola S, Leonarduzzi G, Musso T, et al. Nuclear factor kB is activated by arachidonic acid but not by eicosapentaenoic acid. Biochem Biophys Res Commun. 1996;229:643–7. doi: 10.1006/bbrc.1996.1857. [DOI] [PubMed] [Google Scholar]
- 86.Forman BMCJE Rea. A novel conformational based assay to screen activators of PPAR for their ability to bind directly and to induce DNA binding shows that specific fatty acids, eicosanoids and hypolipidaemic drugs are ligands for PPAR-[alpha] or PPAR-[delta] Proc Natl Acad Sci USA. 2003;94:4312–7. [Google Scholar]
- 87.Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 88.Kliewer SA, Sundseth SS, Jones SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA. 1997;94:4318–23. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs). tissue distribution of PPAR-alpha-beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–66. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 90.Fajas L, Auboeuf D, Raspe E, et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272:18779–89. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 91.Su CG, Wen X, Bailey ST, et al. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999;104:383–9. doi: 10.1172/JCI7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bassaganya-Riera J, Hontecillas R, Beitz DC. Colonic anti-inflammatory mechanisms of conjugated linoleic acid. Clin Nutr. 2002;21:451–9. doi: 10.1054/clnu.2002.0594. [DOI] [PubMed] [Google Scholar]
- 93.Hughes DA, Pinder AC, Piper Z, Johnson IT, Lund EK. Fish oil supplementation inhibits the expression of major histocompatibility complex class II molecules and adhesion molecules on human monocytes. Am J Clin Nutr. 1996;63:267–72. doi: 10.1093/ajcn/63.2.267. [DOI] [PubMed] [Google Scholar]
- 94.Burke DA, Axon AT. Ulcerative colitis and Escherichia coli with adhesive properties. J Clin Pathol. 1987;40:782–6. doi: 10.1136/jcp.40.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Favier C, Neut C, Mizon C, Cortot A, Colombel JF, Mizon J. Fecal beta-D-galactosidase production and Bifidobacteria are decreased in Crohn's disease. Dig Dis Sci. 1997;42:817–22. doi: 10.1023/a:1018876400528. [DOI] [PubMed] [Google Scholar]
- 96.Ringo E, Bendiksen HR, Gausen SJ, Sundsfjord A, Olsen RE. The effect of dietary fatty acids on lactic acid bacteria associated with the epithelial mucosa and from faecalia of Arctic charr, Salvelinus alpinus (L.) J Appl Microbiol. 1998;85:855–64. doi: 10.1046/j.1365-2672.1998.00595.x. [DOI] [PubMed] [Google Scholar]
- 97.Kankaanpaa PE, Salminen SJ, Isolauri E, Lee YK. The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol Lett. 2001;194:149–53. doi: 10.1111/j.1574-6968.2001.tb09460.x. [DOI] [PubMed] [Google Scholar]
- 98.Bomba A, Nemcova R, Gancarcikova S, et al. The influence of omega-3 polyunsaturated fatty acids (omega-3 pufa) on lactobacilli adhesion to the intestinal mucosa and on immunity in gnotobiotic piglets. Berl Munch Tierarztl Wochenschr. 2003;116:312–6. [PubMed] [Google Scholar]
- 99.Hontecillas R, Wannemeulher MJ, Zimmerman DR, et al. Nutritional regulation of porcine bacterial-induced colitis by conjugated linoleic acid. J Nutr. 2002;132:2019–27. doi: 10.1093/jn/132.7.2019. [DOI] [PubMed] [Google Scholar]
- 100.Coakley M, Ross RP, Nordgren M, Fitzgerald G, Devery R, Stanton C. Conjugated linoleic acid biosynthesis by human-derived Bifido-bacterium species. J Appl Microbiol. 2003;94:138–45. doi: 10.1046/j.1365-2672.2003.01814.x. [DOI] [PubMed] [Google Scholar]
- 101.Kankaanpaa PE, Yang B, Kallio HP, Isolauri E, Salminen SJ. Influence of probiotic supplemented infant formula on composition of plasma lipids in atopic infants. J Nutr Biochem. 2002;13:364–9. doi: 10.1016/s0955-2863(02)00185-7. [DOI] [PubMed] [Google Scholar]