Abstract
Increased expression of CD44 variant isoforms have been shown on the inflammatory infiltrates in human and mouse colitis and blockade or deletion of CD44 isoforms inhibit experimental colitis. The objective of this study was to find out if short-term treatment of CD44 antibodies specific to CD44v7, but not to other variant isoforms, suppresses leucocyte–endothelial interaction in chronic dextran sodium sulphate (DSS)-induced colitis in mice. Chronic colitis was induced by oral administration of four cycles of 5% DSS in BALB/c mice. Expression of CD44 was investigated on isolated mononuclear cells of the gut immune system. In established colitis, mice were treated with antibodies against CD44v7 or CD44v4 three times in 7 days. Intravital microscopy was used to study leucocyte–endothelial interactions and leucocyte extravasation. As a marker of inflammatory infiltrates myeloperoxidase was quantified in gut tissue. CD44-induced apoptosis was determined by fluorescence staining of hypodiploidic cell nuclei. In chronic DSS-induced colitis both CD44 variant isoforms, v4 and v7 were significantly up-regulated on mononuclear cells. However, whereas anti-CD44v7 antibody treatment induced a marked restoration of the gut mucosa and significantly reduced endothelial sticking and extravasation of circulating leucocyte in vivo (P < 0·01), application of anti-CD44v4 or an isotype control antibody had no anti-inflammatory effect. A significant reduction of myeloperoxidase activity was detected after blockade of CD44v7, but not v4. Short-term treatment with anti-CD44v7 antibody blocks T cell extravasation and recruitment to the intestinal mucosa and cures established experimental colitis.
Keywords: CD44 variant isoforms, CD44 variant therapy, chronic inflammatory bowel disease
Introduction
Recruitment of leucocytes and lymphocytes from the vessels to the gut mucosa is a multi-step process that leads to the accumulation of cells in the inflamed tissue and is most probably a pivotal step in the initiation and perpetuation of chronic inflammatory bowel disease [1]. The inflammatory process is further promoted by proinflammatory mediators such as tumour necrosis factor-α, NF-κΑ or endotoxin and by the up-regulation of adhesion molecule expression on lymphocytes and local endothelium [2,3]. One of the lymphocyte activation markers which has been suggested to function as a cell adhesion molecule is the transmembrane glycoprotein CD44 [4]. CD44 is expressed by most cell types, including leucocytes, and it is the major cell surface receptor for hyaluronan (HA), which plays a unique role in the maintenance of tissue integrity [5]. At inflammatory sites, CD44 has the ability to recruit leucocytes to vascular endothelium, which is one of the first steps in the inflammatory response. Neutrophils express the standard CD44 isoform. In addition, CD44 functions as a co-stimulatory molecule in T cell activation as a result of its constitutive association with the lck signalling cascade and its association with the T cell receptor upon stimulation and formation of the immunological synapse [6]. In addition to T cells, stimulation through CD44 enhances macrophage production of proinflammatory cytokines [7–9]. Besides the standard form of CD44, CD44 has 10 different splice variants, developed as a result of alternative splicing of variant exons. It has been suggested that expression of specific isoforms may play a role in the regulation of the immune response as well as in the development of autoimmune disorders [10–13]. CD44 molecules containing splice variants v4–7 are aberrantly expressed in many human tumours and have been linked to the metastatic spread of tumour cells [12,14,15]. These data suggest that CD44 variant isoforms might be involved in cell adhesion at inflammatory sites. Furthermore, in experimental colitis expression of the isoform CD44v7, which is rarely expressed in resting immune cells, is increased on mononuclear cells of intestinal inflammatory lesions predominantly in Th-1-polarized inflammation[16,17]. Based on these findings we have shown that blockade or deletion of CD44v7 protects mice from severe intestinal inflammation in trinitro-benzene sulphonic acid (TNBS)-induced experimental colitis, as a model of T cell-dependent acute colitis [18].
Another well-characterized model uses dextran sodium sulphate (DSS) to induce colonic inflammation. DSS induces acute or chronic colitis in BALB/c mice depending on the administration protocol [19]. Whereas acute DSS-colitis predominantly shows toxic effects of DSS on the epithelium and seems to be T cell-independent because it is observed in T cell-deficient SCID mice [18,20], the chronic phase of DSS-colitis reflects a prolonged inflammatory immune response of macrophages and T cells comparable to Crohn's disease. Histologically, the chronic phase is characterized by a mononuclear cell infiltrate, with lymphoid hyperplasia, focal crypt damage and few scattered ulcerations in the epithelial layer [21]. In DSS-induced colitis an increase in mucosal myeloperoxidase activity, as well as increased concentrations of macrophage-derived cytokines, are found [18,21–23]. In the current study we examined whether specifically CD44v7 or arbitrary CD44 variant isoforms play a role in the maintenance of intestinal inflammation in chronic experimental colitis. Furthermore, the effects of neutralization using anti-CD44v4 and v7-antibodies on histological inflammation were examined, as well as leucocyte–endothelium interactions in colonic post-capillary and collecting venules by intravital microscopy.
Materials and methods
Animals
Female BALB/c mice (Charles River; Sulzfeld, Germany) weighing 18–20 g were used for experiments. Animals were housed in a room maintained at 22°C and kept on standard laboratory pellet food (150 mg/kg Vit E, H1003, Alma, Kempten, Germany). All experiments were performed in accordance with the German legislation on the protection of animals.
Induction of DSS-colitis and experimental design
Established protocols were used for induction of DSS-induced chronic colitis [23]. To establish chronic colitis, mice were fed 5% DSS (mol. wt 40 000, ICN, Eschwege, Germany) dissolved in sterile, distilled drinking water ad libitum for 7 days, followed by normal drinking water for 10 days; this treatment cycle was repeated four successive times. The drinking amount per mouse per day was evaluated and found to be equal in each DSS-fed group. Control mice were fed tap water without DSS. Two weeks after the last DSS feeding, mice (n = 6/group) were treated three times over a 7-day period with anti-CD44v7 (clone LN7·1, mouse-IgG1; 40 µg/mouse, intraperitoneally [17]), anti-CD44v4 antibody (clone: 1OD1, rat IgG1, Serotec, Düsseldorf, Germany) or an isotype control (clone W3/25; mouse-IgG1; Serotec, Düsseldorf, Germany). In vivo microscopy was performed 7 days after the last antibody injection. After in vivo microscopy tissue was collected for histology and measurement of myeloperoxidase activity.
Microsurgical technique and in vivo microscopy
After premedication with atropine [0·1 mg/kg body weight subcutaneously (s.c.)], animals were anaesthetized with a constant flow of oxygen (33%), isoflurane (0·4 volume %) and nitrous oxide. Animals were placed in a supine position on a heating pad for maintenance of the body temperature between 36°C and 37°C, as measured via a rectal thermometer. The left carotid artery and jugular vein were cannulated for continuous recording of mean arterial pressure (MAP), for heart rate measurement, for injection of fluorescent dyes (in vivo microscopy) and for substitution of volume loss [40 ml/h/kg Ringer's lactate intravenously (i.v.)]. After transverse laparotomy, the descending colon was mobilized.
In vivo microscopy was performed as described previously [3]. Briefly, the mobilized left colon segment was exteriorized on a specially designed mechanical stage. The stage was placed on a computer-controlled microscope platform, allowing for repeated scanning of the same microvessels during the experiment. Throughout the experiment the tissue was kept moist with 37°C Ringer's lactate solution. We used a technical microscopic setup, as described by Harris et al. [24], including a Zeiss microscope (axiotech vario 100 HD, 20× objective). All images were recorded by a video camera (HG16 PCO, Kelheim, Germany) attached to the microscope. Quantitative off-line analysis was blinded using a custom-designed computer-assisted analysing system.
The microcirculation of the submucosa was visualized for determination of leucocyte endothelium interaction in 10 randomly selected post-capillaries and collecting venules (magnification: 600×). Leucocytes were stained in vivo with an isotonic 0·02% acridine orange (Sigma Chemical, St Louis, MO, USA) solution; the solution was injected intravenously at a concentration of 0·1 mg/kg/min. Leucocytes were subsequently classified as adherent or non-adherent cells with regard to their interaction with the vascular endothelial lining. In each vessel segment visualized, leucocytes were classified as adherent when no movement or detachment was observed for >30 s. Results are given as number of adherent or non-adherent cells per mm2 endothelial surface. To analyse lymphocyte extravasation, a longitudinal incision (approximately 20 mm) along the anti-mesenteric border was performed by microcautery to access the intestinal mucosa. Extravasated leucocytes in the mucosa were quantified by counting the acridine-orange-labelled leucocytes lying close to the mucosal vessels. Results were calculated as leucocytes/mm2 mucosal surface. For all in vivo microscopy experiments, the analysis was performed 20–70 min following laparotomy. At the end of the experiment, animals were killed and tissues were collected.
Histology
Standard haematoxylin and eosin (H&E) staining was performed on colon tissue to assess the degree of inflammation. The scoring was performed by a blinded observer, as described previously [23]. Briefly, a score of 0–8 (8 being the most severe) was assigned for epithelial loss and inflammatory infiltration. Mice were scored individually, with each value representing the mean score of three sections of the distal third of the colon.
Myeloperoxidase activity
Colonic myeloperoxidase (MPO) activity was determined as described previously [25]. Briefly, colonic tissue was homogenized in 1 ml of 50 mmol/l potassium phosphate buffer (pH 6·0) containing 0·5% (wt/vol) hexadecyltrimethylammonium hydroxide and centrifuged at 120 g at 4°C for 20 min; 10 µl of the supernatant was transferred into phosphate buffer (pH 6·0) containing 0·17 mg/ml 3,3′-dimethoxybenzidine and 0·0005% H2O2. MPO activity of the supernatant was determined by measuring the H2O2-dependent oxidation of 3,3′-dimethoxybenzidine and expressed as units per gram of total protein. Total protein content of the samples was analysed using a bicinchoninic acid protein assay kit (Sigma).
Flow cytometric analysis of CD44 expression
Single cell suspensions from mesenteric lymph nodes were made and washed once in phosphate buffered saline (PBS) (Cambrex, Verviers, Belgium) containing 3% fetal calf serum (FCS). The cells were first incubated with mouse Fc-block (clone: 2·4G2, BD Biosciences, Heidelberg, Germany) for 5 min at 4°C to minimize unspecific binding and then labelled with purified antibodies against mouse CD44v7 (polyclonal, rabbit IgG, Chemicon, Chandlers Ford, UK) or CD44v4 (clone: 1OD1, rat IgG1, Serotec, Düsseldorf, Germany) for 30 min at 4°C. Cells were washed twice in PBS/3% FCS and then labelled with anti-mouse CD3-PE (clone: 17A2, rat IgG2b, BD, Heidelberg, Germany) and goat anti-rabbit-Ig-FITC or mouse anti-rat-IgG1-FITC, respectively (both from BD Biosciences). Cells were washed twice in PBS/3%FCS and then analysed using a FACSCalibur (BD Biosciences). The following isotype controls were used: rat IgG2b-PE (BD Biosciences), purified rabbit-IgG (Jackson Immuno Research, West Grove, PA, USA) and purified rat-IgG1 (Caltag, Hamburg, Germany). Data were analysed using WinMDI software version 2·8.
Statistical analysis
In vivo microscopy data, myeloperoxidase activity and histology results are presented as the median values, including the range (as box-plots). Statistical analyses were performed using the Kruskall–Wallis statistic for non-parametric tests. Differences were considered significant at P < 0·05.
Results
Expression of CD44 variant isoforms on gut-associated lymphoid tissue in chronic colitis
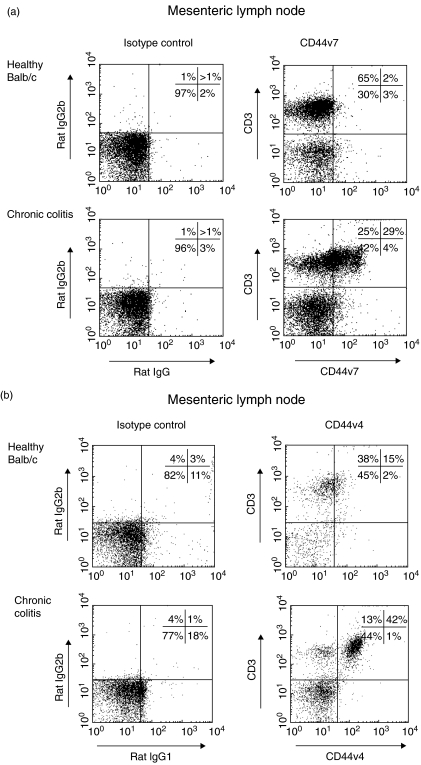
Expression of CD44v4 and CD44v7 was analysed in mesenteric lymph nodes of healthy mice and tissue from mice with chronic colitis via flow cytometric analysis (Fig. 1a,b). Both variant isoforms showed increased expression in mice with chronic colitis, compared to healthy controls. Our results demonstrate that both variant isoforms are up-regulated on T cells of mice with chronic colitis under inflammatory conditions, indicating a role in T cell activation.
Fig. 1.
Representative flow cytometric analysis of CD44 variant isoform 4 (CD44v4) (b) and CD44v7 (a) expression on CD4+ T cells in the mesenteric lymph nodes of mice with chronic colitis. A marked increase in CD44v4 and CD44v7 expression is demonstrated in mice with chronic colitis, compared to T cells in healthy mice.
Anti-CD44v7 antibody but not anti-CD44v4 inhibits leucocyte–endothelial cell interactions in vivo
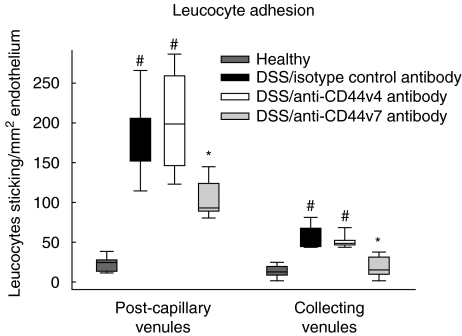
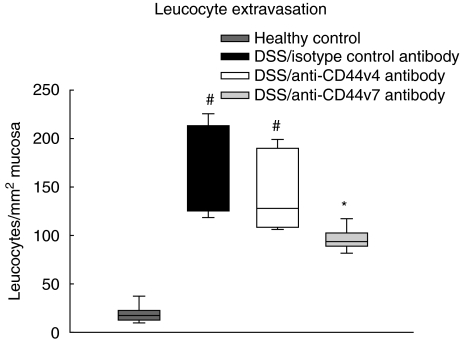
In the next step, leucocyte adhesion and extravasation as a marker of tissue inflammation was examined by intravital microscopy. In DSS-treated mice leucocyte sticking was augmented fivefold in the collecting venules (Fig. 2) and 10-fold in the post-capillary venules compared to healthy controls. To test the role of CD44v7 in the adherence and extravasation processes, in vivo microscopic analysis was performed in mice with chronic colitis after treatment with anti-CD44v7-specific monoclonal antibody. In our study, CD44v7 antibody treatment decreased leucocyte adherence in collecting venules (Fig. 2) and in post-capillary venules by approximately 50%, compared to mice treated with control antibody (P < 0·01). Surprisingly, mice treated with anti-CD44v4 antibody showed unmodified markedly leucocyte adhesion to the endothelium, and subsequent migration into the mucosa comparable to isotype control. We then assessed the effect of CD44v4 and CD44v7 on the extent of leucocyte extravasation into the inflamed colonic tissue. After four cycles of DSS treatment, the number of extravasating leucocytes was increased by 10-fold in diseased mice treated with isotype control antibody, compared to healthy mice (Fig. 3). Again, anti-CD44v7 treatment of DSS-colitis reduced leucocyte extravasation by half in contrast to the anti-CD44v4 antibody-treated group (P > 0·01). Taken together, these results demonstrate a specific role for CD44v7, but maintenance of chronic inflammation for CD44v4, as shown by reduced sticking and extravasation of leucocytes.
Fig. 2.
Four weeks after the last dextran sodium sulphate (DSS) application, in vivo microscopy was performed to investigate leucocyte adhesion in submucosal vessels of the colon. Leucocyte sticking is increased in chronic DSS-induced colitis in submucosal collecting and post-capillary venules compared to healthy control animals (n = 6/group; median and range, #P < 0·01 versus healthy control). Treatment with anti-CD44 variant isoform 7 (CD44v7) antibody but not with anti-CD44v4 antibody significantly down-regulates leucocyte adhesion in collecting and post-capillary venules (*P < 0·01 versus isotype antibody and anti-CD44v4 antibody).
Fig. 3.
Leucocyte extravasation is still markedly increased 4 weeks after the last cycle of dextran sodium sulphate (DSS) in chronic colitis compared to control animals (n = 6/group; median and range, #P < 0·01 versus healthy control). Application of anti-CD44 variant isoform 7 (CD44v7) antibody significantly down-regulates leucocyte extravasation compared to animals which were treated with an isotype-matched antibody or anti-CD44v4 antibody (*P < 0·01 versus isotype antibody and anti-CD44v4 antibody).
Short-term treatment with anti-CD44v7 antibody abrogates an established chronic DSS-induced colitis
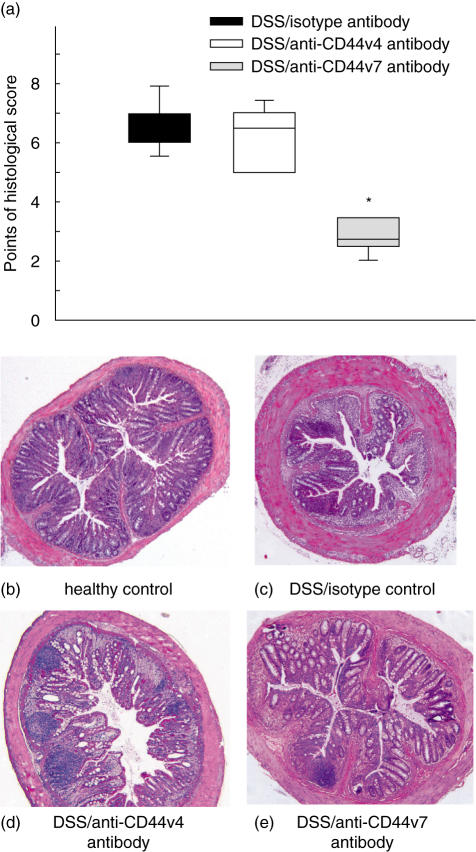
After studying the role of CD44v7 for extravasation and adhesion in chronic DSS-induced colitis, we tested whether the treatment with anti-CD44v7 antibody actually affects the sustained disease. Indeed, treatment of DSS-induced mice with anti-CD44v7 antibody led to a significantly improved histological score (Fig. 4a). More specifically, anti-CD44v7 but not anti-CD44v4 antibody treatment diminished the loss of crypts and reduced the inflammatory infiltrate, compared to the histology of healthy control mice and DSS mice treated with isotype control antibody (Fig. 4b,c). Also, leucocyte infiltration was significantly reduced in mice treated with anti-CD44v7 antibody compared to mice treated with anti-CD44v4 antibody (Fig. 4d,e). These results suggest that CD44v7 expression does influence the development of colitis in mice.
Fig. 4.
For assessment of inflammation histologically, mice were killed 4 weeks after the last dextran sodium sulphate (DSS) cycle. A score of 0–8 (8 being the most severe) was used for each animal by a blinded observer as described by Obermeier et al. [23] (median and range *P < 0·05, versus anti-CD44 variant isoform 4 (CD44v4) antibody and isotype antibody) (a). Representative histological specimens (magnification 5×) of the colon from healthy mice (b) and from mice receiving DSS only (c) are shown. Treatment with anti-CD44v4 antibody of mice with established DSS-colitis (d) does not improve histological changes in contrast to anti-CD44v7 therapy (e), which leads to a decrease of epithelial damage and inflammatory infiltration.
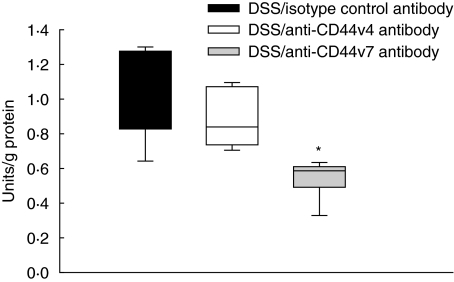
Anti-CD44v7 antibody down-regulates myeloperoxidase activity
The in vivo and histological findings were paralleled by the MPO, which is a marker of neutrophil infiltration. MPO activity in the colon of mice with chronic colitis was significantly decreased after antibody treatment with anti-CD44v7 antibody (Fig. 5), compared to mice treated with antibody against CD44v4 or isotype control. This indicates decreased leucocyte infiltration into the colon after anti-CD44v7 therapy and strongly supports the intravital microscopic and histological results.
Fig. 5.
Myeloperoxidase (MPO) activity in the tissue was significantly down-regulated after blocking with CD44 variant isoform 7 (CD44v7) antibody versus mice treated with anti-CD44v4 antibody (n = 6/group; median and range; *P < 0·01 versus healthy controls and anti-CD44v4 antibody).
Discussion
The aetiological agents causing chronic inflammatory bowel disease have not yet been identified, but the result at chronically inflamed sites is persistent infiltration of mononuclear effector cells, among which T cells play a prominent role. Activated T cells serve as trigger for a cascade of events that lead to amplification of the inflammatory process inducing tissue damage at target sites [26]. The goal of the present study was to investigate the role of CD44 variant isoforms in lymphocyte recruitment and intestinal inflammation in the chronic DSS-model. It is demonstrated clearly that lymphocytes within the mucosal infiltrates strongly up-regulate CD44 variant isoforms, CD44v4, CD44v6 and CD44v7 compared to normal gut-associated lymphoid tissue [17]. However, only blockade of CD44v7 but not of CD44v4 resulted in a strong reduction of chronic inflammation in experimental colitis.
Effector or memory lymphocyte populations demonstrate increased expression of the adhesion molecule CD44 standard. Leucocyte transmigration into inflamed tissue is one of the major functions of the CD44 standard molecule on lymphocytes [27]. CD44 may modulate immunological and inflammatory responses through at least two mechanisms. First, CD44 standard binding to its primary ligand, the glycosaminoglycan hyaluronan, mediates interactions between lymphocytes and endothelial cells. These interactions initiate lymphocyte contact and primary adhesion, or rolling, on endothelial cells under conditions of physiological laminar flow [28,29]. As well as attachment of lymphocytes, adhesion molecules are also involved in local lymphocyte stimulation and antigen presentation within the intestinal mucosa [30]. Recent data suggest that in addition to CD44 standard, CD44v7 associates with the cytoskeletal linker proteins ankyrin and the ezrin, radixin, moesin (ERM) family during lymphocyte activation [31]. However, knowledge of ligands and signal transduction pathways mediated by CD44 variant isoforms is still sparse. Our previous studies on CD44v7 in experimental colitis show that this splice variant is substantially involved in intestinal lymphocyte activation. We have shown that CD44v7 expression is increased in inflammatory infiltrates of Th1-cytokine-mediated experimental colitis and lamina propria mononuclear cells of patients with Crohn's disease compared to healthy controls [32,33]. Furthermore, deletion of CD44v7 induced apoptosis in lamina propria mononuclear cells of inflamed but not normal mucosa in experimental colitis [17]. However, in vitro data suggest that apoptosis of T helper cells is also induced by targeting dendritic cells with a monoclonal anti-CD44v4 antibody [34]. It has been shown recently by our group that the anti-CD44v4 antibody (rat IgG1 anti-mouse CD44v4, clone 10D1) used in our study is functionally active in vitro (unpublished data). Based on the high CD44v4-expression in inflamed mucosa it was meaningful to test whether this antibody ameliorates chronic colitis in vivo.
An intriguing question resulting from our data is how anti-CD44v7 but not anti-CD44v4 antibody treatment inhibits leucocyte migration to inflammatory sites. At least two theories could explain this effect. First, as shown previously [35,36], induction of the regulatory cytokine IL-10 by CD44v7 blockade down-regulates proinflammatory cytokines and chemotactic signals in the mucosa that attract circulating immune cells to adhere and transmigrate the endothelial layer at inflammatory sites. Secondly, interaction of CD44v7 with the cytokine-like molecule osteopontin has been implicated in maintaining the integrity of inflamed tissues. Both osteopontin and CD44 variant expression is associated with a Th-1 type T cell activation, and these osteopontin-mediated events are likely to contribute to the function of CD44 variant v7 during chronic inflammation [37]. Therefore, the loss of the CD44–osteopontin interaction could account for the anti-inflammatory effect of CD44v7-blockade or deletion in experimental colitis. There are two important implications. The first is that CD44v6 and CD44v7, but not CD44v4, have been shown to bind to osteopontin. Furthermore, recent data demonstrate that CD44-mediated osteopontin binding leads to enhanced cell motility and chemotactic behaviour [38]. Thus, osteopontin binding via CD44 variants, that has been implicated in Th-1 mediated inflammation and cell migration, would directly promote cell adhesion by increasing cell motility. These data have significant ramifications for the interpretation of why and how osteopontin and CD44v7 might be associated with lymphocyte adhesion. It is obvious that both CD44 variant isoforms, CD44v4 and CD44v7 exerting similar expression patterns, fulfil different functions in chronic colitis. Whereas CD44v7 is involved in lymphocyte activation and adhesion, the function of CD44v4 is still unknown. Our data demonstrate clearly that short-term treatment with anti-CD44v7 antibody is particularly effective in eliminating pathogenic pre-activated cells in chronic intestinal inflammation.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (AN334/1–1) to M. Anthuber and S. Farkas, by the BMBF (Kompetenznetz CED) to S. Farkas and BM Wittig and by the Broad Medical Research Program (BM Wittig). The authors thank Florian Obermeier for scoring the histological slices and Karoline Edtinger for excellent technical assistance.
References
- 1.Rosemblatt M, Bono MR. Functional consequences of immune cell adhesion to endothelial cells. Curr Pharm Des. 2004;10:109–20. doi: 10.2174/1381612043453487. [DOI] [PubMed] [Google Scholar]
- 2.Spiik AK, Ridderstad A, Axelsson LG, Midtvedt T, Bjork L, Pettersson S. Abrogated lymphocyte infiltration and lowered CD14 in dextran sulfate induced colitis in mice treated with p65 antisense oligonucleotides. Int J Colorectal Dis. 2002;17:223–32. doi: 10.1007/s00384-001-0366-3. [DOI] [PubMed] [Google Scholar]
- 3.Farkas S, Herfarth H, Rossle M, et al. Quantification of mucosal leucocyte endothelial cell interaction by in vivo fluorescence microscopy in experimental colitis in mice. Clin Exp Immunol. 2001;126:250–8. doi: 10.1046/j.1365-2249.2001.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jalkanen S, Jalkanen M, Bargatze R, Tammi M, Butcher EC. Biochemical properties of glycoproteins involved in lymphocyte recognition of high endothelial venules in man. J Immunol. 1988;141:1615–23. [PubMed] [Google Scholar]
- 5.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 6.Foger N, Marhaba R, Zoller M. CD44 supports T cell proliferation and apoptosis by apposition of protein kinases. Eur J Immunol. 2000;30:2888–99. doi: 10.1002/1521-4141(200010)30:10<2888::AID-IMMU2888>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Levesque MC, Haynes BF. Activated T lymphocytes regulate hyaluronan binding to monocyte CD44 via production of IL-2 and IFN-gamma. J Immunol. 2001;166:188–96. doi: 10.4049/jimmunol.166.1.188. [DOI] [PubMed] [Google Scholar]
- 8.Levesque MC, Haynes BF. TNFalpha and IL-4 regulation of hyaluronan binding to monocyte CD44 involves posttranslational modification of CD44. Cell Immunol. 1999;193:209–18. doi: 10.1006/cimm.1999.1456. [DOI] [PubMed] [Google Scholar]
- 9.Hodge-Dufour J, Noble PW, Horton MR, et al. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol. 1997;159:2492–500. [PubMed] [Google Scholar]
- 10.Konig H, Ponta H, Herrlich P. Coupling of signal transduction to alternative pre-mRNA splicing by a composite splice regulator. EMBO J. 1998;17:2904–13. doi: 10.1093/emboj/17.10.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arch R, Wirth K, Hofmann M, et al. Participation in normal immune responses of a metastasis-inducing splice variant of CD44. Science. 1992;257:682–5. doi: 10.1126/science.1496383. [DOI] [PubMed] [Google Scholar]
- 12.Gunthert U, Hofmann M, Rudy W, et al. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 13.Gunthert U, Schwarzler C, Wittig B, et al. Functional involvement of CD44, a family of cell adhesion molecules, in immune responses, tumour progression and haematopoiesis. Adv Exp Med. 1998;451:43–9. doi: 10.1007/978-1-4615-5357-1_7. [DOI] [PubMed] [Google Scholar]
- 14.Barbour AP, Reeder JA, Walsh MD, Fawcett J, Antalis TM, Gotley DC. Expression of the CD44v2–10 isoform confers a metastatic phenotype: importance of the heparan sulfate attachment site CD44v3. Cancer Res. 2003;63:887–92. [PubMed] [Google Scholar]
- 15.Seiter S, Arch R, Reber S, et al. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. 1993;177:443–55. doi: 10.1084/jem.177.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wittig B, Seiter S, Foger N, Schwarzler C, Gunthert U, Zoller M. Functional activity of murine CD44 variant isoforms in allergic and delayed type hypersensitivity. Immunol Lett. 1997;57:217–23. doi: 10.1016/s0165-2478(97)00060-6. [DOI] [PubMed] [Google Scholar]
- 17.Wittig BM, Johansson B, Zoller M, Schwarzler C, Gunthert U. Abrogation of experimental colitis correlates with increased apoptosis in mice deficient for CD44 variant exon 7 (CD44v7) J Exp Med. 2000;191:2053–64. doi: 10.1084/jem.191.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Axelsson LG, Landstrom E, Goldschmidt TJ, Gronberg A, Bylund-Fellenius AC. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice. effects in CD4(+)-cell depleted, athymic and NK-cell depleted SCID mice. Inflamm Res. 1996;45:181–91. doi: 10.1007/BF02285159. [DOI] [PubMed] [Google Scholar]
- 19.Kojouharoff G, Hans W, Obermeier F, et al. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107:353–8. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–52. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 21.Obermeier F, Kojouharoff G, Hans W, Scholmerich J, Gross V, Falk W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238–45. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamamoto N, Maemura K, Hirata I, Murano M, Sasaki S, Katsu K. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1) Clin Exp Immunol. 1999;117:462–8. doi: 10.1046/j.1365-2249.1999.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obermeier F, Dunger N, Strauch UG, et al. Contrasting activity of cytosinguanosin dinucleotide oligonucleotides in mice with experimental colitis. Clin Exp Immunol. 2003;134:217–24. doi: 10.1046/j.1365-2249.2003.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris AG, Hecht R, Peer F, Nolte D, Messmer K. An improved intravital microscopy system. Int J Microcirc Clin Exp. 1997;17:322–7. doi: 10.1159/000179247. [DOI] [PubMed] [Google Scholar]
- 25.Herfarth H, Brand K, Rath HC, Rogler G, Scholmerich J, Falk W. Nuclear factor-kappa B activity and intestinal inflammation in dextran sulphate sodium (DSS)-induced colitis in mice is suppressed by gliotoxin. Clin Exp Immunol. 2000;120:59–65. doi: 10.1046/j.1365-2249.2000.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackay CR. T-cell memory: the connection between function, phenotype and migration pathways. Immunol Today. 1991;12:189–92. doi: 10.1016/0167-5699(91)90051-T. [DOI] [PubMed] [Google Scholar]
- 27.Siegelman MH, Stanescu D, Estess P. The CD44-initiated pathway of T-cell extravasation uses VLA-4 but not LFA-1 for firm adhesion. J Clin Invest. 2000;105:683–91. doi: 10.1172/JCI8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeGrendele HC, Estess P, Picker LJ, Siegelman MH. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: a novel lymphocyte–endothelial cell primary adhesion pathway. J Exp Med. 1996;183:1119–30. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–5. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- 30.Von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–78. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 31.Marhaba R, Bourouba M, Zoller M. CD44v7 interferes with activation-induced cell death by up-regulation of anti-apoptotic gene expression. J Leukoc Biol. 2003;74:135–48. doi: 10.1189/jlb.1202615. [DOI] [PubMed] [Google Scholar]
- 32.Wittig B, Seiter S, Schmidt DS, Zuber M, Neurath M, Zoller M. CD44 variant isoforms on blood leukocytes in chronic inflammatory bowel disease and other systemic autoimmune diseases. Lab Invest. 1999;79:747–59. [PubMed] [Google Scholar]
- 33.Wittig BM, Zeitz M. The gut as an organ of immunology. Int J Colorectal Dis. 2003;18:181–7. doi: 10.1007/s00384-002-0444-1. [DOI] [PubMed] [Google Scholar]
- 34.Termeer C, Averbeck M, Hara H, et al. Targeting dendritic cells with CD44 monoclonal antibodies selectively inhibits the proliferation of naive CD4+ T-helper cells by induction of FAS-independent T-cell apoptosis. Immunology. 2003;109:32–40. doi: 10.1046/j.1365-2567.2003.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wittig B, Schwarzler C, Fohr N, Gunthert U, Zoller M. Curative treatment of an experimentally induced colitis by a CD44 variant V7-specific antibody. J Immunol. 1998;161:1069–73. [PubMed] [Google Scholar]
- 36.Seiter S, Schmidt DS, Zoller M. The CD44 variant isoforms CD44v6 and CD44v7 are expressed by distinct leukocyte subpopulations and exert non-overlapping functional activities. Int Immunol. 2000;12:37–49. doi: 10.1093/intimm/12.1.37. [DOI] [PubMed] [Google Scholar]
- 37.Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults. regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–61. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katagiri YU, Sleeman J, Fujii H, et al. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine–glycine–aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59:219–26. [PubMed] [Google Scholar]