Abstract
We have demonstrated recently that CCL20 was expressed in periodontal diseased tissues and abundant CCR6 positive T cells infiltrated in periodontally diseased tissue. However, it is uncertain which cells can elicit CCL20 production. In the present study, we examined the properties of CCL20 production by human gingival fibroblasts (HGF) culture. Here, we report that interleukin-1 beta (IL-1β), tumour necrosis factor-alpha (TNF-α) and Escherichia coli lipopolysaccharide (LPS) can significantly induce the production of CCL20 by HGF. We found that TNF-α and E. coli LPS enhanced the production of CCL20 by HGF treated with IL-1β. In contrast, interferon-gamma (IFN-γ) dramatically diminished CCL20 production induced by IL-1β. Moreover, we demonstrated that nuclear factor-kappaB (NF-κB), p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinases (ERK) play an important role in mediating the production of CCL20 induced by IL-1β and TNF-α. On the other hand, we found that not only NF-κB, p38 MAPK and ERK but also c-Jun NH2-terminal kinase (JNK) are involved in CCL20 production induced by E. coli LPS. Finally, we found that HGF express CCR6, CCL20 receptor, and CCL20 induced vascular endothelial growth factor (VEGF) by HGF. Taken together, these findings that HGF will be a source of CCL20 in periodontal tissue, and the CCL20 production will be controlled by proinflammatory cytokine and bacterial LPS in periodontally diseased tissue. Thus, CCL20 by HGF might be involved in inflammatory cells infiltration, and promote the progression of periodontal disease.
Keywords: CCL20, fibroblast, periodontal disease
Introduction
Periodontal disease is characterized as chronic inflammation associated with Gram-negative bacteria in the oral cavity [1,2], resulting in soft tissue destruction and periodontal bone resorption. Although host-immune response to these bacteria has been suggested to be associated with alteration or even progress of this disease [3], the mechanism of leucocyte infiltration in periodontally diseased tissues is still unknown.
It has been reported that some chemokines that are involved in leucocyte migration are expressed in periodontally diseased tissues. Constitutive expression of monocyte chemotactic protein-1 (MCP-1) and interleukin-8 (IL-8), along with lesser expression of growth-related gene product gamma (GRO-γ), macrophage inflammatory protein-1 alpha (MIP-1α) and macrophage inflammatory protein-1 beta (MIP-1β) were measured by reverse transcription-polymerase chain reaction (RT-PCR) in healthy gingivae [4]. Up-regulated production of MCP-1 and MIP-1α in diseased tissue was observed to correlate with the degree of inflammation [5,6]. Furthermore, we have reported recently that CCL20 and its receptor, CCR6, positive T cells infiltrated into periodontally diseased tissues [7]. However, it is uncertain which cells can elicit CCL20 production in periodontally diseased tissues.
CCL20 is expected to play a crucial role in trafficking and homing of not only memory T cells but also some kinds of leucocytes, including immature dendritic cells, into inflammatory sites such as atopic dermatitis [8], hepatitis [9], arthritis [10,11] and periodontal disease [7]. CCL20 exerts its activity through binding to CCR6 [12–16], which is not shared by any other known chemokine, but nevertheless binds a member of the structurally unrelated β-defensins [17]. CCR6 is found to be expressed on immature dendritic cells and memory T lymphocytes as well as on B lymphocytes in various lymphoid organs, and in pancreas [12–15,18,19]. Up-regulation of CCR6 expression on human neutrophils by cytokines can explain the slight chemotactic response of these cells to CCL20 [20].
Fibroblasts were previously considered important connective tissue cells that construct a supporting framework crucial for tissue integrity and repair. Recently, fibroblasts have been suggested to be important sentinel cells in immune systems [21]. Fibroblasts actively define the structure of tissue microenvironments and regulate inflammatory response by the production of cytokines such as IL-1β and IL-8 [22]. However, there is still uncertainty about CCL20 production.
In the present study, we focused on human gingival fibroblasts (HGF). We examined the production of CCL20 by HGF stimulated with proinflammatory cytokines, lipopolysaccharide (LPS) and lipoteichoic acid (LTA). Moreover, we examined the signalling pathways involved in CCL20 production by HGF. Furthermore, we investigated the role of CCL20 on HGF, especially vascular endothelial growth factor (VEGF) production.
Materials and methods
Cells and culture condition
HGF was prepared from the explants of clinically normal gingiva from patients (three females, aged 26–40 years old) during routine distal wedge surgical procedure, with informed consent. The patients had no periodontal disease, and they were systemically healthy with no evidence of known systemic modifiers of periodontal disease (types 1 and 2 diabetes mellitus, osteoporosis and medications known to influence periodontal tissues). Exclusion criteria included those patients who had taken systemic antibiotic, anti-inflammatory, hormonal or other assisted drug therapies in the 6 months prior to the study. Explants were cut into pieces and culture in 100-mm diameter tissue cultured dishes in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; JRH Bioscience, Lenexa, KA, USA), penicillin 50 IU/ml and streptomycin 50 µg/ml with a medium change every 3 days for 10–15 days until confluent cell monolayers were formed. The cells were detached with 0·25% trypsin-ethylenediamine tetraacetic acid (EDTA), washed with phosphate buffered saline (PBS) and subcultured in plastic flasks. After three to four subcultures by trypsinization, homogeneous, slim spindle-shaped cells grown in characteristic swirls were obtained. The cells were used as confluent monolayers at subculture levels 5–15. HGF were stimulated with IL-1β (0·1–100 ng/ml; Peprotech, Rocky Hill, NJ, USA), TNF-α (0·1–100 ng/ml; Peprotech), IFN-γ (0·1–100 ng/ml; Peprotech), Esherichia coli LPS (0·1–100 µg/ml; serotype 026:B6, purchased from Sigma, St Louis, MO, USA), Porphyromonas gingivalis (Pg) w83 LPS (1–100 µg/ml), Staphylococcus aureus (Sa) LTA (1–100 µg/ml; Sigma) and Streptococcus mutans (Sm) LTA (1–100 µg/ml; Sigma) for the periods indicated in the figure legends. At a predetermined time, cell-free supernatants were harvested and stored at −80°C for cytokine determination. In selected experiments, HGF were cultured for 1 h in the presence or in the absence of SB203580 (0·2–20 µM; Santacruz, Santa Cruz, CA, USA), PD98059 (0·2–20 µM; Calbiochem, La Jolla, CA, USA) and SP600125 (0·2–20 µ M; Sigma), MG-132 (0·5–50 µM; Calbiochem) prior to incubation with the various stimuli. The study was performed with the approval and compliance of the Tokushima University Ethical Committee.
Cytokine determination
CCL20 and VEGF concentration in the culture supernatant was measured by enzyme-linked immunosorbent assay (ELISA). Duoset (R&D Systems, Minneapolis, MI, USA) was used for CCL20 detection. The human VEGF ELISA development kit (Peprotech) was used for VEGF detection. The assay was performed according to the manufacture's instructions. The data were determined by using a standard curve prepared for each assay.
RNA extraction and RT-PCR analysis
Total RNA was prepared from HGF using an RNeasy total RNA isolation Kit (Qiagen, Hilden, Germany). Single-strand cDNA for a PCR template was synthesized from 48 ng of total RNA using primer oligo(dT)12−18 (Invitrogen, Carlsbad, CA, USA) and the superscript III reverse transcriptase (Invitrogen) under the conditions indicated by the manufacture. Specific primers were designed from cDNA sequence for CCL20 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Each cDNA was amplified by PCR using Hot star Taq DNA polymerase (Qiagen). The sequences of the primers were as follows: CCL20-F (5′-TTG CTC CTG GCT GCT TTG-3′), CCL20-R (5′-ACC CTC CAT GAT GTG CAA G-3′), GAPDH-F (5′-TGA AGG TCG GAG TCA ACG GAT TTG GT-3′) and GAPDH-R (5′-CAT GTG GGC CAT GAG GTC CAC CAC-3′). The conditions for PCR were 1× (95°C, 15 min), 35× (94°C, 40 s, 55°C, 40 s, 72°C, 1 min) and 1× (72°C, 10 min). The products were analysed on a 2·0% agarose gel containing ethidium bromide. The expected size for the PCR products for CCL20 and GAPDH were 367 base pairs (bp) and 985 bp, respectively.
Flow cytometric analyses
Cultured HGF were detached by using PBS-4 mmol/l EDTA and washed twice with PBS. Detached cells were incubated with anti-human CCR6 monoclonal antibody (mAb) (R&D Systems) or isotype control antibody (Dako, Kyoto, Japan) on ice for 30 min. After washing three times with PBS-1% bovine serum albumin (BSA; Sigma), the cells were incubated with fluoroscein isothiocyanate (FITC)-conjugated rabbit anti-mouse F(ab’) 2 fragment (Dako) for 30 min on ice. After washing three times with PBS-1% BSA, the cells were analysed immediately by flow cytometry (Epics XL-MCL; Coulter, Hialeah, FL, USA).
Statistical analysis
Data are presented as mean values ± standard deviation (SD). Differences between the two groups were calculated using Student's t-test. P-values of less than 0·05 were considered significant.
Results
Induction of CCL20 mRNA by IL-1β or TNF-α
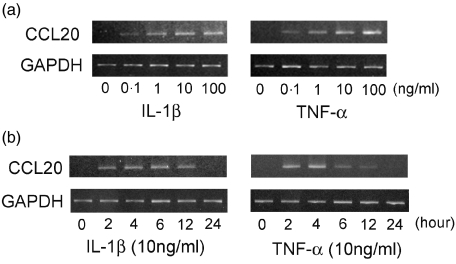
As shown in Fig. 1a, CCL20 mRNA was induced by both IL-1β and TNF-α in a dose-dependent fashion. CCL20 mRNA expression was enhanced at 2 h stimulation, peaked at 4 h stimulation and returned weak levels at 24 h stimulation (Fig. 1b).
Fig. 1.
Induction of CCL20 mRNA by interleukin (IL)-1β and tumour necrosis factor (TNF)-α. (a) Total RNA was prepared from human gingival fibroblasts (HGF) treated without or with IL-1β (0·1–100 ng/ml) or TNF-α (0·1–100 ng/ml) as indicated. After 4 h, reverse transcription-polymerase chain reaction (RT-PCR) analysis was carried out for CCL20 and GAPDH. (b) Total RNA was prepared from HGF treated without or with IL-1β (10 ng/ml) or TNF-α (10 ng/ml) for 2, 4, 6, 12 and 24 h. RT-PCR analysis was carried out for CCL20 and GAPDH. Similar results were obtained in three repeated experiments.
CCL20 release by stimulated HGF
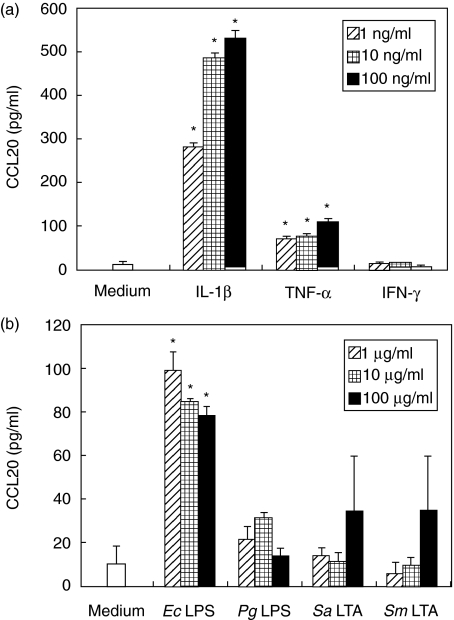
We examined whether HGF could produce the CCL20 protein by ELISA. We stimulated HGF with proinflammatory cytokines (Fig. 2a), LPS or LTA (Fig. 2b). IL-1β and TNF-α induced a dose dependent increase in CCL20 release. However, IFN-γ could not induce CCL20 (Fig. 2a). E. coli LPS could induce CCL20 at low concentrations. On the other hand, Pg LPS, Sa LTA and Sm LTA could not induce CCL20 from HGF significantly (Fig. 2b).
Fig. 2.
Release of CCL20 by human gingival fibroblasts (HGF). HGF was cultured in the absence or presence of interleukin (IL)-1β (1–100 ng/ml), tumour necrosis factor (TNF)-α (1–100 ng/ml), interferon (IFN)-γ (1–100 ng/ml), Escherichia coli lipopolysaccharide (LPS) (1–100 µg/ml), Porphyromonas gingivalis LPS (1–100 µg/ml), Staphylococcus aureus lipoteichoic acid (LTA) (1–100 µg/ml) and Streptococcus mutans LTA (1–100 µg/ml) for 24 h at 37°C. Medium was removed and assayed for CCL20 release by enzyme-linked immunosorbent assay. Data are presented as the mean ± SD (n = 3; by Student's t-test, *P < 0·05, stimulated versus unstimulated). Similar results were obtained in three repeated experiments.
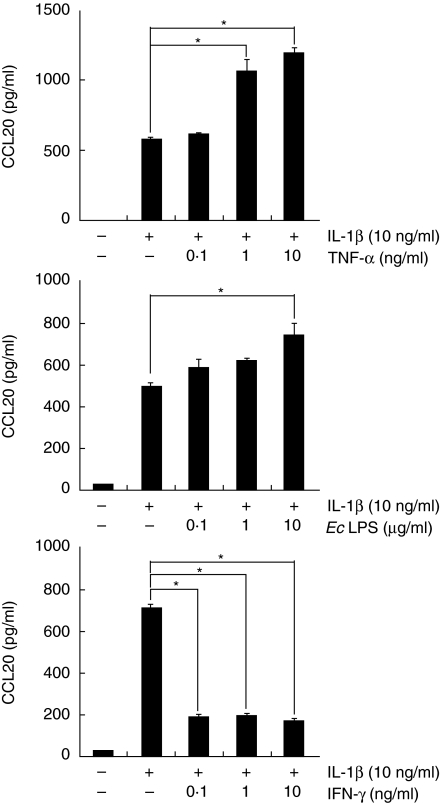
Effects of TNF-α, E. coli LPS and IFN-γ on the production of CCL20 by IL-1β-stimulated HGF
Figure 2 shows that IL-1β, TNF-α and E. coli LPS can induce CCL20 by HGF. Next, we examined whether TNF-α, E. coli LPS and IFN-γ modulate CCL20 production induced by IL-1β. Figure 3 shows that TNF-α and E. coli LPS could enhance IL-1β-induced CCL20 secretion in a dose-dependent fashion. On the other hand, IL-1β-induced CCL20 production was dramatically diminished by IFN-γ treatment (Fig. 3). We examined whether IFN-γ induced cell death by using cell viability assay. IFN-γ treatment did not induce cell death of HGF (data not shown). IFN-γ treatment did not change CCL20 production by HGF stimulated with TNF-α or E. coli LPS (data not shown).
Fig. 3.
Effects of tumour necrosis factor (TNF)-α, Escherichia coli lipopolysaccharide and interferon (IFN)-γ on CCL20 release by interleukin (IL)-1β−stimulated human gingival fibroblasts (HGF). HGF was stimulated with IL-1β (10 ng/ml) in the presence or absence of TNF-α (0·1–10 ng/ml), E. coli LPS (0·1–10 µg/ml) or IFN-γ (0·1–10 ng/ml) for 24 h at 37°C. Medium was removed and assayed for CCL20 release by enzyme-linked immunosorbent assay. Data are presented as the mean ± SD (n = 3; by Student's t-test, *P < 0·05). Similar results were obtained in three repeated experiments.
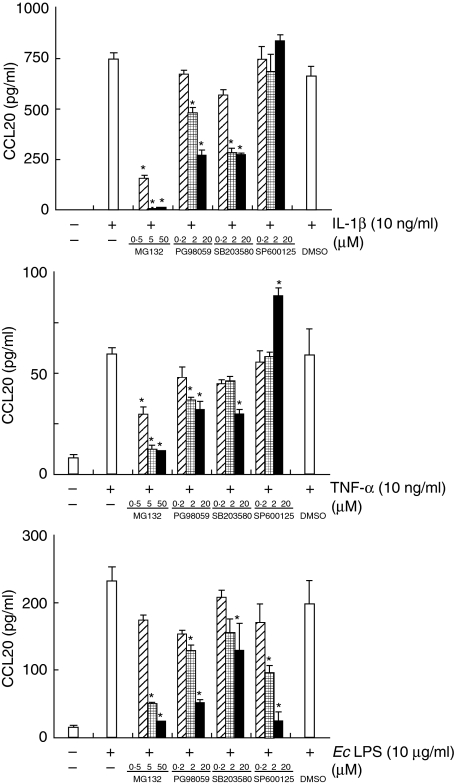
Involvement of p38 MAPK, ERK, JNK and NF-κB in induction of CCL20 in HGF stimulated with IL-1β, TNF-α and E. coli LPS
To determine whether p38 MAPK and/or ERK and/or JNK and/or NF-κB are required for CCL20 production in response to IL-1β, TNF-α or E. coli LPS, the effects of several inhibitors on CCL20 production by HGF were examined. At a concentration of 50 µ M, MG-132, a cell-permeable peptide-aldehyde protease inhibitor that blocks NF-κB activation via its effect on the proteasome, completely prevented the induction of CCL20 production stimulated with IL-1β, TNF-α and E. coli LPS (Fig. 4). PD98059, a specific inhibitor that binds inactive forms of MEK and prevents their activation and phosphorylation resulting in inhibition of ERK, partially inhibited CCL20 production by HGF stimulated with IL-1β, TNF-α and E. coli LPS. SB203580, a selective inhibitor of p38 MAPK, binds with high affinity to p38 MAPK near the ATP-binding site, thus rendering p38 MAPK inactive, partially blocked induction of CCL20 by HGF in response to IL-1β, TNF-α or E. coli LPS. SP600125, a selective JNK inhibitor, blocked CCL20 production by HGF stimulated with only E. coli LPS. On the other hand, CCL20 production induced by TNF-α was enhanced by SP600125 treatment (Fig. 4).
Fig. 4.
Effects of signalling pathway inhibitors the production of CCL20 production by stimulated human gingival fibroblasts (HGF). HGF was pretreated for 1 h with or without MG-132 (0·5–50 µ M), PD98052 (0·2–20 µ M), SB203580 (0·1–10 µ M), SP600125 (0·2–20 µ M) or dimethylsulphoxide (1 : 2000 dilution) as a control, and treated with interleukin (IL)-1β (10 ng/ml), tumour necrosis factor (TNF)-α (10 ng/ml) or Escherichia coli lipopolysaccharide (10 µg/ml) for 24 h at 37°C. Medium was removed and assayed for CCL20 release by enzyme-linked immunosorbent assay. Data are presented as the mean ± SD (n = 3, by Student's t-test, *P < 0·05 versus stimulated). Similar results were obtained in three repeated experiments.
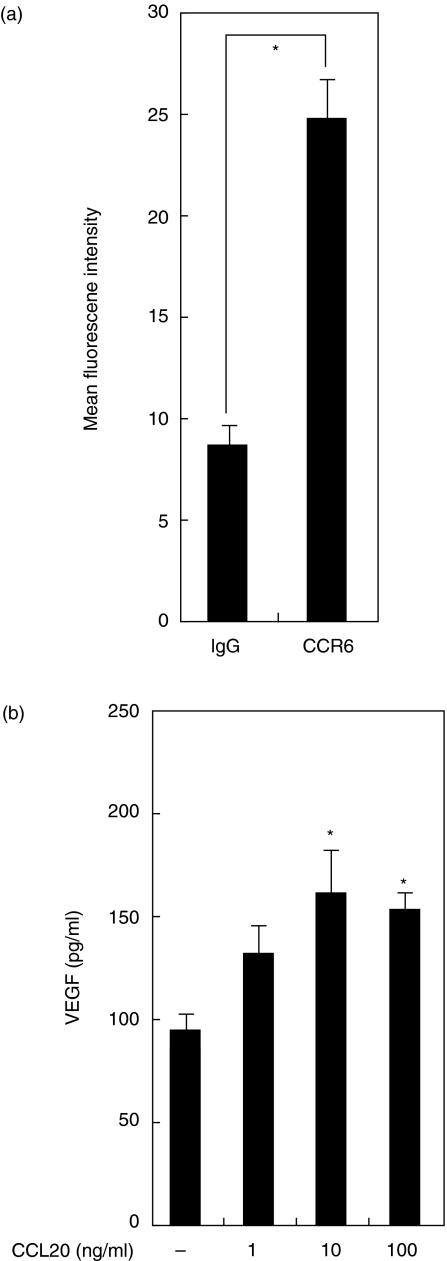
CCL20 induces VEGF production by HGF
We examined CCR6 expression on HGF using flow cytometry. Flow cytometric analysis revealed that CCR6 was expressed on non-stimulated HGF (Fig. 5a). To determine the effects of CCL20 on HGF, HGF was stimulated with CCL20 and the production of VEGF was analysed by ELISA. Figure 5b shows that VEGF levels were significantly higher in HGF cultures treated with CCL20 (10 ng/ml or 100 ng/ml) compared to the unstimulated HGF culture. At the same time, we examined IL-8 production and ICAM-1 expression by HGF stimulated with CCL20. However, CCL20 treatment did not induce IL-8 production and ICAM-1 expression by HGF (data not shown).
Fig. 5.
Release of vascular endothelial growth factor (VEGF) by human gingival fibroblasts (HGF) treated with CCL20. (a) CCR6 expression on HGF. HGF was incubated with human CCR6 mouse antibody or isotype-matched mouse IgG. Cells were then stained with fluoroscein isothiocyanate (FITC)-labelled goat antimouse IgG and analysed with flow cytometry to determine the expression of CCR6. Data are presented as the mean ± SD (n = 3; by Student's t-test, *P < 0·05). Similar results were obtained in three repeated experiments. (b) HGF was stimulated in the absence or presence of CCL20 (1–100 ng/ml) for 24 h at 37°C. Medium was removed and assayed for VEGF release by enzyme-linked immunosorbent assay. Data are presented as the mean ± SD (n = 3; by Student's t-test, *P < 0·05, stimulated versus unstimulated). Similar results were obtained in three repeated experiments.
Discussion
It has been reported that CCL20 is a CC chemokine expressed mainly by surface-lining cells, such as mucosal epithelial cells and epidermal keratinocytes [8,23,24]. Its receptor CCR6 is expressed on immature dendritic cells, B cells and memory T cells [13]. Thus, it is likely that CCL20 is an important mediator for both the initiation and effector phases of immune responses. In this study, we examined the mechanism of CCL20 production by HGF. We have shown that CCL20 is highly inducible by proinflammatory cytokines such as IL-1β and TNF-α. Previously, Nakayama et al. have also shown that CCL20 is inducible in epidermal keratinocytes by IL-1β and TNF-α[8]. Thus, these proinflammatory cytokines might be universal inducers of CCL20 in human cells. TNF-α was much less effective than IL-1β for induction of CCL20 by HGF. It is reported that the opposite was true in the case of T84 cells (human colon carcinoma cell lines) [25]. Thus, the effects of IL-1β and TNF-α on induction of CCL20 may be dependent on the cellular background.
We showed that E. coli LPS could induce CCL20 production by HGF. It is reported that E. coli LPS-treated peripheral blood mononuclear cells [26] or neutrophils [27] could produce CCL20. On the other hand, E. coli LPS could not induce CCL20 production by epithelial-type cells [25]. This means that the effects of E. coli LPS on induction of CCL20 will be dependent upon the type of cells.
We reported that IFN-γ could not induce CCL20 by HGF and inhibited CCL20 production induced by IL-1β. Schutyser et al. reported that IFN-γ could not induce CCL20 by epithelial cell, monocyte and skin fibroblasts [26] and Fujiie et al. reported that IFN-γ consistently suppressed expression of CCL20 in Caco-2 (human colon carcinoma cell line) and T84 cells [25]. Therefore, our results agree with their studies.
We have demonstrated further that induction of CCL20 by IL-1β, TNF-α and E. coli LPS is mediated critically by NF-κB. NF-κB is an important transcriptional factor for inflammatory and immunological responses. NF-κB is known to be involved in expression of various cytokines, acute-phase proteins and adhesion molecules [28]. NF-κB has also been shown to play an essential role in the induction of a number of inflammatory chemokines such as IL-8, monocyte chemoattractant protein-1 (MCP-1)/CCL2, regulated upon activation, normal T-cell expressed and secreted (RANTES)/CCL5 and eotaxin/CCL11 [29–32]. The present results extend its list of targets to CCL20.
Activation of ERK and p38 MAPK pathways by IL-1β, TNF-α and E. coli LPS has been well described. Reibman et al. reported that ERK and p38 MAPK pathways were related to CCL20 production by airway epithelial cells stimulated with IL-1β or TNF-α[33]. Our results show that HGF use the same signalling pathways to release CCL20 as epithelial cells.
Rhee et al. reported that the JNK inhibitor did not modulate CCL20 production induced by flagellin [34]. We report here that CCL20 production induced by E. coli LPS was inhibited by the JNK inhibitor; it has been reported that treatment of fibroblasts with E. coli LPS caused activation of JNK [35]. The difference between our report and Sang's may be dependent upon the type of cells, because CCL20 production pattern was totally different from each cell type. Further investigation will be necessary concerning the signalling pathway involved in CCL20 production.
Recently, Shiba et al. reported that human pulp fibroblasts express CCR6 [36]. Our reports add to the data showing that HGF also express CCR6. We also show that CCL20 induce VEGF. It is reported CCL20 stimulation induced osteopontin expression in human pulp fibroblasts [36]. Fujiie et al. reported that CCL20 enhanced the growth of Huh7 cells (hepatocellular carcinoma cells) [25]. CCL20 may be related to tissue remodelling by inducing VEGF production, cell proliferation, extracellular matrix including osteopontin as well as leucocyte infiltration.
In summary, these data describe the synthesis and release of CCL20 by HGF. The wide range of stimuli, including proinflammatory cytokines and LPS, suggest that this chemokine may serve an important role in the progression of periodontal disease. CCL20 released by HGF might be important for CCR6 positive leucocytes, including memory T cells and immature dendritic cells infiltration in periodontal tissues, and might be involved in the pathogenesis of periodontal disease.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) (16591915) from the Japan Society for the Promotion of Science.
References
- 1.Dzink JL, Tanner AC, Haffajee AD, Socransky SS. Gram negative species associated with active destructive periodontal lesions. J Clin Periodontol. 1985;12:648–59. doi: 10.1111/j.1600-051x.1985.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 2.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 3.Taubman MA, Eastcott JW, Shimauchi H, Takeichi O, Smith DJ. Modulatory role of T lymphocytes in periodontal inflammation. In: Genco RJ, Hamada S, Mergenhagen SE, et al., editors. Molecular pathogenesis of periodontal disease. Washington, DC: American Society for Microbiology; 1994. p. 14757. [Google Scholar]
- 4.Zehnder M, Greenspan JS, Greenspan D, Bickel M. Chemokine gene expression in human oral mucosa. Eur J Oral Sci. 1999;107:231–5. doi: 10.1046/j.0909-8836.1999.eos107401.x. [DOI] [PubMed] [Google Scholar]
- 5.Yu X, Graves DT. Fibroblasts, mononuclear phagocytes, and endothelial cells express monocyte chemoattractant protein-1 (MCP-1) in inflamed human gingiva. J Periodontol. 1995;66:80–8. doi: 10.1902/jop.1995.66.1.80. [DOI] [PubMed] [Google Scholar]
- 6.Yu X, Antoniades HN, Graves DT. Expression of monocyte chemoattractant protein 1 in human inflamed gingival tissues. Infect Immun. 1993;61:4622–8. doi: 10.1128/iai.61.11.4622-4628.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosokawa Y, Nakanishi T, Yamaguchi D, et al. Macrophage inflammatory protein 3alpha-CC chemokine receptor 6 interactions play an important role in CD4+ T-cell accumulation in periodontal diseased tissue. Clin Exp Immunol. 2002;128:548–54. doi: 10.1046/j.1365-2249.2002.01865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakayama T, Fujisawa R, Yamada H, et al. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3 alpha/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int Immunol. 2001;13:95–103. doi: 10.1093/intimm/13.1.95. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu Y, Murata H, Kashii Y, et al. CC-chemokine receptor 6 and its ligand macrophage inflammatory protein 3alpha might be involved in the amplification of local necroinflammatory response in the liver. Hepatology. 2001;34:311–9. doi: 10.1053/jhep.2001.26631. [DOI] [PubMed] [Google Scholar]
- 10.Matsui T, Akahoshi T, Namai R, et al. Selective recruitment of CCR6-expressing cells by increased production of MIP-3 alpha in rheumatoid arthritis. Clin Exp Immunol. 2001;125:155–61. doi: 10.1046/j.1365-2249.2001.01542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruth JH, Shahrara S, Park CC, et al. Role of macrophage inflammatory protein-3alpha and its ligand CCR6 in rheumatoid arthritis. Lab Invest. 2003;83:579–88. doi: 10.1097/01.lab.0000062854.30195.52. [DOI] [PubMed] [Google Scholar]
- 12.Power CA, Church DJ, Meyer A, et al. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3alpha from lung dendritic cells. J Exp Med. 1997;186:825–35. doi: 10.1084/jem.186.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol. 1999;162:186–94. [PubMed] [Google Scholar]
- 14.Baba M, Imai T, Nishimura M, et al. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem. 1997;272:14893–8. doi: 10.1074/jbc.272.23.14893. [DOI] [PubMed] [Google Scholar]
- 15.Greaves DR, Wang W, Dairaghi DJ, et al. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3alpha and is highly expressed in human dendritic cells. J Exp Med. 1997;186:837–44. doi: 10.1084/jem.186.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao F, Alderson R, Su J, Ullrich SJ, Kreider BL, Farber JM. STRL22 is a receptor for the CC chemokine MIP-3alpha. Biochem Biophys Res Commun. 1997;236:212–7. doi: 10.1006/bbrc.1997.6936. [DOI] [PubMed] [Google Scholar]
- 17.Yang D, Chertov O, Bykovskaia SN, et al. Beta-defensins. linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 18.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleeff J, Kusama T, Rossi DL, et al. Detection and localization of Mip-3alpha/LARC/Exodus, a macrophage proinflammatory chemokine, and its CCR6 receptor in human pancreatic cancer. Int J Cancer. 1999;81:650–7. doi: 10.1002/(sici)1097-0215(19990517)81:4<650::aid-ijc23>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Yamahiro SJM, Wang WH, Gong D, Yang Kamohara H, Yoshimura T. Expression of functional CCR6 in cytokine-stimulated human neutrophils. Cytokine. 1999;11:918. [Abstract]. [Google Scholar]
- 21.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–22. [PMC free article] [PubMed] [Google Scholar]
- 22.Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki A, Kelsall BL. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–94. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka Y, Imai T, Baba M, et al. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur J Immunol. 1999;29:633–42. doi: 10.1002/(SICI)1521-4141(199902)29:02<633::AID-IMMU633>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Fujiie S, Hieshima K, Izawa D, et al. Proinflammatory cytokines induce liver and activation-regulated chemokine/macrophage inflammatory protein-3alpha/CCL20 in mucosal epithelial cells through NF-kappaB [correction of NK-kappaB] Int Immunol. 2001;13:1255–63. doi: 10.1093/intimm/13.10.1255. [DOI] [PubMed] [Google Scholar]
- 26.Schutyser E, Struyf S, Menten P, et al. Regulated production and molecular diversity of human liver and activation-regulated chemokine/macrophage inflammatory protein-3 alpha from normal and transformed cells. J Immunol. 2000;165:4470–7. doi: 10.4049/jimmunol.165.8.4470. [DOI] [PubMed] [Google Scholar]
- 27.Scapini P, Laudanna C, Pinardi C, et al. Neutrophils produce biologically active macrophage inflammatory protein-3alpha (MIP-3alpha)/CCL20 and MIP-3beta/CCL19. Eur J Immunol. 2001;31:1981–8. doi: 10.1002/1521-4141(200107)31:7<1981::aid-immu1981>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 29.Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kB-like binding sites of the interleukin 8 gene. J Biol Chem. 1992;267:22506–11. [PubMed] [Google Scholar]
- 30.Martin T, Cardarelli PM, Parry GC, Felts KA, Cobb RR. Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-kappa B and AP-1. Eur J Immunol. 1997;27:1091–7. doi: 10.1002/eji.1830270508. [DOI] [PubMed] [Google Scholar]
- 31.Rovin BH, Lu L, Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem Biophys Res Commun. 1999;259:344–8. doi: 10.1006/bbrc.1999.0796. [DOI] [PubMed] [Google Scholar]
- 32.Moriuchi H, Moriuchi M, Fauci AS. Nuclear factor-kappa B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J Immunol. 1997;158:3483–91. [PubMed] [Google Scholar]
- 33.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol. 2003;28:648–54. doi: 10.1165/rcmb.2002-0095OC. [DOI] [PubMed] [Google Scholar]
- 34.Rhee SH, Keates AC, Moyer MP, Pothoulakis C. MEK is a key modulator for TLR5-induced interleukin-8 and MIP3alpha gene expression in non-transformed human colonic epithelial cells. J Biol Chem. 2004;279:25179–88. doi: 10.1074/jbc.M400967200. [DOI] [PubMed] [Google Scholar]
- 35.Sugano N, Ito K, Murai S. Cyclosporin A inhibits collagenase gene expression via AP-1 and JNK suppression in human gingival fibroblasts. J Periodont Res. 1998;33:448–52. doi: 10.1111/j.1600-0765.1998.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 36.Shiba H, Mouri Y, Komatsuzawa H, et al. Macrophage inflammatory protein-3alpha and beta-defensin-2 stimulate dentin sialophosphoprotein gene expression in human pulp cells. Biochem Biophys Res Commun. 2003;306:867–71. doi: 10.1016/s0006-291x(03)01075-1. [DOI] [PubMed] [Google Scholar]