Abstract
The study of the singular hypersensitivity reactions to Anisakis simplex (A.s) proteins, may help us to undestand many of the unknown immune interactions between helmiths infections and allergy. We have developed a murine model of allergy to A. simplex, that mimics human A. simplex allergy to study the specific aspects of anaphylaxis induced by parasites. Male C3H/HeJ mice were intraperitoneally sensitized to A. simplex. Mice were then intravenous or orally challenged with A. simplex. Antigen-specific immunoglobulins, polyclonal IgE, anaphylactic symptoms, plasma histamine levels and cytokine profiles were determined. Comparative IgE immunoblot analyses were also performed. Specific IgE, IgG1 and IgG2a were detected in sensitized mice since week 3. Polyclonal IgE raised and peaked with different kinetics. Intravenous A. simplex challenge produced anaphylaxis in mice, accompanied by plasma histamine release. Oral A. simplex challenge in similarly sensitized mice did not caused symptoms nor histamine release. Numerous A. simplex allergens were recognized by sensitized mouse sera, some of them similar to human serum. The A. simplex stimulated splenocytes released IL-10, IFN-γ, IL-4, IL-13 and IL-5. We describe a new animal model of anaphylaxis. It exhibits characteristics of type I hypersensitivity reactions to Anisakis simplex similar to those observed in allergic humans. Different responses to i.v. or oral A. simplex challenges emerged, which did not reflect a window tolerization period. The cytokine profile developed (mixed Th1/Th2 pattern) differed from the observed in classical models of anaphylaxis or allergy to food antigens. This model may permit to investigate the peculiar allergic reactions to parasitic proteins.
Keywords: allergy to parasites, anaphylaxis, animal model, Anisakis simplex, helminths
Introduction
Epidemiological and immunological studies reported over the past decades have demonstrated complex interactions between allergy and helminth infections. The human immune system has evolved in the presence of intense helminth infections and has developed regulatory mechanisms to limit the harmful inflammation that can be caused by these potent allergens secreted by chronic pathogens [1]. This tolerization process induced by parasite antigens could consequently suppress allergic responses to common inhalant allergens. It has therefore been proposed that infections with helminths may protect from the development of allergic diseases [2]. However, so far, epidemiological and experimental studies have yield conflicting results.
The protection from allergic diseases by parasitic proteins may operate through many different mechanisms. Helminth infections generate a polyclonal stimulus of IgE, which was first thought to mediate the parasite protection from allergic diseases. This IgE receptor-blocking theory was lately discarded in studies in Gabon, where confounders were included [3]. A heminth-induced modified Th2 response with high titres of IgG4 antibodies, have also been invoked to mediate this immune protection [4,5]. High titres of IgG4 are present in asymptomatic helminth infections, and are able to inhibit IgE-mediated degranulation of effector cells [6]. Blocking IgG4 levels, however, do not correlate with the lack of skin reactivity in the epidemiological studies [6]. Heminth-induced anti-inflammatory T regulatory cytokines may mediate allergy protection. IL-10 has been shown to inversely correlate with allergy. Decreased local levels of IL-10 have been reported in allergic individuals [7], and suppression of allergen-induced airway eosinophilia and reduction of eotaxin production by parasites infection is abolished in IL-10 deficient mice [8]. Furthermore, worm-modulated IL-10-producing B cells from IL-4 deficient mice, confer complete resistance to anaphylaxis when transferred to naïve mice [9]. In addition, innate CD25+CD4+ regulatory cells have been recently shown to contribute to Th2 polarization during helminth infections by specifically suppression of Th1 development by mature dendritic cells [10,11]. This regulatory network produced by a persistent immune challenge, would offer unifying explanations for the inverse association of parasitic infections and allergic diseases [12].
Paradoxically, allergic reactions have been described against these immunimodulatory parasitic allergens [13,14]. The morbility and severity of helminth infections have been drastically reduced in westernized countries, but for many years little interest has been paid to clinical allergic responses to certain parasites, which are still prevalent in modernized countries. Human infection by Anisakis simplex, has become frequent nowadays due to the overall high rate of fish contamination and the new eating habits. This helminth infection has a strong impact in allergy [13,15–19]. In Spain, it has been estimated that A. simplex allergy may be more prevalent than any specific food allergy in the adult population [20] and compromises as much as 10% of the idiopathic anaphylaxis. In addition, a high rate (13%) of blood donors are sensitized to these larval proteins [21] as well as 50% of the fishmongers and the fishermen in Italy [22]. In the Madrid area, 23% of the patients attending to an allergy clinic are sensitized to A. simplex [18]. Curiously, only 20% of them develop allergic reactions.
Some A. simplex allergens are being identified [23–26]. Although many of them are thermostable and deep frying dose not fully eliminate them, most of the authors believe that the live larvae is needed to produce allergic reactions [27]. This is mainly based on different negative oral challenges performed in allergic patients with lyophilized larva [28], somatic [29] or excretion-secretion antigens [30] in sensitized patients, partly explained by their allergenic susceptibility to pepsin digestion. This sensitivity to pepsin may justify that many sensitized patients are safely eating frozen contaminated fish. Contrarily, other authors report reactions after eating well cooked fish in some of their patients [31].
There are specific clinical features which distinguish in A. simplex allergy from other allergies caused by common antigens in humans. A. simplex sensitization is not more frequent among the atopic population [18]. Secondly, many patients with high exposure to A. simplex larvae (big consumers of fish, living in highly fish contaminated areas), are sensitized but do not refer clinical symptoms (subclinical sensitization). Third, the allergic patients develop symptoms only in very few occasions, and finally, only a small percentage of the patients who suffer gastric or intestinal anisakiasis, develop urticaria [32,33] as it is reported in large series of Japanese patients [34].
In previous research on A. simplex allergy it has not taken into account that parasite proteins are also immunomodulatory agents. Therefore the peculiarities of A. simplex allergy and the absence of response to A. simplex after specific oral challenges may have different explanations. After allergic reactions to A. simplex or after A. simplex infections, a sensitized patient may develop a prolonged determining immune modulation with the result of a tolerization period to further antigen challenges. Systemic challenges with A. simplex extracts, to mimic larval infections, and be able to answer this questions have not been performed in patients for ethical reasons.
We have generated a murine model with numerous similarities to the A. simplex allergic patients to better understand this peculiar allergy, as well as to explore specific immune responses following A. simplex sensitization.
Materials and methods
Mice and reagents
Sixty-two male, 6-week-old, C3H/HeJ mice were pursached from Charles River laboratories (Barcelona, Spain), and maintained under a 12-h light-dark cycle with free access to water and standard laboratory food. All mice were kept at the animal department of Experimental Medicine Unit of Hospital Gregorio Marañón (Madrid, Spain), which followed the European Regulations for Animal Experimentation (Directive: 86/609/EEC). The experiments were approved by the animal care committee of our institution.
A. simplex crude extract, regularly used for human skin testing (I.P.I. Diagnostics, Madrid. Spain), was used for the sensitization, challenge and ELISA experiments. This extract was produced, after thorough washings with sterile water, by homogenization of stage 3 larva followed by sonication and delipidation as elsewhere described [35]. The A. simplex extract was next biologically standardized and it is now in use in allergy clinics. It has been tested in fish allergic patients with negative responses. Pertussis toxin (PT) and Concanavalin A were pursached from Sigma-Aldrich Inc. (Missouri, USA).
Sensitization and challenge protocols
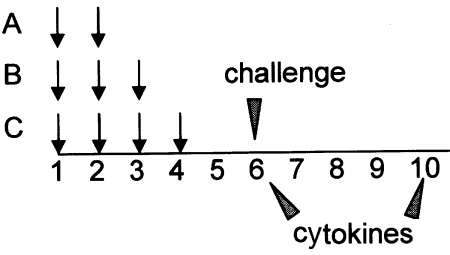
Different groups of mice received two, three or four weekly intraperitoneal (i.p) injections of A. simplex (100 µg), PT (300 ng) and alum (1 mg) in 200 µl of phosphate buffered saline (PBS) (Protocols A, B and C) (Fig. 1). Control mice received PT with alum or saline. Six weeks after the initial sensitization dose, a subset of mice was intravenously (i.v) challenged with 200 µg of A. simplex in 50 µl of saline solution. A second group fasted overnight and was challenged with 2 mg of A. simplex by intragastric gavage (i.g). The mice were then observed for 2 h.
Fig. 1.
Mice received two, three or four weekly intraperitoneal injections of A. simplex (100 µg), PT (300 ng) and alum (1 mg) in 200 µl of phosphate buffered saline (PBS) (Protocols A, B and C) (Fig. 1). Control mice received PT with alum or saline. Six weeks after the initial sensitization dose, a subset of mice was intravenously challenged with 200 µg of A. simplex in 50 µl of saline solution. A second group fasted overnight and was challenged with 2 mg of A. simplex by intragastric gavage. The mice were then observed for 2 h. Cytokines from stimulated splenocytes were measured on week 6 and 10.
Antibody assays
Mice were weekly anaesthetized with inhaled isoflurane (Abbott, IL, USA) and bled from the retro-ocular plexus in order to measure A. simplex specific IgE (sIgE), IgG1 and IgG2a as well as total IgE levels by ELISA. Briefly, for IgG1 and IgG2a 96-well plates were coated with 50 µl of 1·6 ng/ml of A. simplex in 0·2 M borate-buffered saline solution, pH 8·2 (BBS) overnight at 4 °C. Plates were then blocked with 1% BSA in PBS at 37 °C for 2 h, washed with BBS-0·05% Tween-20 (Sigma) and incubated with the standards and samples overnight at 4 °C. Alkaline phosphatase-linked anti-IgG1 or G2a (Southern Biotechnology Associates, Birmingham, USA) were added at 1 : 2000 dilutions, washed and then incubated with 2·63 mg/ml p-nitrophenyl phosphate (Boehringer Mannhein, GmbH, Germany). Absorbance at 405–650 nm was read at 1 and 18 h, respectively.
A. simplex specific serum IgE titres were also measured by ELISA. To remove antigen specific IgG, serum samples were previously incubated with protein-G sepharose beads according to manufacturer recommendations (Pharmacia, Uppsala, Sweden). Coated plates were saturated with 1% BSA in PBS. Protein G-absorbed sera were added and incubated overnight at 4 °C. Plates were incubated with goat biotinylated anti-mouse IgE at 6 µg/ml (Becton Dickinson, PharMingen, San Diego, CA, USA), subsequently washed and incubated with horseradish peroxidase-linked streptavidin at 0·62 µg/ml (Zymed, San Francisco, CA, USA). TMB peroxidase was added (3,3′,5,5′-tetramethyl benzidine, KirKegaard and Perry Laboratories, Gaithersburg, MD, USA). The reaction was stopped with phosphoric acid. Absorbance was read at 450–650. Sample concentrations in both assays were calculated by comparison with a standard curve on each plate using a Delta-SOFT II Pc (V 1·71·2, BioMethalics Inc (Pricenton, USA). Results were expressed in units per milliliter on the basis of pooled high-titre standards obtained from a group of extensively sensitized mice, which were given arbitrary concentrations and expressed as U/ml. Total IgE levels were quantified in ng/ml using purified mouse IgE (PharMingen) for the standard curve and antimouse IgE monoclonal antibody to coat plates.
IgE immunoblot analyses
A. simplex allergenic proteins were determined by a 15% sodium dodecyl sulphate-polyacrylamyde electrophoresis (SDS-PAGE) according to standard procedures as described elsewhere [30]. Blotted membranes were incubated with the pooled, IgG absorbed, positive and negative mice sera. The membranes were incubated with goat biotinylated anti-mouse IgE at 1 µg/ml (PharMingen), subsequently washed and incubated with horseradish peroxidase-conjugated streptavidin. Finally, the membranes were incubated in chemiluminescent substrate (Supersignal® West Pico Chemiluminescent Substrate, Pierce®, Rockford, USA) for 5 min, and then were exposed to radiographic films. A similar procedure, with various reactive and dilution modifications was applied to perform a human A. simplex IgE immunoblotting as previously described [30].
Assessment of anaphylactic responses
Symptoms were evaluated by using a scoring system described by Li et al. [36]. 0: no symptoms; 1: scratching and rubbing around the nose and head; 2: puffiness around the eyes and mouth, diarrhea, pillar erecti, reduced activity and/or decreased activity with increased respiratory rate; 3: wheezing, laboured respiration, and cyanosis around the mouth and the tail; 4: no activity after prodding or tremor and convulsion; 5: death.
A subset of mice was bled 8 min after the i.v. challenge or 8 and 30 min after the i.g. challenges. Blood was collected into chilled tubes containing 7·5% potassium-EDTA. After centrifugation (900 g) for 10 min at 4 °C, plasma aliquots were frozen at −80 °C until use. Histamine levels were determined by using an enzyme immunoassay kit (ImmunoTECH Inc., Marseille, France), as recommended by the manufacturer. Blood for basal histamine values were collected 24 h before the challenge. The mice used to assess histamine release were not used in the final analyses of anaphylactic symptoms.
Splenocyte cytokine profiles
Mouse spleens were harvested 6 or 10 weeks after the first sensitization dose, teased to prepare single cell suspensions, and resuspended in RPMI-1640 supplemented with 10% heat-inactivated FCS, 2 mmol/l glutamine, 0·05 mM M2-mercaptoethanol, and 1% penicillin-streptomycin (complete medium). Splenocytes were incubated at 5 × 105 cells per well in 96-well plates in a final volume of 250 µl of complete medium with A. simplex or concavalin A added at 10 and 2·5 µg/ml, respectively, or saline, at 37 °C in a 5% CO2 atmosphere. Culture supernatants were collected at 72 h. Samples were tested for the presence of IL-4, IL-5, IFN-γ, IL-13 and IL-12 by using ELISA with capture and biotinylated detecting antibodies (Genzyme, Boston MA, USA and PharMingen). Washing, blocking, and detection steps were analogous to those used in the immunoglobulin E ELISA. A standard curve was generated by using known amounts of recombinant cytokines (PharMingen).
Statistical procedures
Data were analysed by using a Graph Pad Prism software V 3·06 (San Diego, USA). Anaphylactic scores and histamine analyses were determined by anova test. For comparison of immunoglobulin patterns Pearson correlation was applied. Differences with P < 0·05 were judged significant. All experiments were repeated at least twice.
Results
Production of Anisakis simplex specific immunoglobulins and polyclonal IgE
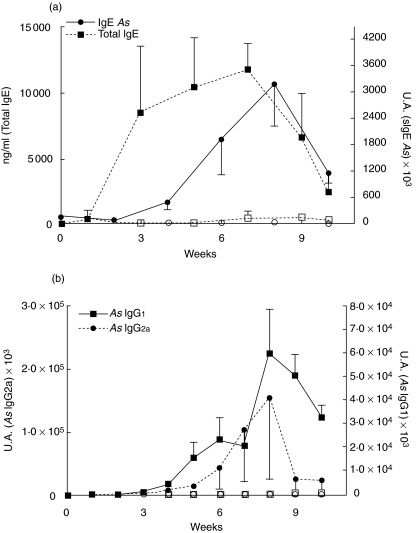
Mice receiving 3 i.p. doses of A. simplex, were bled weekly to investigate the profile of immunoglobulin production. They produced specific IgE (sIgE), IgG1 (sIgG1) and IgG2a (sIgG2a) by 3–4 weeks which peaked at week 8 (Fig. 2). Levels of sIgE significantly correlated to sIgG1 (r: 0·79, P < 0·0001) and sIgG2a (r: 0·42, P < 0·032). No specific immunoglobulin production was detected in the control groups during the 10 week-follow up period. Total IgE raised and peaked earlier with a different kinetics (Fig. 2). Total IgE levels did not correlate with specific immunoglobulins.
Fig. 2.
(a) Serum levels of total IgE (□, ▪) and specific IgE (○, •) were measured in the sensitized group (▪, •) and the saline group (□, ○) for 10 weeks. (b) Serum levels of specific IgG1 and IgG2a in both groups. Results are expressed as the mean of 5 mice ± SEM.
Systemic anaphylaxis after Anisakis simplex challenge
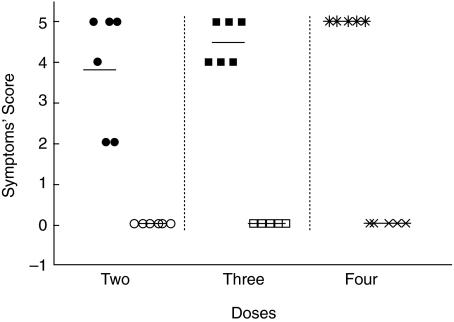
Six weeks after the initial sensitization dose, 5–6 mice in each group (protocols A, B and C), as well as the corresponding control groups, were i.v. challenged with 200 ng of A. simplex in 50 µl of saline. Symptoms scores are plotted in Fig. 3. All mice receiving A. simplex during the sensitizing period had anaphylactic symptoms, and the mean symptoms’ score increased with the number of sensitizing doses. Anaphylactic symptoms started between 3 and 11 min and lasted up to 30 min. In a previous experiment i.v. challenges with 100 ng of A. simplex produced clinical scores of 2–3 in sensitized mice (data not shown).
Fig. 3.
Sensitized mice (•, ▪, ) and control mice (○, □, ×) were intravenously challenged with 200 ng of Anisakis simplex extract. Post-challenge anaphylaxis symptoms’ score in groups of 5–6 mice receiving 2, 3 or 4 sensitizing Anisakis simplex doses.
) and control mice (○, □, ×) were intravenously challenged with 200 ng of Anisakis simplex extract. Post-challenge anaphylaxis symptoms’ score in groups of 5–6 mice receiving 2, 3 or 4 sensitizing Anisakis simplex doses.
A group of mice following each protocol were i.g. challenged with 2 mg of antigen and were observed for two hours. No clinical symptoms were recorded. Intravenous and i.g. A. simplex challenges were negative in the adjuvant and the saline control mice.
Histamine release after Anisakis simplex challenge
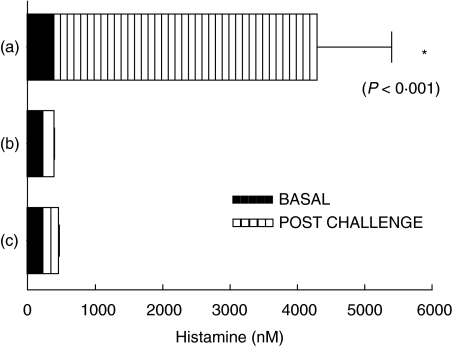
Plasma histamine levels obtained 8 min after the i.v. challenge of the sensitized group, increased by 480% from basal values (Fig. 4). No plasma histamine variations were detected 8 min (data not shown) or 30 min after the sensitized group was challenged by i.g. A. simplex gavages (Fig. 4). Histamine levels from the control mice groups remained also unchanged. Mice used to asses histamine release were not used in the final analyses of anaphylactic symptoms.
Fig. 4.
Plasma histamine release after Anisakis simplex challenges. Hatched bars represent plasma histamine levels (mean of 5 mice ± SEM) in sensitized mice (a) 8 min after iv Anisakis simplex challenge, (b) 30 min after oral challenge in the same group and (c) 8 min after i.v. Anisakis simplex challenge in the control mice. Close bars represent basal plasma histamine levels in each group (mean of 5 mice ± SEM).
Comparison of mouse to human allergenic pattern
A. simplex allergenic proteins to mouse were analysed by IgE immunoblot. Multiple IgE binding molecules were detected with a pooled serum from A. simplex sensitized mice (Fig. 5). This pattern was compared to the allergenic proteins detected by an A. simplex allergic patient. Mouse specific IgE pattern had similarities with the one observed with human serum. Nevertheless, this immunoblot analyses only compares the molecular weight of the allergens recognized by a group of mice to the antigens detected by a unique allergic patient who was representative of a group of allergic subjects reacting to multiple allergens [30].
Fig. 5.

Anisakis simplex IgE immunoblot. Comparison of mouse (M) and human (H) Anisakis simplex IgE binding molecules resolved by a 15% SDS-PAGE.
Production of Th2 and Th1 cytokine profiles after A. simplex sensitization
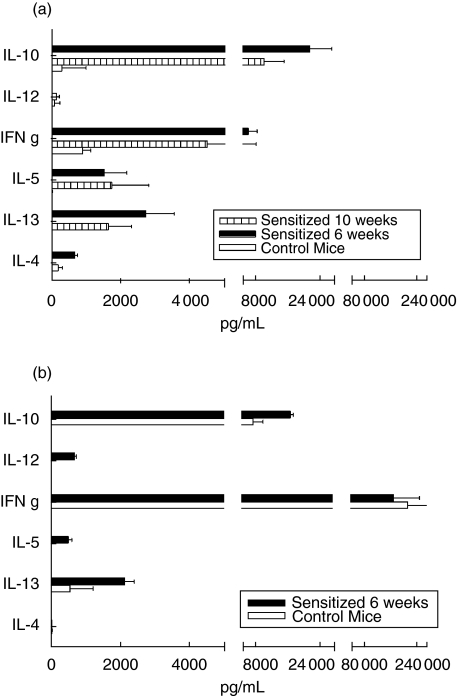
Cytokines from A. simplex stimulated splenocytes were measured the sixth and tenth weeks after the first immunizing dose in mice following protocol B. Th2 cytokines (IL-4, IL-5 and IL-13) were detected on week 6 and still were present 4 weeks later on mice sensitized with A. simplex (Fig. 6). Surprisingly, an important release was observed of IFN-γ in the absence of IL-12. Significant high levels of IL-10 were detected upon A. simplex activation. No cytokine release was detected from splenocytes derived from control mice. A non specific stimuli, concanavalin A, caused important release of IFN-γ, and in a lesser extend IL-10 from sensitized and naïve mice. IL-12, IL-13 and IL-5 but not IL-4 was exclusively released by splenocytes of sensitized mice upon the concanavalin A booster.
Fig. 6.
Splenocyte cytokine profile. (a) Splenocytes from sensitized and control mice (□) were stimulated by Anisakis simplex crude extract, on week 6 (▪) or 10 ( ). (b) A non specific stimulus (concanavalin A) was used to stimulate splenocytes from both groups on week 6: sensitized (▪) and control (□). Supernatant cytokine levels are expressed as ng/ml (mean of 4 mice ± SEM)
). (b) A non specific stimulus (concanavalin A) was used to stimulate splenocytes from both groups on week 6: sensitized (▪) and control (□). Supernatant cytokine levels are expressed as ng/ml (mean of 4 mice ± SEM)
Discussion
The intimate relationship between allergic diseases and parasitic infections remains unresolved. We have generated a murine model of anaphylaxis after sensitization with proteins of the larval parasite Anisakis simplex. This model exhibits characteristics of a type I hypersensitivity reaction, very similar to those observed in humans after A. simplex infection. After i.v. challenge, anaphylactic symptoms were apparent in 3–12 min and involved different organ systems. This challenge, which tried to mimic the larva gastric incursion through the mucosa, evoked symptoms in all sensitized mice and these were accompanied by an important histamine release. The three different protocols demonstrated a correlation between the number of sensitizing doses and the clinical score. In contrast, a much larger amount of antigen (×10), through the oral route, did not cause any symptoms in allergic mice, nor a histamine release.
The first consequence we learned from this new model is the fact that in mice, the unresponsiveness to oral A. simplex allergens, as it apparently happens in allergic patients, dose not depend on a systemic tolerization state. A distinct pattern of systemic immunoglobulins or cytokines between the two groups of allergic mice (immunized and challenged by the oral or the i.v. route) does not explain the different responses, as all mice were equally sensitized. On addition to the destroying effects of the activated gastric pepsin on A. simplex allergens [30], local immune responses by the gut-associated lymphoid tissue [37] for this lack of response may be present. Intestinal down-regulation of allergic responses driven by IL-10 by direct effects of both DC [11,38,39] or mast cells [40] as elsewhere suggested [41], may hamper the allergic response to oral allergens. This unresponsiveness to oral A. simplex antigens reinforce the need of a live larva to elicit most of the allergic reactions in man.
The two Th2 immunoglobulins which have been related to anaphylaxis in mice, specific IgE and specific IgG1, were strongly correlated in these mice, and lasted for more than 10 weeks. The IgE A. simplex specifity of mouse had similarities to the pattern described for some allergic human subjects. The specific IgG mice response to somatic and secreted A. simplex antigens was already characterized by other authors [42].
As expected, parasite proteins induced a polyclonal stimulation of IgE which followed a different kinetics than specific immunoglobulins. It is curious though, that the important release of the Th1 specific immunoglobulin, IgG2a was not able to block the allergic reaction. The adjuvants, alum hydroxide and pertussis toxin assigned to create this model were similar to other already described murine models for anaphylaxis [43]. They were important to enhance the allergic nature, but they did not impede the induction of the specific IgG2a by the parasite allergens.
When interpreting the character of the immune response in this allergy model, the role of the adjuvants has to be taken into account. As in other allergy models which pursue the appearance of anaphylactic symptoms, the addition of adjuvants was necessary to record a clinical allergic response. Parallel experiments with A. simplex proteins or with larval infection on the absence of adjuvants generated a similar specific immunoglobulin profile, but serum levels of this response were far below (data not shown). Some of the clinical and immunological features in this model are similar to other allergy murine models in which adjuvants were used [36,43–46]. But in this case, a different nature of the immune response was observed. The usual cytokine profile in food allergy models reveals a clear-cut Th2 response with the prominence of IL-4, IL-13 and IL-5 and the absence of IFN-γ [43,44,46,47]. On the contrary, in the A. simplex model, cytokines reflected a balanced Th1/Th2 response. Indeed there was a production of IL-4 and IL-13 probably responsible for the B lymphocyte shift to IgE production and long-term of IgE-secreting B cells survival [48], and IL-5 surely implicated in the eosinophilic production and accumulation at the parasite locations demonstrated in human anisakiasis [49]. But at the same time there was an important release of IFN-γ, and IL-10 which were not able to avoid the allergic systemic reaction. A non specific stimuli (concanavalin A) caused a preferential release of IFN-γ and IL-10 with no distinction between allergic and naïve mice, with or without adjuvants.
Experiments run in the same strain of mice by Bashir et al. [41] revealed a clinical protection of peanut oral allergy by enteric heminth infections. The protection was suggested to be conducted by IL-10 which blocked allergen-specific IgE. In the case of allergy to A. simplex, IL-10 or IFN-γ did not block sIgE synthesis and did not prevent from allergic symptoms induced by the parasitic proteins.
There is a delicate balance between parasitic induction of protective regulatory effects and detrimental IL-4 allergic responses. Indeed, parasites are often long-lived and inhabit immunocompetent host for prolonged periods, and is not surprising that they posses modulatory molecules that ameliorate host responses to enhance their survival. The role of T reg cell in regulating allergen-specific Th2 responses is still controversial, and it is even possible that, at least under certain conditions, these cells favour, rather than inhibit such type of responses [50].
In conclusion, we have generated a murine model of anaphylaxis to immunomodulatory Anisakis simplex proteins, which open new possibilities to study the human allergic reactions over parasite infections.
Acknowledgments
We want to thank I.P.I. Diagnostics, Madrid, Spain, for having provided us the Anisakis simplex extract and Dr Francisco del Cañizo for his help on the figure's design. This work has been funded by the Instituto de Salud Carlos III (Ministry of Health) Spain. Grant: FIS 02/1223.
References
- 1.Cooper PJ. The potential impact of early exposures to geohelminth infections on the development of atopy. Clin Rev Allergy Immunol. 2004;26:5. doi: 10.1385/CRIAI:26:1:5. [DOI] [PubMed] [Google Scholar]
- 2.Masters S, Barrett-Connor E. Parasites and asthma predictive or protective? Epidemiol Rev. 1985;7:49. doi: 10.1093/oxfordjournals.epirev.a036285. [DOI] [PubMed] [Google Scholar]
- 3.Scrivener S, Yemaneberhan H, Zebenigus M, et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case-control study. Lancet. 2001;358:1493. doi: 10.1016/S0140-6736(01)06579-5. [DOI] [PubMed] [Google Scholar]
- 4.Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 5.Rook GA, Ristori G, Salvetti M, Giovannoni G, Thompson EJ, Stanford JL. Bacterial vaccines for the treatment of multiple sclerosis and other autoimmune disorders. Immunol Today. 2000;21:503. doi: 10.1016/s0167-5699(00)01700-x. [DOI] [PubMed] [Google Scholar]
- 6.Hussain R, Poindexter RW, Ottesen EA. Control of allergic reactivity in human filariasis. Predominant localization of blocking antibody to the IgG4 subclass. J Immunol. 1992;148:2731. [PubMed] [Google Scholar]
- 7.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol. 1996;97:1288. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 8.Wohlleben G, Trujillo C, Muller J, Ritze Y, Grunewald S, Tatsch U, Erb KJ. Helminth infection modulates the development of allergen-induced airway inflammation. Int Immunol. 2004;16:585. doi: 10.1093/intimm/dxh062. [DOI] [PubMed] [Google Scholar]
- 9.Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol. 2004;173:6346. doi: 10.4049/jimmunol.173.10.6346. [DOI] [PubMed] [Google Scholar]
- 10.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 11.Oldenhove G, de Heusch M, Urbain-Vansanten G, Urbain J, Maliszewski C, Leo O, Moser M. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J Exp Med. 2003;198:259. doi: 10.1084/jem.20030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 13.Kasuya S, Hamano H, Izumi S. Mackerel-induced urticaria and Anisakis. Lancet. 1990;335:665. doi: 10.1016/0140-6736(90)90455-e. [DOI] [PubMed] [Google Scholar]
- 14.Matheu V, Gracia Bara MT, Rodríguez V, Olalde S, Baeza ML. Shock anafiláctico secundario a rotura espontánea de quiste hidatídico esplénico. Rev Esp Alergol Inmunol Clin. 1997;12:242. [Google Scholar]
- 15.Audicana MT, Fernández de Corres L, Muñoz D, Fernández E, Navarro JA, del Pozo MD. Recurrent anaphylaxis caused by Anisakis simplex parasitizing fish. J Allergy Clin Immunol. 1995;96:558. doi: 10.1016/s0091-6749(95)70301-2. [DOI] [PubMed] [Google Scholar]
- 16.Mendizabal-Basagoiti L. Hypersensitivity to Anisakis simplex: apropos of 36 cases. Allerg Immunol (Paris) 1999;31:15. [PubMed] [Google Scholar]
- 17.Gracia-Bara MT, Matheu V, Zubeldia JM, et al. Anisakis simplex-sensitized patients: should fish be excluded from their diet? Ann Allergy Asthma Immunol. 2001;86:679. doi: 10.1016/S1081-1206(10)62298-3. [DOI] [PubMed] [Google Scholar]
- 18.López Sáez MP, Zubeldia JM, Matheu V, et al. Sensibilización a Anisakis simplex: prevalencia en una consulta de alergia hospitalaria de Madrid. Rev Esp Alergol Inmunol Clin. 1999;14:23. [Google Scholar]
- 19.Montoro A, Perteguer MJ, Chivato T, Laguna R, Cuéllar C. Recidivous acute urticaria caused by Anisakis simplex. Allergy. 1997;52:985. doi: 10.1111/j.1398-9995.1997.tb02418.x. [DOI] [PubMed] [Google Scholar]
- 20.Fernández de Corres L, Audicana M, Del Pozo MD, Muñoz D, Fernández E, Navarro JA, García M, Díez J. Anisakis simplex induces not only anisakiasis. report on 28 cases of allergy caused by this nematode. J Invest Allergol Clin Immunol. 1996;6:315. [PubMed] [Google Scholar]
- 21.Del Pozo MD, Audicana M, Díez JM, et al. Anisakis simplex, a relevant etiologic factor in acute urticaria. Allergy. 1997;52:576. doi: 10.1111/j.1398-9995.1997.tb02603.x. [DOI] [PubMed] [Google Scholar]
- 22.Purello D'Ambrosio F, Pastorello E, Gangemi S, Lombardo G, Ricciardi L, Fogliani O, Merendino RA. Incidence of sensitivity to Anisakis simplex in a risk population of fishermen/fishmongers. Ann Allergy Asthma Immunol. 2000;84:439. doi: 10.1016/S1081-1206(10)62278-8. [DOI] [PubMed] [Google Scholar]
- 23.Caballero ML, Moneo I. Specific IgE determination to Ani s 1, a major allergen from Anisakis simplex, is a useful tool for diagnosis. Ann Allergy Asthma Immunol. 2002;89:74. doi: 10.1016/S1081-1206(10)61914-X. [DOI] [PubMed] [Google Scholar]
- 24.Shimakura K, Miura H, Ikeda K, Ishizaki S, Nagashima Y, Shirai T, Kasuya S, Shiomi K. Purification and molecular cloning of a major allergen from Anisakis simplex. Mol Biochem Parasitol. 2004;135:69. doi: 10.1016/j.molbiopara.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Pérez J, Fernández-Caldas E, Marañón F, Sastre J, Bernal ML, Rodríguez J, Bedate CA. Molecular cloning of paramyosin, a new allergen of Anisakis simplex. Int Arch Allergy Immunol. 2000;123:120. doi: 10.1159/000024442. [DOI] [PubMed] [Google Scholar]
- 26.Moneo I, Caballero ML, González-Muñoz M, Rodríguez-Mahillo AI, Rodríguez-Pérez R, Silva A. Isolation of a heat-resistant allergen from the fish parasite Anisakis simplex. Parasitol Res. 2005;96:285. doi: 10.1007/s00436-005-1362-2. [DOI] [PubMed] [Google Scholar]
- 27.Alonso-Gómez A, Moreno-Ancillo A, López-Serrano MC, Suárez de Parga JM, Daschner A, Caballero MT, Barranco P, Cabañas R. Anisakis simplex only provokes allergic symptoms when the worm parasitises the gastrointestinal tract. Parasitol Res. 2004;93:378. doi: 10.1007/s00436-004-1085-9. [DOI] [PubMed] [Google Scholar]
- 28.Daschner A, Alonso GA, Cabañas R, Suárez de Parga JM, López Serrano MC. Gastroallergic anisakiasis. borderline between food allergy and parasitic disease-clinical and allergologic evaluation of 20 patients with confirmed acute parasitism by Anisakis simplex. J Allergy Clin Immunol. 2000;105:176. doi: 10.1016/s0091-6749(00)90194-5. [DOI] [PubMed] [Google Scholar]
- 29.Sastre J, Lluch-Bernal M, Quirce S, Arrieta I, Lahoz C, Del Amo A, Fernández-Caldas E, Marañón F. A double-blind, placebo-controlled oral challenge study with lyophilized larvae and antigen of the fish parasite, Anisakis simplex. Allergy. 2000;55:560. doi: 10.1034/j.1398-9995.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- 30.Baeza ML, Rodríguez A, Matheu V, et al. Characterization of allergens secreted by Anisakis simplex parasite: clinical relevance in comparison with somatic allergens. Clin Exp Allergy. 2004;34:296. doi: 10.1111/j.1365-2222.2004.01883.x. [DOI] [PubMed] [Google Scholar]
- 31.Audicana MT, Ansótegui IJ, Fernández de Corres LF, Kennedy MW. Anisakis simplex: dangerous – dead and alive? Trends Parasitol. 2002;18:20. doi: 10.1016/s1471-4922(01)02152-3. [DOI] [PubMed] [Google Scholar]
- 32.Cocheton JJ, Cabou I, Lecomte I. Anisakiasis and Anisakis infections. Ann Med Interne (Paris) 1991;142:121. [PubMed] [Google Scholar]
- 33.Sugimachi K, Inokuchi K, Ooiwa T, Fujino T, Ishii Y. Acute gastric anisakiasis. Analysis of 178 cases. J Am Med Assoc. 1985;253:1012. [PubMed] [Google Scholar]
- 34.Sun SZ, Koyama T, Kagei N. Anisakidae larvae found in marine fishes and squids from the Gulf of Tongking, the East China Sea and the Yellow Sea. Jpn J Med Sci Biol. 1991;44:99. doi: 10.7883/yoken1952.44.99. [DOI] [PubMed] [Google Scholar]
- 35.Rodero M, González ML, Esteban MI, Cuéllar C. Estandarización biológica del extracto de Anisakis simplex. Alergol Inmunol Clin. 2000;15:000–000. [Google Scholar]
- 36.Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA. A murine model of IgE-mediated cow's milk hypersensitivity. J Allergy Clin Immunol. 1999;103:206. doi: 10.1016/s0091-6749(99)70492-6. [DOI] [PubMed] [Google Scholar]
- 37.Matricardi PM, Rosmini F, Riondino S, Fortini M, Ferrigno L, Rapicetta M, Bonini S. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. Brit Med J. 2000;320:412. doi: 10.1136/bmj.320.7232.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellinghausen I, Brand U, Steinbrink K, Enk AH, Knop J, Saloga J. Inhibition of human allergic T-cell responses by IL-10-treated dendritic cells: differences from hydrocortisone-treated dendritic cells. J Allergy Clin Immunol. 2001;108:242. doi: 10.1067/mai.2001.117177. [DOI] [PubMed] [Google Scholar]
- 39.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 40.Royer B, Varadaradjalou S, Saas P, Guillosson JJ, Kantelip JP, Arock M. Inhibition of IgE-induced activation of human mast cells by IL-10. Clin Exp Allergy. 2001;31:694. doi: 10.1046/j.1365-2222.2001.01069.x. [DOI] [PubMed] [Google Scholar]
- 41.Bashir ME, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol. 2002;169:3284. doi: 10.4049/jimmunol.169.6.3284. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy MW, Tierney JYeP, McMonagle FA, McIntosh A, McLaughlin D, Smith JW. The secreted and somatic antigens of the third stage larva of Anisakis simplex, and antigenic relationship with Ascaris suum, Ascaris lumbricoides, and Toxocara canis. Mol Biochem Parasitol. 1988;31:35. doi: 10.1016/0166-6851(88)90143-0. [DOI] [PubMed] [Google Scholar]
- 43.Horner AA, Nguyen MD, Ronaghy A, Cinman N, Verbeek S, Raz E. DNA-based vaccination reduces the risk of lethal anaphylactic hypersensitivity in mice. J Allergy Clin Immunol. 2000;106:349. doi: 10.1067/mai.2000.107933. [DOI] [PubMed] [Google Scholar]
- 44.Li XM, Serebrisky D, Lee SY, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 45.Li XM, Zhang TF, Huang CK, Srivastava K, Teper AA, Zhang L, Schofield BH, Sampson HA. Food Allergy Herbal Formula-1 (FAHF-1) blocks peanut-induced anaphylaxis in a murine model. J Allergy Clin Immunol. 2001;108:639. doi: 10.1067/mai.2001.118787. [DOI] [PubMed] [Google Scholar]
- 46.Srivastava KD, Kattan JD, Zou ZM, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115:171. doi: 10.1016/j.jaci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Morafo V, Srivastava K, Huang CK, Kleiner G, Lee SY, Sampson HA, Li AM. Genetic susceptibility to food allergy is linked to differential TH2-TH1 responses in C3H/HeJ and BALB/c mice. J Allergy Clin Immunol. 2003;111:1122. doi: 10.1067/mai.2003.1463. [DOI] [PubMed] [Google Scholar]
- 48.Hajoui O, Janani R, Tulic M, Joubert P, Ronis T, Hamid Q, Zheng H, Mazer BD. Synthesis of IL-13 by human B lymphocytes: regulation and role in IgE production. J Allergy Clin Immunol. 2004;114:657. doi: 10.1016/j.jaci.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 49.del Pozo V, Arrieta I, Tuñón T, et al. Immunopathogenesis of human gastrointestinal infection by Anisakis simplex. J Allergy Clin Immunol. 1999;104:637. doi: 10.1016/s0091-6749(99)70336-2. [DOI] [PubMed] [Google Scholar]
- 50.Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol. 2004;113:395. doi: 10.1016/j.jaci.2003.11.025. [DOI] [PubMed] [Google Scholar]