Abstract
Chlamydia trachomatis infects epithelial cells at the mucosal surface. While in vitro and animal studies have shown changes in mucosal TH1-associated cytokines in the presence of C. trachomatis infection and with its progression to the upper genital tract or clearance, in vivo cytokine responses to chlamydial infection in humans are not well understood. Using a quantitative enzyme-linked immunosorbent assay (ELISA), we examined the endocervical production of two TH1-associated cytokines, i.e. interleukin (IL)-2 and IL-12, in relation to C. trachomatis infection in adolescents. At a randomly selected visit for 396 females, median endocervical IL-2 levels were significantly lower (190 versus 283 pg/ml, P = 0·02) and median IL-12 levels significantly higher (307 versus 132 pg/ml, P < 0·001) in subjects testing positive versus negative for C. trachomatis. These divergent TH1-associated cytokine responses were: (1) confirmed in paired analyses of 96 individuals before and after infection within 6-month intervals, (2) reversible in 97 patients who cleared infection during consecutive visits, (3) not attributable to sociodemographic factors or other genital infections and (4) independent of common genetic variants at the IL2 and IL12B loci associated previously with differential gene expression. From these findings we infer that increased IL-12 and decreased IL-2, observed commonly during mucosal inflammation, are important features of mucosal immune defence against C. trachomatis infection.
Keywords: Chlamydia, cytokines/interleukins, helper T cells
Introduction
Genital Chlamydia trachomatis infection is the most common sexually transmitted bacterial infection, with an estimated 90 million new cases each year around the world [1]. The complications of chlamydial infection are seen disproportionately in women, who may develop pelvic inflammatory disease (PID), followed by ectopic pregnancy, tubal infertility and chronic pelvic pain [2]. While chlamydial infection persists in some individuals, spontaneous clearance of infection has been observed in others without therapy [3], suggesting that adaptive immune responses are often effective in controlling chlamydial infection.
In vitro and animal studies have established that cellular rather than humoral immunity plays the central role in clearing genital chlamydial infection [4,5]. Cellular immune responses in such studies have been shown to be mediated by CD8+ cytotoxic T-lymphocytes (CTLs) and augmented by type 1 CD4+ T helper (TH1) cells (through cytokine production) [6]. Recent work localizing cytokine responses to C. trachomatis infection primarily to the genital tract has made it difficult to correlate mucosal with plasma cytokine levels [7], but endocervical cytokines may have a more direct effect on chlamydial infection given that chlamydial organisms infect epithelial cells of the cervix. In a previous study we found the endocervical concentration of IL-10, a TH2 cytokine, significantly increased after genital chlamydial infection, and then decreased after infection clearance [8]. The concentrations of endocervical IL-10 were also influenced by the genetic background of the host (i.e. polymorphisms in the IL-10 cytokine gene promoter) [8]. Other TH2 cytokines, such as IL-4 and IL-5, may have a minimal effect on chlamydial infection and outcomes [9,10], whereas some TH1-associated cytokines, such as tumour necrosis factor (TNF)-α, can inhibit chlamydia growth in vitro, facilitate earlier eradication of genital chlamydial infection in murine models, and may have higher concentrations in the genital tract of C. trachomatis positive infertile women [11–13].
Most human studies to date have documented the endocervical cytokine profile in Chlamydia-infected patients with upper genital tract infection and associated complications [13,14]. Few studies have dealt with uncomplicated endocervical infection [15], and none have examined cytokine profiles longitudinally during the evolution of chlamydial infection, particularly TH1-associated cytokines, which may play a more critical role in outcomes of chlamydial infection. Here we report our investigation of (1) changes in endocervical IL-2 and IL-12 concentrations in female adolescents before and after acquisition and before and after clearance of chlamydial infection, (2) the relationships between endocervical cytokine levels and other host factors including demographics and the presence of bacterial vaginosis and other sexually transmitted infections and (3) the relationships between endocervical cytokine levels and genetic polymorphisms that may influence cytokine production [16] and/or clinical manifestations of infection [17,18].
Materials and methods
Study population and C. trachomatis detection
This study was conducted as a nested substudy of the 396 females enrolled in the Reaching for Excellence in Adolescent Care and Health (REACH) study [19]. The REACH study, conducted in 15 centres in 13 US cities, enrolled HIV-positive youth predominantly infected through sexual risk behaviours between the ages of 12 and 19 at enrolment as well an HIV-uninfected cohort with a similar behavioural risk profile. This work was approved by institutional review boards at 15 participating institutions in 13 US cities, and informed consent was obtained from all adolescents. Participants were considered to be at high risk of sexually transmitted infections (STIs) through sex and drug use, as judged by the incidence and prevalence of chlamydia, HPV and gonorrhoea infections [20].
Diagnosis of C. trachomatis infection was based on C. trachomatis ligase chain reaction (LCR) testing (Abbott Laboratories, Abbott Park, IL, USA) of cervical swab specimens collected at least once every 6 months from 1996 to 2000 [21]. Test results generated from a central laboratory were returned to clinicians at variable time intervals. All chlamydia-positive individuals were prescribed appropriate anti-chlamydial therapy by their physicians.
Endocervical specimen collection, processing and cytokine quantification
A sample of endocervical secretions was collected every 6 months in a Weck-cel sponge placed around the cervix following speculum insertion, as described previously [22]. Specimen sponges were kept on ice before being frozen at −70°C and shipped to a central laboratory, where they were equilibrated in phosphate-bufered saline (PBS) +0·25 M NaCl with 10% Aprotinin (Sigma) for 30 min at 4°C, and secretions were extracted from each sponge by centrifuging at 16 000 g for 20 min using a spin-x centrifuge filter unit. A dilution factor for the final extract was determined by adjusting the dry weight of sponges and the volume of the extraction buffer used. This dilution factor was used to calculate the final concentration of cytokines when quantitative enzyme-linked immunosorbent assay (ELISA) kits (BioSource International, Camarillo, CA, USA) were used to measure the concentrations of IL-2, IL-10 and IL-12 [23]. The lower detection limits were 5, 0·2 and 0·8 pg/ml for IL-2, IL-10 and IL-12, respectively [21]. The findings on endocervical IL-10 responses to chlamydial infection and the related differential effect from the genetic variants encoding for IL-10 were published elsewhere [8]. Cytokines other than IL-2, IL-10 and IL-12 were not measured in the REACH study. CD4+ T cell counts were completed with basic flow cytometry at each clinical site in laboratories that were certified through the Immunology Quality Assurance Program supported by National Institute of Allergy and Infectious Diseases, as reported previously [24].
DNA extraction and genotyping of cytokine gene variants
Genomic DNA was extracted from 2 × 106 peripheral blood mononuclear cells using the QIAamp Blood Kit (Qiagen, Valencia, CA, USA) [25]. Several single nucleotide polymorphisms (SNPs) at the IL2 and IL12B loci encoding IL-2 and IL-12 subunit p40 (IL-12β), respectively, were typed by PCR with sequence-specific primers, following procedures recommended by the manufacturer (Department of Transplantation Immunology, University of Heidelberg, Heidelberg, Germany) and the 13th International Histocompatibility Workshop (IWHG) [26]. The insertion/deletion variants in IL12B promoter sequence were also analysed; the two alleles (L for long and S for short) were assigned according to apparent fragment sizes differing by 4 base pairs (bp) after polymerase chain reaction (PCR) amplification and denaturing gel electrophoresis on the ALFexpress system (Amersham Pharmacia Biotech, Piscataway, NJ, USA) [27].
Statistical analysis
Statistical analyses of chlamydial infection and cytokine responses were performed initially for a visit selected randomly for each subject. We subclassified the study population further in order to address the longitudinal cytokine responses to chlamydial infection. Group I subjects (n = 281) had at least four visits when cytokine responses were measured. Group II subjects (n = 96) acquired chlamydia infection within two consecutive study visits. Group III subjects (n = 97) cleared chlamydial infection within two consecutive study visits. Because mucosal cytokine concentrations did not follow a normal distribution [28], non-parametric methods, Mann–Whitney U-test or Wilcoxon signed-rank test, were used to compare differences in cytokine profiles by infection status (random visit and Group I) or by time (Groups II and II), respectively. A parametric method, Pearson's χ2 test, was used to assess the dichotomous distribution of demographic characteristics, behavioural factors, human immunodeficiency virus type 1 (HIV-1) infection status, degree of immunosuppression (i.e. CD4+ T cell count), cytokine gene variants (Groups II and III only), bacterial vaginosis and other STIs. Finally, the relationship of the above factors with cytokine profiles was assessed further through a multivariate regression model, with logarithmic transformation of cytokine values.
Results
Characteristics of the study population and subpopulations
The vast majority of the 396 female adolescents were African American, 17 years of age or older, HIV-1 positive, and with a relatively normal immune status (CD4+ T cell count > 400/µl) (Table 1). Compared with Group I patients, a greater proportion of those in Group II (P < 0·05) and Group III (P < 0·01) had CD4+ T cell counts exceeding 400/µl. Group III subjects were slightly younger and had more sexual partners but were otherwise similar to Groups I and II (Table 1).
Table 1. Characteristics of the study population and several subpopulations.
| All females:n(%) | Group Ia: n(%) | Group IIb: n(%) | Group IIIc: n(%) | |
|---|---|---|---|---|
| Race | ||||
| ″African American | 297 (75·0) | 209 (74·4) | 80 (83·3) | 75 (77·3) |
| ″Other | 99 (25·0) | 72 (25·6) | 16 (16·7) | 22 (22·7) |
| Age at enrolment | ||||
| ″< 17 years | 144 (36·4) | 108 (38·4) | 44 (45·8) | 47 (48·5)e* |
| ″≥ 17 years | 252 (63·6) | 173 (61·6) | 52 (54·2) | 50 (51·5) |
| Sexual partners | ||||
| ″< 2 | 297 (75·0) | 210 (74·7) | 70 (72·9) | 63 (64·9)e* |
| ″≥ 2 | 99 (25·0) | 71 (25·3) | 26 (27·1) | 34 (35·1) |
| HIV-1 infection | ||||
| ″Seropositive | 254 (64·1) | 195 (69·4) | 60 (62·5) | 58 (59·8) |
| ″Seronegative | 142 (35·9) | 86 (30·6) | 36 (37·5) | 39 (40·2) |
| Oral contraceptive in past 3 monthsd | ||||
| ″No | 219 (51·6) | 160 (57·8) | 51 (54·3) | 56 (59·0) |
| ″Yes | 168 (43·4) | 117 (42·2) | 43 (45·7) | 39 (41·1) |
| Genital wartsd | ||||
| ″No | 292 (80·2) | 204 (78·2) | 77 (89·5) | 73 (84·9) |
| ″Yes | 72 (19·8) | 57 (21·8) | 9 (10·5) | 13 (15·1) |
| Prior chlamydial infectiond | ||||
| ″No | 232 (63·7) | 157 (60·1) | 49 (57·0) | 47 (54·6) |
| ″Yes | 132 (36·3) | 104 (39·9) | 37 (43·0) | 39 (45·4) |
| Gonorrhoead | ||||
| ″No | 281 (77·2) | 194 (74·3) | 61 (70·9) | 65 (75·6) |
| ″Yes | 83 (22·8) | 67 (25·7) | 25 (29·1) | 21 (24·4) |
| Bacterial vaginosisd | ||||
| ″No | 277 (76·1) | 196 (75·1) | 65 (75·6) | 68 (79·1) |
| ″Yes | 87 (23·9) | 65 (24·9) | 21 (24·4) | 18 (20·9) |
| CD4+ T cells count (cells/µl)d | ||||
| ″< 400 | 83 (21·5) | 58 (21·1) | 10 (10·8)e* | 9 (9·5)e** |
| ″≥ 400 | 304 (78·6) | 217 (78·9) | 83 (89·2) | 86 (90·5) |
Subjects with at least four visits of endocervical cytokine data available (Group I).
Subjects who acquired Chlamydia trachomatis within two consecutive visits (C. trachomatis negative visit followed immediately by C. trachomatis positive visit).
Subjects cleared C. trachomatis infection within two consecutive visits (C. trachomatis positive visit followed immediately by C. trachomatis negative visit).
Few subjects had missing information.
P < 0·05 (*) or P < 0·01 (**) compared with all subjects.
Relationship of endocervical TH1-associated cytokine concentrations to C. trachomatis infection in randomly selected visits and repeated measures
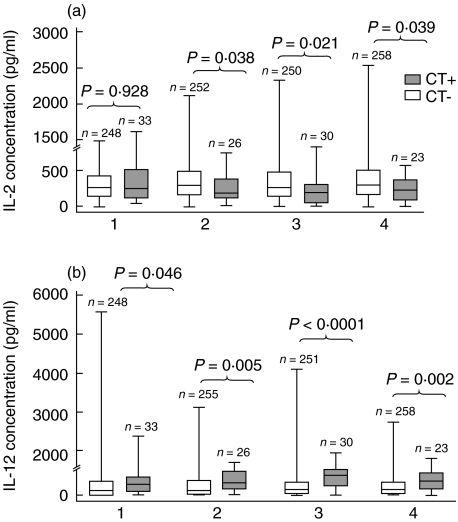
In the 396 subjects randomly selected visits with endocervical cytokine data, the median IL-2 concentration was significantly higher in subjects without (n = 352) versus with (n = 44) chlamydial infection (283 pg/ml versus 190 pg/ml, P = 0·02). The median IL-12 concentration was lower in subjects without versus with chlamydial infection (132 pg/ml versus 307 pg/ml, P = 0·0009). In addition, in subjects with endocervical cytokine data available at least four visits (Group I, n = 281), the median endocervical IL-2 concentrations were significantly higher in chlamydia-negative versus chlamydia-positive adolescents for visits 2–4 (P = 0·04, 0.02 and 0.04, respectively) (Fig. 1a). In contrast, median IL-12 concentrations were significantly lower in chlamydia-negative than chlamydia-positive subjects at all 4 visits (P = 0·05, 0·005, < 0·0001 and .002, respectively) (Fig. 1b).
Fig. 1.
Visits with enzyme-linked immunosorbent assay (ELISA) and tests for chlamydial infection in Group I subjects. Endocervical interleukin (IL)-2 (a) and IL-12 (b) concentrations in adolescent females with at least four visits of data available (Group I, n = 281). Cytokine levels are compared between chlamydia-positive (CT+) and chlamydia-negative (CT−) subjects for each of the four visits available. The interquartile ranges (boxes), medians (bars within boxes) and entire ranges (vertical lines) are shown here. P-values are based on non-parametric Mann–Whitney U-test between patient groups within each visit.
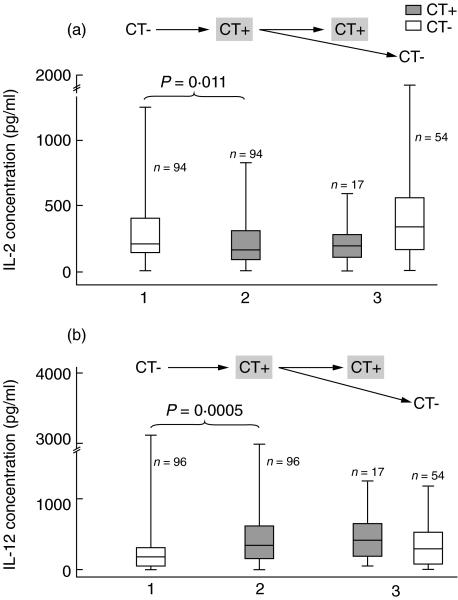
Changes of endocervical TH1-associated cytokine concentrations in subjects acquiring chlamydial infection within two consecutive visits
In 96 subjects (Group II) who acquired chlamydial infection between two consecutive visits, the median IL-2 concentration decreased from 216 pg/ml to 166 pg/ml (P = 0·011). This change in IL-2 was also seen in 17 subjects who were again chlamydia-positive in the next visit (median = 190 pg/ml), whereas clearance of chlamydial infection in the next visit led to median IL-2 concentrations increasing back up to 344 pg/ml in 54 subjects (Fig. 2a).
Fig. 2.
Visits with enzyme-linked immunosorbent assay (ELISA) and tests for chlamydial infection in Group II subjects. Endocervical interleukin (IL)-2 (a) and IL-12 (b) concentrations in response to acquisition of Chlamydia trachomatis (CT) infection, with patients (Group II, n = 96) converting from chlamydia negative (CT−) to chlamydia positive (CT+) within two consecutive visits. Vertical lines correspond to the range of interleukin level (pg/ml) and the interquartile ranges are boxed. Horizontal lines within the boxes represent the median observed values (see text). P-values are based on nonparametric Wilcoxon signed-rank test on changes in cytokine concentration within these two visits.
In response to new chlamydial infection, median endocervical IL-12 levels increased from 181 pg/ml before infection to 339 pg/ml after infection within two consecutive visits (P = 0·0005) (Fig. 2b). When chlamydial infection persisted at the next visit, increased IL-12 (411 pg/ml) was seen again, whereas clearance of chlamydial infection at the next visit led to median IL-12 concentrations decreasing back to 294 pg/ml.
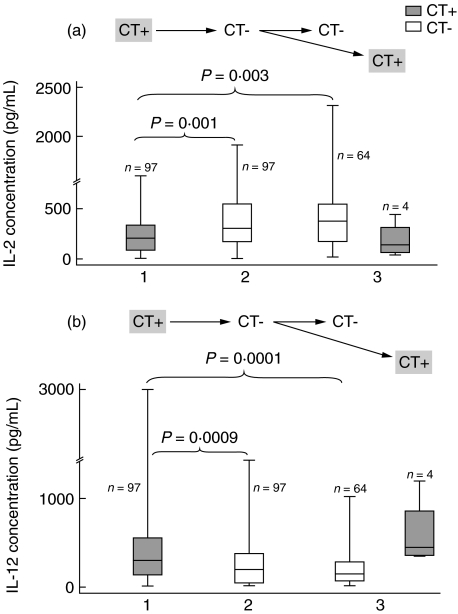
Changes of endocervical TH1-associated cytokine concentrations in subjects clearing chlamydial infection within two consecutive visits
The above patterns of response to chlamydial infection were confirmed in 97 adolescents (Group III) who cleared chlamydial infection within two consecutive visits (Fig. 3). For example, the median IL-2 level measured at the chlamydia-positive visit (208 pg/ml) significantly increased (to 300 pg/ml) after chlamydial clearance (P = 0·001). Higher median IL-2 level persisted in subjects who remained chlamydia-negative at the next visit (381 pg/ml) (P = 0·003) but decreased (to 135 pg/ml) with recurrent C. trachomatis infection (Fig. 3a). For IL-12, the median level (303 pg/ml) at the chlamydia-positive visit declined significantly (to 192 pg/ml) after chlamydial clearance (P = 0·0009). When C. trachomatis infection was noted again at the next visit, median IL-12 increased (to 439 pg/ml); but in subjects who were chlamydia-negative at the next visit, it remained lower (132 pg/ml) (P = 0·0001 compared with baseline level) (Fig. 3b).
Fig. 3.
Visits with enzyme-linked immunosorbent assay (ELISA) and tests for chlamydial infection in Group III subjects. Endocervical interleukin (IL)-2 (a) and IL-12 (b) concentrations in response to clearance of Chlamydia trachomatis infection, with patients (Group III, n = 97) converting from chlamydia positive (CT+) to chlamydia negative (CT−) within two consecutive visits. Illustrated variables and statistics are the same as those shown in Fig. 2.
Relationship of cytokine gene variants to endocervical TH1-associated cytokine concentrations
Our analyses did not reveal any significant association of common variants of IL2 and IL12B genes with endocervical IL-2 and IL-12 concentrations in response to chlamydial infection acquisition (in Group II subjects) or clearance (in Group III subjects).
Confirmation through multivariable analyses of repeated measures
In the multivariable analysis of longitudinal data, presence/absence of C. trachomatis infection was the independent variable in the models; the log10-transformed, repeated measures of endocervical IL-2 and IL-12 in all 396 subjects were dependent variables, and other STIs [gonorrhoea, human papillomavirus (HPV) and HIV-1], bacterial vaginosis, demographic and behavioural factors [age, race, number of sexual partners and oral contraceptive (OCP) use] and immune status (CD4+ T cell count) were covariates. The multivariate model revealed that despite independent associations of OCP use, bacterial vaginosis, gonorrhoea and CD4+ count with one or both endocervical TH1 cytokines analysed, genital chlamydial infection still predicted a significant decrease in IL-2 and increase in IL-12 (P < 0·001 and < 0·0001, respectively) independent of these potential confounding covariates; the slopes (β) for these changes were −0·169 and 0·422, respectively (Table 2).
Table 2. Multivariable analyses on the repeated measures of endocervical interleukin (IL)-2 and IL-12 concentrations in response to Chlamydia trachomatis infectiona.
| IL-2 (n = 1660c) | IL-12 (n = 1680c) | |||
|---|---|---|---|---|
| Covariates | βd | P-value | βd | P-value |
| Race (African American versus other) | 0·057 | 0·071 | 0·024 | –b |
| Age (< 17 versus ≥ 17) | 0·006 | –b | 0·002 | –b |
| Sexual partner (≥ 2 versus < 2) | 0·007 | –b | − 0·043 | –b |
| CD4+ T cell count (< 400 versus ≥ 400 cells/µl) | 0·037 | –b | − 0·008 | –b |
| Oral contraceptive in past 3 months (yes versus no) | 0·102 | 0·0002 | 0·206 | 0·002 |
| HIV-1 infection (yes versus no) | 0·001 | –b | − 0·164 | 0·044 |
| Cervical HPV infection (yes versus no) | 0·019 | –b | 0·026 | –b |
| Bacterial vaginosis (yes versus no) | 0·093 | 0·001 | 0·034 | –b |
| Trichomonas infection (yes versus no) | − 0·018 | –b | − 0·255 | 0·079 |
| Cervical gonorrhoea infection (yes versus no) | − 0·155 | 0·03 | 0·524 | 0·002 |
| Cervical chlamydial infection (yes versus no) | − 0·169 | 0·0002 | 0·422 | < 0·0001 |
The interleukin (IL)-2 and IL-12 concentrations are log10 transformed in the analyses here.
P values = 0·20 are omitted and shown as ‘-’.
Number of measures available.
Slopes of cytokines in response to chlamydial infection.
Discussion
In our comprehensive analyses of longitudinal IL-2 and IL-12 data from a large cohort of high-risk, female adolescents, the rise in IL-12 and fall in IL-2 concentrations in endocervical secretions coincided closely with genital C. trachomatis infection. These changes were remarkably uniform at four levels of analysis: (1) comparison of random individual visits stratified by diagnosis of infection, (2) paired data before and after acquisition of infection, (3) paired data before and after clearance of infection and (4) multivariable analyses of repeated measures with further statistical adjustments for other infections (i.e. gonorrhoea and bacterial vaginosis) and related factors (i.e. OCP use). In addition, there was no evidence that common genetic (heritable) variants at the IL2 and IL12B loci (promoter and 3′ untranslated regions, in particular) accounted for the divergent TH1-associated cytokine production in response to genital chlamydial infection.
The decrement in IL-2 was consistent with previous reports on the inhibition of IL-2 production in cervical secretions during chlamydial cervicitis and in fallopian tube and cervical secretions from patients with infertility attributed to chlamydia infection [13,15]. The increment in IL-12 concentrations in cervical secretions has also been reported in patients with infertility and follicular inflammation [13,29,30]. The external consistency indicates that such TH1-associated cytokine responses are independent of the staging or disease manifestations of genital chlamydial infection. For our study population, chlamydial infection and cytokine production were detected at 6-month intervals; subtle changes within this time-window could not be revealed.
Both IL-2 and IL-12 regulate adaptive immune responses to a wide range of infectious (viral, bacterial and parasitic) agents. More specifically, IL-2 is a T cell growth factor secreted primarily by TH1 lymphocytes and drives the expansion of antigen-specific T cells, B cells and NK cells [31]. IL-12, on the other hand, is primarily produced by activated macrophages and dendritic cells; it further induces interferon (IFN)-γ production to favour the differentiation of TH1 cells [32,33]. IFN-γ is a critical immune regulatory factor, influencing both chlamydial infection acquisition and outcome; it inhibits chlamydial proliferation in vitro, thereby accelerating clearance of chlamydia from epithelial cells [34], and can have a protective effect against chlamydial infection in women [35]. Decrease in IL-2 accompanied by an increase in IL-12 production after genital chlamydial infection may compromise IFN-γ (the TH1 immune effector) secretion and thereby impair cell-mediated immunity in the genital mucosa, as reported in chlamydial cervicitis [13,15,29]. Our earlier work had also revealed that recurrent genital chlamydial infection is associated with increased IL-10 (an anti-inflammatory cytokine) [8], which could impair protective immune responses. However, unlike the endocervical IL-2 and IL-12 responses to genital chlamydial infection, IL-10 responses did vary according to the IL10 promoter genotypes (haplotypes) [8]. Collectively, decreased local T cell response and IFN-γ production may promote latent C. trachomatis infection and inflammation or immunopathology [6]. The potential contribution to these phenomena by other cytokines including IL-4, IL-5 and TNF-α cannot be excluded at this stage of our work.
The specific host cells and chlamydial antigens responsible for differential cytokine responses following genital chlamydial infection remain to be defined. Epithelial cells would be the logical epicentre for the local immune defence against chlamydial infection in the female genital tract [36,37]. Infiltrates of genital mucosal T cells, macrophages and dendritic cells can also dictate cytokine responses. The timing and dynamics of major cytokine responses and their relationships to pathogenesis or development of protective immunity during genital C. trachomatis infection deserve further elucidation.
Acknowledgments
We thank investigators and staff (listed in [38]) of the Adolescent Medicine HIV/AIDS Research Network (1994–2001) and the youth who participated in the REACH project for their valuable contributions to various aspects of this work. We are further indebted to E. Lobashevsky, W. Shao, A. D. Myracle and C. A. Rivers for excellent technical assistance. Dr W. M. Geisler is supported by the Sexually Transmitted Disease Faculty Expansion Program (grant R30 CCR421113) from Centers for Disease Control and Prevention. The Reaching for Excellence in Adolescent Care and Health (REACH) project was supported by grant U01 HD32830 from the National Institute of Child Health and Human Development, with additional funding from the National Institute on Drug Abuse, National Institute of Allergy and Infectious Diseases and National Institute of Mental Health.
References
- 1.Norman J. Epidemiology of female genital Chlamydia trachomatis infections. Best Pract Res Clin Obstet Gynaecol. 2002;16:775–87. doi: 10.1053/beog.2002.0325. [DOI] [PubMed] [Google Scholar]
- 2.McNeeley SG., Jr Pelvic inflammatory disease. Curr Opin Obstet Gynecol. 1992;4:682–6. [PubMed] [Google Scholar]
- 3.Parks KS, Dixon PB, Richey CM, Hook EW. 3rd. Spontaneous clearance of Chlamydia trachomatis infection in untreated patients. Sex Transm Dis. 1997;24:229–35. doi: 10.1097/00007435-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey KH, Soderberg LS, Rank RG. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320–5. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Igietseme JU, Ramsey KH, Magee DM, Williams DM, Kincy TJ, Rank RG. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg Immunol. 1993;5:317–24. [PubMed] [Google Scholar]
- 6.Loomis WP, Starnbach MN. T cell responses to Chlamydia trachomatis. Curr Opin Microbiol. 2002;5:87–91. doi: 10.1016/s1369-5274(02)00291-6. [DOI] [PubMed] [Google Scholar]
- 7.Castle PE, Hildesheim A, Bowman FP, et al. Cervical concentrations of interleukin-10 and interleukin-12 do not correlate with plasma levels. J Clin Immunol. 2002;22:23–7. doi: 10.1023/a:1014252402630. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Tang J, Geisler WM, Crowley-Nowick PA, Wilson CM, Kaslow RA. Human leukocyte antigen and cytokine gene variants as predictors of recurrent Chlamydia trachomatis infection in high-risk adolescents. J Infect Dis. 2005;191:1084–92. doi: 10.1086/428592. [DOI] [PubMed] [Google Scholar]
- 9.Yang X. Role of cytokines in Chlamydia trachomatis protective immunity and immunopathology. Curr Pharm Des. 2003;9:67–73. doi: 10.2174/1381612033392486. [DOI] [PubMed] [Google Scholar]
- 10.Williams DM, Grubbs BG, Pack E, Kelly K, Rank RG. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect Immun. 1997;65:2876–82. doi: 10.1128/iai.65.7.2876-2882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shemer-Avni Y, Wallach D, Sarov I. Inhibition of Chlamydia trachomatis growth by recombinant tumor necrosis factor. Infect Immun. 1988;56:2503–6. doi: 10.1128/iai.56.9.2503-2506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darville T, Andrews CW, Jr, Sikes JD, Fraley PL, Rank RG. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect Immun. 2001;69:3556–61. doi: 10.1128/IAI.69.6.3556-3561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy BS, Rastogi S, Das B, Salhan S, Verma S, Mittal A. Cytokine expression pattern in the genital tract of Chlamydia trachomatis positive infertile women − implication for T cell responses. Clin Exp Immunol. 2004;137:552–8. doi: 10.1111/j.1365-2249.2004.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen CR, Plummer FA, Mugo N, et al. Increased interleukin-10 in the the endocervical secretions of women with non-ulcerative sexually transmitted diseases: a mechanism for enhanced HIV-1 transmission? Aids. 1999;13:327–32. doi: 10.1097/00002030-199902250-00004. [DOI] [PubMed] [Google Scholar]
- 15.Mittal A, Kapur S, Gupta S. Host immune response in chlamydial cervicitis. Br J Biomed Sci. 1996;53:214–20. [PubMed] [Google Scholar]
- 16.Seegers D, Zwiers A, Strober W, Pena AS, Bouma G. A TaqI polymorphism in the 3′UTR of the IL-12 p40 gene correlates with increased IL-12 secretion. Genes Immun. 2002;3:419–23. doi: 10.1038/sj.gene.6363919. [DOI] [PubMed] [Google Scholar]
- 17.Cohen CR, Gichui J, Rukaria R, Sinei SS, Gaur LK, Brunham RC. Immunogenetic correlates for Chlamydia trachomatis-associated tubal infertility. Obstet Gynecol. 2003;101:438–44. doi: 10.1016/s0029-7844(02)03077-6. [DOI] [PubMed] [Google Scholar]
- 18.Kinnunen AH, Surcel HM, Lehtinen M, et al. HLA DQ alleles and interleukin-10 polymorphism associated with Chlamydia trachomatis-related tubal factor infertility: a case–control study. Hum Reprod. 2002;17:2073–8. doi: 10.1093/humrep/17.8.2073. [DOI] [PubMed] [Google Scholar]
- 19.Rogers AS, Futterman DK, Moscicki AB, Wilson CM, Ellenberg J, Vermund SH. The REACH Project of the Adolescent Medicine HIV/AIDS Research Network: design, methods, and selected characteristics of participants. J Adolesc Health. 1998;22:300–11. doi: 10.1016/s1054-139x(97)00279-6. [DOI] [PubMed] [Google Scholar]
- 20.Vermund SH, Wilson CM, Rogers AS, Partlow C, Moscicki AB. Sexually transmitted infections among HIV infected and HIV uninfected high-risk youth in the REACH study. Reaching for Excellence in Adolescent Care and Health. J Adolesc Health. 2001;29:49–56. doi: 10.1016/s1054-139x(01)00296-8. [DOI] [PubMed] [Google Scholar]
- 21.Crowley-Nowick PA, Ellenberg JH, Vermund SH, Douglas SD, Holland CA, Moscicki AB. Cytokine profile in genital tract secretions from female adolescents: impact of human immunodeficiency virus, human papillomavirus, and other sexually transmitted pathogens. J Infect Dis. 2000;181:939–45. doi: 10.1086/315311. [DOI] [PubMed] [Google Scholar]
- 22.Crowley-Nowick PA, Bell MC, Brockwell R, et al. Rectal immunization for induction of specific antibody in the genital tract of women. J Clin Immunol. 1997;17:370–9. doi: 10.1023/a:1027312223474. [DOI] [PubMed] [Google Scholar]
- 23.Hildesheim A, McShane LM, Schiffman M, et al. Cytokine and immunoglobulin concentrations in cervical secretions: reproducibility of the Weck-cel collection instrument and correlates of immune measures. J Immunol Meth. 1999;225:131–43. doi: 10.1016/s0022-1759(99)00037-x. [DOI] [PubMed] [Google Scholar]
- 24.Douglas SD, Rudy B, Muenz L, et al. Peripheral blood mononuclear cell markers in antiretroviral therapy-naive HIV-infected and high risk seronegative adolescents. Adolesc Med HIV/AIDS Res Netw AIDS. 1999;13:1629–35. doi: 10.1097/00002030-199909100-00005. [DOI] [PubMed] [Google Scholar]
- 25.Tang J, Wilson CM, Meleth S, et al. Host genetic profiles predict virological and immunological control of HIV-1 infection in adolescents. AIDS. 2002;16:2275–84. doi: 10.1097/00002030-200211220-00007. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Song W, Lobashevsky E, et al. Cytokine and chemokine gene polymorphisms among ethnically diverse North Americans with HIV-1 infection. J Acquir Immune Defic Syndr. 2004;35:446–54. doi: 10.1097/00126334-200404150-00002. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Tang J, Song W, Lobashevsky E, Wilson CM, Kaslow RA. HLA and cytokine gene polymorphisms are independently associated with responses to hepatitis B vaccination. Hepatology. 2004;39:978–88. doi: 10.1002/hep.20142. [DOI] [PubMed] [Google Scholar]
- 28.Shrier LA, Bowman FP, Lin M, Crowley-Nowick PA. Mucosal immunity of the adolescent female genital tract. J Adolesc Health. 2003;32:183–6. doi: 10.1016/s1054-139x(02)00536-0. [DOI] [PubMed] [Google Scholar]
- 29.Kinnunen A, Surcel HM, Halttunen M, et al. Chlamydia trachomatis heat shock protein-60 induced interferon-gamma and interleukin-10 production in infertile women. Clin Exp Immunol. 2003;131:299–303. doi: 10.1046/j.1365-2249.2003.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bobo L, Novak N, Mkocha H, Vitale S, West S, Quinn TC. Evidence for a predominant proinflammatory conjunctival cytokine response in individuals with trachoma. Infect Immun. 1996;64:3273–9. doi: 10.1128/iai.64.8.3273-3279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–76. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 32.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 33.Heufler C, Koch F, Stanzl U, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–68. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 34.Morrison RP. Differential sensitivities of Chlamydia trachomatis strains to inhibitory effects of gamma interferon. Infect Immun. 2000;68:6038–40. doi: 10.1128/iai.68.10.6038-6040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen CR, Koochesfahani KM, Meier AS, et al. Immunoepidemiologic profile of Chlamydia trachomatis infection: importance of heat-shock protein 60 and interferon-gamma. J Infect Dis. 2005;192:591–9. doi: 10.1086/432070. [DOI] [PubMed] [Google Scholar]
- 36.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen SJ, Eckmann L, Quayle AJ, et al. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.List of investigators and research staff of the Adolescent Medicine HIV/AIDS Research Network. J Adolesc Health. 2001;29(Suppl.):5–6. [Google Scholar]