Abstract
Uncontrolled studies have found intravenous immunoglobulin (IVIG) to be effective in the treatment of pemphigus vulgaris (PV). The aim of this study was to evaluate the role of IVIG in preventing IgG autoantibodies binding to desmoglein-3 and blister formation using a controlled experimental design. The ability of IVIG to affect the binding of IgG affinity purified from two patients with PV to desmoglein-3 in comparison to IgG from one donor, was conducted by enzyme-linked immunosorbent assay (ELISA). The specificity was confirmed by competition assay. We assessed the effect of IVIG on the induction of experimental-PV in CD1 newborn mice by subcutaneous subjection of IgG affinity purified from two patients with PV. The treatment was conducted by subcutaneous administration of IVIG together with IgG from the pemphigus patients or appropriate control. The skin of the newborns was examined 24–48 h later for blisters, and samples of the affected areas were analysed by immunohistochemistry. IVIG as a whole molecule and its F(ab)2 portion inhibited the binding of anti-desmoglein-3 antibody to recombinant desmoglein-3 in a dose-dependent manner. The specificity was confirmed by competition assays. In-vivo, IVIG and its F(ab)2 portion prevented blister formation in the newborn mice. Cutaneous lesions were noted only in the groups of newborn mice who were injected with IgG fractions from the PV patients. Immunopathological evaluation revealed that IVIG prevented the formation of acanthylosis with IgG deposition in the intercellular spaces. These results point to the efficacy of IVIG in the prevention of blister formation in an experimental PV model.
Keywords: autoantibodies, autoimmunity, desmoglein, IVIG, pemphigus
Introduction
Pemphigus is a group of organ-specific autoimmune mucocutaneous disorders with an established immunological basis. Its clinical hallmark is the presence of intraepithelial blisters and erosions of the skin and the mucous membranes. Histologically, IgG autoantibodies directed against adhesion molecules desmoglein-1 and -3 in the affected epithelium cause cell-to-cell detachment of epidermal and mucosal epithelial cells (acantholysis) [1–3]. The goal of therapy is to eliminate these pathogenic autoantibodies [4]. At present, there are no available selective inhibitors of desmoglein autoantibodies, and therapy is therefore based on non-specific immunosuppression.
Pemphigus vulgaris (PV), left untreated, has a natural history of relentless progression, with 50% mortality at 2 years and almost 100% at 5 years [5]. Since the 1950s, substantial progress has been made in the development of immunomodulatory agents to manage organ transplant rejection, autoimmunity and inflammatory disorders. The survival of PV patients improved remarkably with the introduction of corticosteroids and cytotoxic drugs, which have powerful anti-inflammatory and immunomodulatory effects. However, their use is severely limited by immunosuppression, myelosuppression, and numerous side effects.
Intravenous immunoglobulin (IVIG), a blood product prepared from donor serum, is used as a replacement therapy in immunodeficient conditions. Recent studies have revealed an extremely wide spectrum of IVIG antibody activity. Not only does IVIG recognize a large number of bacterial, viral and other infectious agent antigens, it also exhibits anti-idiotypic specificity [6,7]. Commercial IVIG preparations contain multiple anti-idiotypic antibodies, such as anti-factor VIII antibodies [8], anti-DNA autoantibodies [9–11], anti-intrinsic factor antibodies [11], anti-thyroglobulin (Tg) autoantibodies [11], anti-neutrophil cytoplasmic antibodies [12], anti-microsomal antibodies [13], anti-neuroblastoma antibodies [14], anti-phospholipid antibodies [15], anti-platelet antibodies [16], anti-Sm idiotype (ID-434) [17] and anti-GM1 antibody [18]. Therefore, in the last decade, IVIG has been increasingly used as an immunomodulatory agent in the treatment of patients with autoimmune and systemic inflammatory diseases, including systemic lupus erythematosus (SLE), dermatomyositis and polymyositis, multiple sclerosis, myasthenia gravis, Guillain–Barre syndrome, and antiphospholipid syndrome [19,20].
Anti-idiotypic antibodies are effective in the treatment or prevention of disease manifestations because they inhibit the binding of the pathogenic autoantibodies to their corresponding antigen in vitro [10,11,21,22] and in vivo [15,17,23]. Their value may also be attributable to their inhibitory effect on the spontaneous secretion of anti-desmoglein by peripheral B lymphocytes, as was demonstrated in vitro in systemic lupus erythmatosus [24]. Soluble circulating immune complexes may also become aggregated and insoluble following IVIG treatment via the idiotypic network mechanism, thereby increasing their removal by the reticuloendothelial system.
We believe that the idiotypic network is an important mechanism for controlling the immune repertoire, as indicated by mice models of SLE treated with monoclonal anti-idiotypic antibodies [25–29]. In PV, two uncontrolled studies have so far demonstrated that anti-desmoglein antibodies decline significantly during IVIG therapy [30,31]. However, the clinical efficacy of IVIG and the indications for its use in PV remain unclear, and no controlled double-blind or animal studies have been performed. The aim of the present study was to investigate the beneficial effect of IVIG using an in-vitro controlled design.
Methods
Production of recombinant desmoglein-3
The plasmid pVL1393 containing the extracellular domain of desmoglein-3 (Dsg3) was a generous gift from Dr L. Diaz, Medical College of Wisconsin, Milwaukee, WI, USA. The extracellular portion of Dsg3 (ecDsg3) was excised, cloned into the baculovirus expression vector pFastBac1 and transposed into DH10Bac cells allowing the use of the Bac-to-Bac baculovirus expression system (Invitrogen, Carlsbad, CA, USA). This vector was engineered to contain a stretch of six-histidine codons (His-tag) immediately downstream of the inserted Dsg3 extracellular domain. The junction regions and inserts in the vector pFastBac1-HC2-ecDsg3 were sequenced to ensure sequence integrity. The recombinant viral DNA was transfected into SF9 insect cells, virus amplified and the recombinant protein produced in High5 insect cells. The culture medium (200 ml) containing the recombinant ecDsg3 was incubated with 2 ml Ni-NTA agarose (Qiagen, Chatsworth, CA, USA) at 4°C for 2 h with gentle shaking. The protein was eluted with 200 mM imidazole in a 20 mM Tris buffer pH 7·5, 100 mM NaCl. The eluted protein of MW 70 kDa was concentrated by ultracentrifugation on Centricon YM-30 (Amicon, Danvers, MA, USA).
Affinity purification of PV-IgG
Sera from two patients with active PV, diagnosed in accordance with established criteria, and a healthy individual (control) were obtained from the Dermatology Department of Rabin Medical Center in Israel. Total IgG was affinity-purified from plasmapheresis taken at an active stage of the disease. In brief, plasma was loaded on a protein-G sepharose column (Pharmacia Biotech, Norden AB Sollentuna, Sweden) at 4°C. The column was then washed with phosphate buffer pH 7 and the bound antibodies were eluted using glycine-HCl buffer 0·2 M pH 2·7 and neutralized with Tris pH 9. The eluted immunoglobulins were dialysed against phosphate-buffered saline (PBS).
The anti-desmoglein-3 binding of the affinity-purified IgG was detected by enzyme-linked immunosorbent assay (ELISA) and immunoblot. ELISA plates (Maxisorp, Nunclon, Upsala Sweden) were coated with recombinant desmoglein-3 (r.desmoglein-3) 5 µg/ml in PBS, expressed in a baculovirus system, incubated overnight at 4°C. The plates were blocked with 3% bovine serum albumin (BSA) in PBS for 1 h at 37°C. The affinity-purified IgG were subjected to the plates at different concentrations for 2 h at room temperature. The binding was probed with goat-anti-human-IgG conjugated to alkaline phosphatase (Jackson, Research Laboratory Inc. West Grove, PA, USA), followed by the addition of appropriate substrate, P-nitro-phenylphosphate (Sigma Chemical Co., St Louis, MO, USA). The colour reaction was read in a Titertrek ELISA reader (SLT Labinstruments, Salzburg, Austria) at OD 405 nm.
The specificity of IgG obtained from the pemphigus patients (PV-IgG) was confirmed by binding to recombinant extracellular portion of desmoglein-3 (r.desmoglein-3) expressed in a baculovirus system by immunoblots of epidermal membrane proteins separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).
Preparation of F(ab)2 and Fc fragments of IVIG
F(ab)2 and Fc fragments were prepared from commercial IVIG Omrigam (Omrix Biopharmaceuticals™ Ltd, Weizmann Science Park, Nes-Ziona, Israel). IVIG was dialysed against 100 mM Na-acetate buffer, pH-4·0, and digested with pepsin (2% W/W; Sigma) for F(ab)2 or papain for Fc (2% W/W; Sigma) at 37°C 18 h [32]. Any remaining traces of undigested IgG and Fc fragments were removed by binding to a protein-A column (Pharmacia Biotech, Norden AB, Sollentuna, Sweden). The efficiency of the digestion was confirmed by 10% SDS-PAGE.
IVIG inhibition of IgG binding to r.desmoglein-3
PV-IgG, 5 µg/ml, was incubated with competitor IVIG as a whole molecule, F(ab)2 and Fc portions of IVIG or control IgG at different concentrations overnight at 4°C. The mixtures were added to plates coated with r.desmoglein-3, 5 µg/ml in PBS, overnight at 4°C and then blocked with 3% BSA in PBS for 1 h at 37°C. Following overnight incubation at 4°C, the binding was probed with anti-human IgG conjugated to alkaline phosphatase and appropriate substrate. The results were expressed in OD at 405 nm. The percentage of inhibition was calculated as follows:
CD1 mice model of PV and treatment with IVIG
Fifty pregnant female CD1 mice (8 ± 10 weeks old) were purchased from Harlan Laboratory (Jerusalem, Israel). The experimental PV was induced by subcutaneous PV-IgG injection or control IgG, 8 mg/mouse. The treated group received IVIG, or F(ab)2-IVIG, or Fc-IVIG, or control IgG, 5 mg/mouse, or PV-IgG (8 mg) premixed with r.desmoglein-3 (2 mg) overnight at 4°C with shaking. Between 24 and 48 h after treatment, the newborn mice were inspected for the formation of blisters and erosions, and skin sections were cut from lesional and perilesional areas and stored in 4% formalin. Immunoglobulin deposition was analysed by immunohistochemistry.
Microscopic and immunofluorescence studies
The formalin-embedded skin samples were cut into four slices, deparaffinized and blocked with 3% BSA. Anti-human IgG-fluorescein isothicyanate was added to the slides for 2 h at room temperature, and the slides were washed and analysed by confocal fluorescence microscopy.
Results
Inhibition of PV-IgG binding to r.desmoglein-3 by IVIG
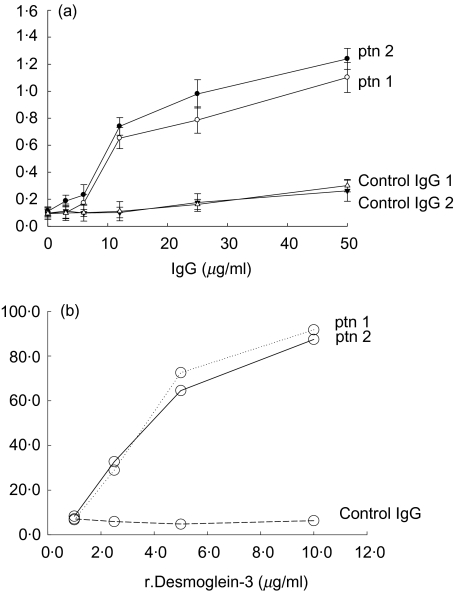
Total IgG was affinity purified on protein-G column from sera derived from two patients with PV at an active stage of the disease. The affinity-purified IgG, named PV-IgG bound r.desmoglein-3 in a dose-dependent manner conducted by ELISA (Fig. 1a). The anti-r.desmoglein-3 binding was specific P < 0·001, when PV-IgG affinity purified from the two patients was compared to the binding of IgG affinity-purified from healthy donors at concentration of 12 µg/ml, Fig. 2a. No binding was detected to irrelevant recombinant protein − SM22α (e.g. anti-SM22α at 12 µg/ml bound 1·472 OD at 405 nm, PV-IgG bound 0·212, 0·179 at OD 405 nm, respectively). The anti-r.desmoglein-3 binding specificity of the purified PV-IgG was confirmed by inhibition assay (Fig. 1b) (P < 0·001 for both patients). IgG from a healthy individual did not bind r.desmoglein-3 (P > 0·05).
Fig. 1.
(a) Direct binding of IgG affinity purified from two pemphigus vulgaris (PV) patients, to recombinant desmoglein-3 (r.desmoglein-3): enzyme-linked immunosorbent assay (ELISA) was employed to detect the binding of PV-IgG to r.desmoglein-3. IgG affinity purified from two healthy donors were used as negative controls. The data are presented in OD at 405 nm, mean ± s.d. of three separate experiments. (b) PV-IgG inhibition of binding to r.desmoglein by r.desmoglein: PV-IgG purified from two PV patients, and control IgG were incubated with different concentrations of r.desmoglein-3. The r.desmoglein-3 unbound PV-IgG was tested by ELISA on r.desmoglein-3 coated plates. The data are presented in OD at 405 nm, mean ± s.d. of three separate experiments.
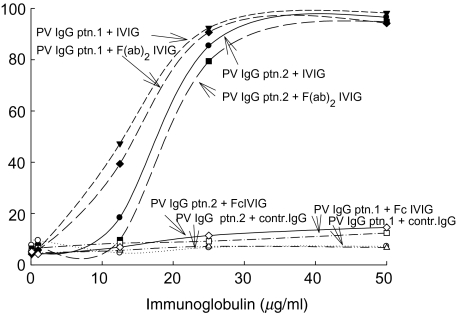
Fig. 2.
Inhibition of pemphigus vulgaris (PV)-IgG binding to recombinant.desmoglein-3 (r.desmoglein-3) by IVIG: inhibition assay demonstrating the neutralization activity of IVIG and its F(ab)2 portion on the PV-IgG binding to r.desmoglein-3. The data are presented in OD at 405 nm, mean ± s.d. of three separate experiments.
IVIG inhibited the binding of PV-IgG to r.desmoglein-3 (Fig. 2). IVIG as a whole molecule and F(ab)2 fraction of IVIG inhibited the binding of PV-IgG from patient 1 at 5 µg/ml (50% binding) by 85·4–92·4%, respectively, at concentration of 25 µg/ml, in comparison to 9·5% inhibition by IgG from one individual (P < 0·002) (Fig. 2). The r.desmogelin-3 binding by PV-IgG derived from patient 2 was inhibited by 79·4–90·7% using whole IVIG or its F(ab)2 derivative, respectively, at a concentration of 25 µg/ml, in comparison to 8·4% inhibition by IgG from one individual (P < 0·002) (Fig. 2). The Fc portion of IVIG did not have any effect on the PV-IgG binding to r.desmogelin-3 (P > 0·05).
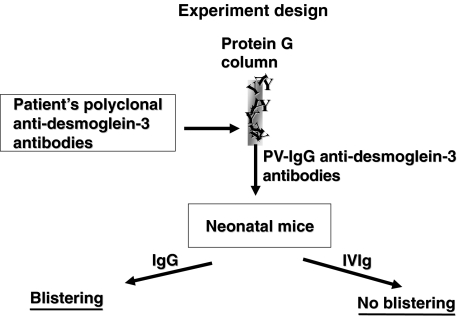
Prevention of blistering and erosions by IVIG (Fig. 3)
Fig. 3.
A flow chart of the experimental design.
As shown in Table 1, cutaneous lesions consisting of blisters and erosions appeared in the newborn mice which were injected with IgG fractions from the two patients (positive findings in seven of 10 tested mice for patient 1, eight of 10 for patient 2). No cutaneous lesions appeared in any of the neonates in the other experimental groups.
Table 1. Incidence of blisters and erosions in newborn mice.
| Source of IgG injected to newborn mice | Treatment of newborn mice | No. of mice with cutaneous lesions |
|---|---|---|
| Patient 1 | IVIG | 0/10 |
| Patient 1 | Normal IgG | 8/10 |
| Patient 1 | F(ab)2 | 3/10 |
| Patient 1 | Fc | 0/10 |
| Patient 2 | IVIG | 0/10 |
| Patient 2 | Normal IgG | 9/10 |
| Patient 2 | F(ab)2 | 4/10 |
| Patient 2 | Fc | 0/10 |
| Patient 1 premixed with r.desmoglein 3 | None | 0/10 |
| Patient 2 premixed with r.desmoglein 3 | None | 0/10 |
| Control IgG | IVIG | 0/10 |
| Control IgG | Normal IgG | 0/10 |
| Control IgG | F(ab)2 | 0/10 |
| Control IgG | Fc | 0/10 |
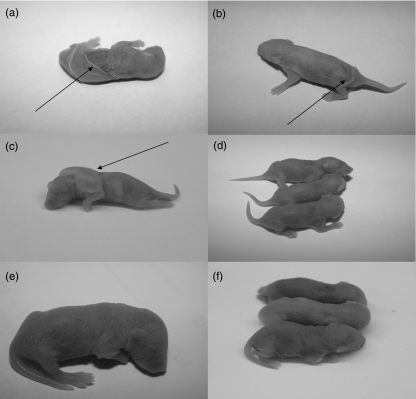
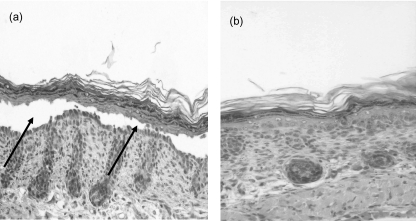
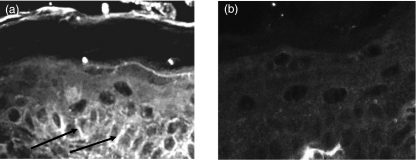
Lesions first appeared 16–48 h after injection. They consisted clinically of either discrete cutaneous vesicles or extensive sloughing of the skin with positive Nikolsky sign (Fig. 4). Histological analysis revealed typical suprabasilar acantholysis (Fig. 5), and immunohistochemistry demonstrated immunoglobulin deposition in the intercellular spaces (Fig. 6). Analyses of skin taken from the other groups of newborn mice did not show any IgG deposition in the intercellular spaces.
Fig. 4.
IVIG effect on the mouse skin in vivo: cutaneous lesions expressed by extensive sloughing of the skin (arrow) induced in CD1 neonatal mice by injection of IgG fractions affinity purified from pemphigus patients (a, b). Blistering (arrow) induced in neonatal mice injected with pemphigus vulgaris PV-IgG and F(ab)2 (c). No lesions in normal serum IgG (d), no lesions in mice injected with PV-IgG and IVIG (e) and in mice injected with PV-IgG and Fc portion of IVIG and PV-IgG premixed with r.desmoglein-3 (f).
Fig. 5.
Histological analysis of the IVIG effect on the mouse skin in vivo: (100×) Histological examination of the affected mice showing subepidermal clefting (arrow) with acantholysis (a) and no clefting in control mice (b).
Fig. 6.
IgG deposition in the pemphigus vulgaris PV-IgG injected mice treated with IVIG: immunohistochemistry slides demonstrating intercellular binding (a) (arrow), and negative staining in control mice (b).
Discussion
This study offers strong clinical and immunopathological evidence that IVIG contain anti-anti-desmogelin-3 activity (anti-desmoglein-3 anti-idiotypes) capable of neutralizing the binding of PV-IgG to Dsg3. Furthermore, IVIG is a useful agent in the prevention of blister formation in PV experimental model in-vivo.
Dsg3 is an adhesion molecule predominantly expressed in the basal layers of the human epidermis, the site of suprabasilar acantholysis in PV [33]. Antibodies reactive with Dsg3 are considered to be a highly specific serological marker for diagnosis. In the individual patient, antibody levels correlate with disease activity, showing a remarkable increase during exacerbations and a drop during remission [34].
An important clue to the pathogenicity of Dsg3 antibodies was provided by the study of Anhalt et al. [1], wherein the passive transfer of IgG from patients with PV to newborn mice resulted in the development of suprabasilar acantholysis. More recent studies using the same experimental model showed that the immunoadsorption of anti-desmoglein-3 IgG from sera of PV patients with r.desmoglein-3 abolished blister formation [2]. Comparison of mouse and human Dsg3 amino acid sequences demonstrated high homology [35]. Furthermore, it has been shown that antibodies against Dsg3 cause acantholysis in vivo in neonatal mice [36].
It is important to emphasize that in PV, the antibodies are those which cause the tissue injury, in the absence of any inflammatory mediators [1,37,38]. Therefore, in order to reduce anti-desmoglein-3 autoantibody synthesis, only agents that are known to suppress antibody production, alter their action, inhibit binding to antigen or encourage antibody catabolism have a rational basis for use. There are only a limited number of drugs that have this effect, and none is restricted to desmoglein autoantibodies. Therefore, therapy for PV is still based on non-specific immunosuppression.
Several uncontrolled clinical studies have demonstrated the efficacy of IVIG in patients with moderate to severe pemphigus disease [39,40]. Furthermore, the influence of IVIG administered to patients with PV was correlated strongly with the clinical status and the reduction of desmoglein-1 and -3 titres [30,31].
The mechanism of action of IVIG is complex, involving modulation of expression and function of Fc receptors, interference with complement activation and the cytokine network, provision of anti-idiotypic antibodies and modulation of T and B cell activation, differentiation and effector functions [7]. Its main mechanism in PV appears to be the manipulation of the idiotypic network by its anti-idiotypic antibodies.
In summary, IVIG has been suggested previously to be a safe and effective agent for the treatment of PV. To the best of our knowledge, this is the first study to confirm these findings in a controlled set-up. Our findings prove that IVIG has a specific inhibitory effect on anti-desmogleins-3, as demonstrated in the competition assay, and that it prevents blister formation in PV-experimental mice models.
References
- 1.Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306:1189–96. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- 2.Amagai M, Hashimoto T, Shimizu N, Nishikawa T. Absorption of pathogenic autoantibodies by the extracellular domain of pemphigus vulgaris antigen (Dsg3) produced by baculovirus. J Clin Invest. 1994;94:59–67. doi: 10.1172/JCI117349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishii K, Amagai M, Hall RP, et al. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010–7. [PubMed] [Google Scholar]
- 4.Mimouni D, Anhalt GJ. Pemphigus. Dermatol Ther. 2002;15:362–8. [Google Scholar]
- 5.Stanley JR. Pemphigus. In: Freedberg IM, Eisen AZ, Wolff K, et al., editors. Fitzpatrick's dermatology in general medicine. 5. New York: McGraw-Hill; 1999. pp. 654–66. [Google Scholar]
- 6.Krause I, Wu R, Sherer Y, Patanik M, Peter J, Shoenfeld Y. In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations − a potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases. Transfus Med. 2002;12:133–9. doi: 10.1046/j.1365-3148.2002.00360.x. [DOI] [PubMed] [Google Scholar]
- 7.Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747–55. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- 8.Sultan Y, Kazatchkine MD, Maissoneuve P, Nydegger UE. Anti-idiotypic suppression of autoantibodies to factor VIII (antihaemophilic factor) by high-dose intravenous gammaglobulin. Lancet. 1984;2:765–8. doi: 10.1016/s0140-6736(84)90701-3. [DOI] [PubMed] [Google Scholar]
- 9.Krause I, Blank M, Shoenfeld Y. Anti-DNA and antiphospholipid antibodies in IVIG preparations: in vivo study in naive mice. J Clin Immunol. 1998;18:52–60. doi: 10.1023/a:1023239904856. [DOI] [PubMed] [Google Scholar]
- 10.Evans MJ, Suenaga R, Abdou NI. Detection and purification of antiidiotypic antibody against anti-DNA in intravenous immune globulin. J Clin Immunopathol. 1991;11:291–5. doi: 10.1007/BF00918187. [DOI] [PubMed] [Google Scholar]
- 11.Rossi F, Kazatchkine MD. Antiidiotypes against autoantibodies in pooled normal human polyspecific IgG. J Immunol. 1989;143:4104–9. [PubMed] [Google Scholar]
- 12.Rossi F, Jayne DR, Lockwood CM, Kazatchkine MD. Anti-idiotypes against anti-neutrophil cytoplasmic antigen autoantibodies in normal human polyspecific IgG for therapeutic use and in the remission sera of patients with systemic vasculitis. Clin Exp Immunol. 1991;83:298–303. doi: 10.1111/j.1365-2249.1991.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tandon N, Jayne DR, McGregor AM, Weetman AP. Analysis of anti-idiotypic antibodies against anti-microsomal antibodies in patients with thyroid autoimmunity. J Autoimmun. 1992;5:557–70. doi: 10.1016/0896-8411(92)90153-h. [DOI] [PubMed] [Google Scholar]
- 14.Lundkvist I, van Doorn PA, Vermeulen M, Brand A. Spontaneous recovery from the Guillain–Barre syndrome is associated with anti-idiotypic antibodies recognizing a cross-reactive idiotype on anti-neuroblastoma cell line antibodies. Clin Immunol Immunopathol. 1993;67:192–8. doi: 10.1006/clin.1993.1064. [DOI] [PubMed] [Google Scholar]
- 15.Caccavo D, Vaccaro F, Ferri GM, Amoroso A, Bonomo L. Anti-idiotypes against antiphospholipid antibodies are present in normal polyspecific immunoglobulins for therapeutic use. J Autoimmun. 1994;7:537–48. doi: 10.1006/jaut.1994.1039. [DOI] [PubMed] [Google Scholar]
- 16.Mehta YS, Badakere SS. In-vitro inhibition of antiplatelet autoantibodies by intravenous immunoglobulins and Rh immunoglobulins. J Postgrad Med. 1996;42:46–9. [PubMed] [Google Scholar]
- 17.DeKeyser F, DeKeyser H, Kazatchkine MD, Rossi F, Dang H, Talal N. Pooled human immunoglobulins contain anti-idiotypes with reactivity against the SLE-associated 4B4 cross-reactive idiotype. Clin Exp Rheumatol. 1996;14:587–91. [PubMed] [Google Scholar]
- 18.Yuki N, Miyagi F. Possible mechanism of intravenous immunoglobulin treatment on anti-GM1 antibody-mediated neuropathies. J Neurol Sci. 1996;139:160–2. [PubMed] [Google Scholar]
- 19.Shoenfeld Y, Krause I. IVIG for autoimmune, fibrosis, and malignant conditions. our experience with 200 patients. J Clin Immunol. 2004;24:107–14. doi: 10.1023/b:joci.0000019809.55787.ec. [DOI] [PubMed] [Google Scholar]
- 20.Levy Y, Sherer Y, Ahmed A, et al. A study of 20 SLE patients with intravenous immunoglobulin − clinical and serological response. Lupus. 1999;8:705–12. doi: 10.1191/096120399678841007. [DOI] [PubMed] [Google Scholar]
- 21.Terryberry JF, Shoenfeld Y, Sherer Y, Levy Y, Fabrizio F, Ahmed A, Peter JB. Detection of antibodies to gangliosides and glycolipids in various intravenous immunoglobulin (IVIg) preparations. Immunol Invest. 2000;29:337–47. doi: 10.3109/08820130009060871. [DOI] [PubMed] [Google Scholar]
- 22.Sherer Y, Wu R, Krause I, Peter JB, Shoenfeld Y. Antiphospholipid antibody levels in intravenous immunoglobulin (IVIG) preparations. Lupus. 2001;10:568–70. doi: 10.1191/096120301701549705. [DOI] [PubMed] [Google Scholar]
- 23.Silvestris F, Cafforio P, Dammacco F. Pathogenic anti-DNA idiotype-reactive IgG in intravenous immunoglobulin preparations. Clin Exp Immunol. 1994;97:19–25. doi: 10.1111/j.1365-2249.1994.tb06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans M, Abdou NI. In vitro modulation of anti-DNA secreting peripheral blood mononuclear cells of lupus patients by anti-idiotypic antibody of pooled human intravenous immune globulin. Lupus. 1993;2:371–5. doi: 10.1177/096120339300200607. [DOI] [PubMed] [Google Scholar]
- 25.Mahana W, Guilbert B, Avrameas S. Suppression of anti-DNA antibody production in MRL mice by treatment with anti-idiotypic antibodies. Clin Exp Immunol. 1987;70:538–45. [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn BH, Ebling FM. Suppression of murine lupus nephritis by administration of an anti-idiotypic antibody to anti-DNA. J Immunol. 1984;132:187–90. [PubMed] [Google Scholar]
- 27.Morland C, Michael J, Adu D, et al. Anti-idiotype and immunosuppressant treatment of murine lupus. Clin Exp Immunol. 1991;83:126–32. doi: 10.1111/j.1365-2249.1991.tb05601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoenfeld Y. The idiotypic network in autoimmunity: antibodies that bind antibodies that bind antibodies. Nat Med. 2004;10:17–18. doi: 10.1038/nm0104-17. [DOI] [PubMed] [Google Scholar]
- 29.Shoenfeld Y, Rauova L, Gilburd B, et al. Efficacy of IVIG affinity-purified anti-double-stranded DNA anti-idiotypic antibodies in the treatment of an experimental murine model of systemic lupus erythematosus. Int Immunol. 2002;14:1303–11. doi: 10.1093/intimm/dxf099. [DOI] [PubMed] [Google Scholar]
- 30.Sami N, Bhol KC, Ahmed RA. Influence of intravenous immunoglobulin therapy on autoantibody titers to desmoglein 3 and desmoglein 1 in pemphigus vulgaris. Eur J Dermatol. 2003;13:377–81. [PubMed] [Google Scholar]
- 31.Herzog S, Schmidt E, Goebeler M, Brocker EB, Zillikens D. Serum levels of autoantibodies to desmoglein 3 in patients with therapy-resistant pemphigus vulgaris successfully treated with adjuvant intravenous immunoglobulins. Acta Derm Venereol. 2004;84:48–52. doi: 10.1080/00015550310005861. [DOI] [PubMed] [Google Scholar]
- 32.Parham P. Preparation and purification of active fragments from mouse monoclonal antibodies. In: Herzenberg L, Blackwell C, Herzenberg L, editors. Handbook of experimental immunology, Cellular immunology. Vol. 2. Oxford: Blackwell Scientific; 1994. p. 14. [Google Scholar]
- 33.Amagai M, Koch PJ, Nishikawa T, Stanley JR. Pemphigus vulgaris antigen (Desmoglein 3) is localized in the lower epidermis, the site of blister formation in patients. J Invest Dermatol. 1996;106:351–5. doi: 10.1111/1523-1747.ep12343081. [DOI] [PubMed] [Google Scholar]
- 34.Cheng SW, Kobayashi M, Tanikawa A, Kinoshita-Kuroda K, Amagai M, Nishikawa T. Monitoring disease activity in pemphigus with enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3. Br J Dermatol. 2002;147:261–5. doi: 10.1046/j.1365-2133.2002.04838.x. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa H, Li K, Tamai K, Sawamura D, Uitto J. Cloning of the mouse desmoglein 3 gene (Dsg3): interspecies conservation within the cadherin superfamily. Exp Dermatol. 2000;9:229–39. doi: 10.1034/j.1600-0625.2000.009004229.x. [DOI] [PubMed] [Google Scholar]
- 36.Amagai M, Nishikawa T, Nousari HC, Anhalt GJ, Hashimoto T. Antibodies against desmoglein 3 (pemphigus vulgaris antigen) are present in sera from patients with paraneoplastic pemphigus and cause acantholysis in vivo in neonatal mice. J Clin Invest. 1998;102:775–82. doi: 10.1172/JCI3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiltz JR, Michel B. Production of epidermal acantholysis in normal human skin in vitro by the IgG fraction from pemphigus serum. J Invest Dermatol. 1976;67:254–60. doi: 10.1111/1523-1747.ep12513454. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto K, Shafran KM, Webber PS, Lazarus GS, Singer KH. Anti-cell surface pemphigus autoantibody stimulates plasminogen activator activity of human epidermal cells. J Exp Med. 1983;157:259–72. doi: 10.1084/jem.157.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sami N, Qureshi A, Ruocco E, Ahmed AR. Corticosteroid-sparing effect of intravenous immunoglobulin therapy in patients with pemphigus vulgaris. Arch Dermatol. 2002;138:1158–62. doi: 10.1001/archderm.138.9.1158. [DOI] [PubMed] [Google Scholar]
- 40.Bystryn JC, Jiao D, Natow S. Treatment of pemphigus with intravenous immunoglobulin. J Am Acad Dermatol. 2002;47:358–63. doi: 10.1067/mjd.2002.122735. [DOI] [PubMed] [Google Scholar]