Abstract
The importance of the innate immune system, including mannose-binding lectin and the complement system, in common variable immunodeficiency is unclear. The objective of this study was to evaluate mannose-binding lectin and the complement system in relation to clinical and immunological parameters in patients with common variable immunodeficiency. Circulating levels of mannose-binding lectin, complement components, complement activation products and functional capacity of complement pathways were correlated to clinical features within 71 patients and compared with 30 healthy controls. The main findings were; the patients had signs of increased complement activation significantly associated with signs of autoimmunity and immunological hyperactivity; there were no signs of deficiencies of the classical and alternative complement pathways in the patient group; the prevalence of lectin pathway deficiency was the same in patients and controls, but patients with increased frequency of lower respiratory tract infections or bronchiectasis had lower capacity of the lectin pathway than patients without these features (P = 0·002 and 0·004, respectively); the serum concentration of mannose-binding lectin was inversely correlated to the frequency of lower respiratory tract infections (P = 0·002) and bronchiectasis (P = 0·01). We conclude that patients with common variable immunodeficiency have no increased frequency of complement deficiencies but signs of increased complement activation. Our findings suggest that mannose-binding lectin and the lectin complement pathway may protect against lower respiratory tract infection and bronhiectasis in patients with common variable immunodeficiency.
Keywords: immune deficiency syndromes, mannose-binding lectin, complement, bronchiectasis, respiratory tract infections
Introduction
Common variable immunodeficiency (CVID) is a heterogeneous syndrome characterized by failure of B cell differentiation and defective immunoglobulin (Ig) production leading to recurrent bacterial infections, particularly in the respiratory tract. Although reduced Ig secretion from B cells is the hallmark of CVID, other immunological abnormalities such as T cell dysfunction and monocyte/macrophage hyperactivity are seen in a considerable proportion of patients. These abnormalities may be of importance for both the B cell deficiency as well as for some of the clinical manifestations in these patients such as increased frequency of autoimmune disorders, granulomatous inflammation and malignant and nonmalignant lymphoid hyperplasia [1].
The complement system, consisting of more than 30 proteins, represents an important component of the innate immune system and plays a central role in the host defence against microbes. However, enhanced complement activation may also induce tissue damage and inflammation. Accordingly, a dysregulated complement system has been associated both with increased susceptibility to infections and autoimmune diseases [2,3].
Mannose-binding lectin (MBL), another important part of innate immunity, is a key component of the lectin pathway of the complement system. Serum levels of MBL are closely correlated to polymorphisms in promotor regions as well as mutations in the MBL gene [4–6]. Some studies have found that children with recurrent sinopulmonary infections have low levels of MBL [7,8], but low MBL levels do not seem to increase mortality or the occurrence of infectious disease in an adult population [9]. On the other hand, altered MBL levels have been associated with persistent inflammation and tissue destruction, potentially contributing to the pathogenesis of some inflammatory disorders [10,11].
Because of the role of the complement system, including the MBL pathway, in the innate immune response, dysregulation of this system may be involved in the pathogenesis of CVID by contributing to both susceptibility to infections and increased frequency of autoimmune and inflammatory manifestations. Indeed, Andersen et al. [12] have recently reported an association between structural mutations of the mbl2 gene and increased frequency of severe respiratory tract infections in CVID, and others have reported an association between low producing MBL alleles and earlier age of disease onset and autoimmunity in CVID [13]. There are some other studies of single complement components in CVID [14,15], but to our knowledge no systematic study of the complement system in CVID has been published. The aim of this study was to evaluate MBL and all three pathways of complement activation in relation to clinical and immunological parameters in a relatively large population of CVID patients.
Methods
Patients and controls
During 2003 and 2004, 71 patients with CVID, according to the criteria of the World Health Organization expert group for primary immunodeficiencies [1], attending the Section of Clinical Immunology and Infectious Diseases, Medical Department, Rikshospitalet University Hospital, Oslo, were consecutively included in the study. The patients did not have any clinically apparent infection when recruited and did not receive prophylactic treatment with antibiotics. Clinical data of the patients are summarized in Table 1. Twenty-six of these patients were randomly selected for longitudinal testing (median observation time 18·5 months, range 9–29 months). Diagnoses of splenomegaly, granulomatous disease and nodular lymphoid hyperplasia were defined as previously described [16]. The diagnosis of bronchiectasis was based on typical findings on high-resolution computed tomography of the thorax [17,18]. Increased frequency of upper and lower respiratory tract infection (RTI) was defined as > 2 episodes of clinically verified infection treated with antibiotics over the last three years. The definition of autoimmune disease included idiopathic thrombocytopenic purpura, autoimmune thyroiditis, haemolytic anaemia and autoimmune colitis. The patient group was matched with a control group of 30 healthy blood donors according to sex (male/female: 40/31 versus 15/15, patients and controls, respectively; P = 0·559) and age (median age (interquartile range): 44 (29–56) years versus 40 (32–49) years, patients and controls, respectively; P = 0·598). Blood samples were taken as previously described [19]. Informed consent to blood sampling was obtained from all subjects. The study was conducted according to the ethical guidelines at our hospital, which comply with the Helsinki declaration, and was approved by the hospital's authorized representative.
Table 1. Clinical features of the CVID patients (n = 71).
| Demographics | |
| ″Gender (Male/Female, %) | 56/44 |
| ″Age (years; median, 25th to 75th percentiles) | 44 (29–56) |
| ″Age at debut (years; median, 25th to 75th percentiles) | 18 (6–35) |
| Clinical symptoms | |
| ″Upper respiratory tract infection (%)* | 65 |
| ″Lower respiratory tract infection (%)* | 54 |
| ″Bronchiectasis (%) | 52 |
| ″Splenomegaly (%) | 42 |
| ″Autoimmune disease (%)† | 41 |
| ″Granulomatous disease (%) | 13 |
| Therapy | |
| ″Age at start of therapy (years, median and 25th to 75th percentiles)‡ | 36 (20–49) |
| ″IVIG or IVIG/SCIG (%) | 32 |
| ″SCIG only (%) | 68 |
> 2 clinically verified upper/lower respiratory infections with use of antibiotics over the last 3 years.
Cases include any history of idiopathic thrombocytopenic purpura, autoimmune thyroiditis, haemolytic anaemia and autoimmune colitis.
Start of therapy defined as start of IVIG/SCIG. Some patients received intramuscular immunoglobulin therapy prior to this. IVIG, Intravenous immunoglobulin therapy; SCIG, Subcutaneous immunoglobulin therapy.
Quantification of individual complement components and C-reactive protein
Serum concentrations of MBL were quantified by enzyme linked immunosorbent assay (ELISA; MBL Oligomer ELISA Kit, Antibodyshop/Statens seruminstitut, Copenhagen, Denmark). Serum concentrations of C3 and C4 were quantified by nephelometry (Behring, Liederbach, Germany). Serum concentrations of C1q and factor B were quantified by radial immunodiffusion (Human complement C1q Bindarid™ Radial Immunodiffusion kit and Human factor B ‘NL’ Bindarid™ Radial Immunodiffusion kit, respectively; The Binding Site Ltd, Birmingham, UK). Serum concentrations of high sensitivity C-reactive protein (hsCRP) were quantified by ELISA as described elsewhere [20], using a polyclonal rabbit antihuman CRP antibody (DakoCytomation, Glostrup, Denmark) as capture antibody and a rabbit antihuman CRP/HRP antibody (DakoCytomation, Glostrup, Denmark) as detection antibody.
Assay of functional capacity of the classical, lectin and alternative pathways in the complement system
The functional capacity of the classical, lectin and alternative pathways in the complement system were quantified by ELISA (Wielisa®– kit: Total Complement System screen Classical, MBL, Alternative Pathways; Wieslab, Lund, Sweden). Classical pathway deficiency was defined as < 40% capacity and lectin and alternative pathway deficiency was defined as < 10% capacity, both with reference to the manufacturer's standard.
Analysis of complement activation products
Plasma concentrations of C3a, C4a and C5a were quantified by flow cytometry (Human Anaphylatoxin Kit: BD™ Cytometric Bead Array; BD Biosciences Pharmingen, San Diego, CA, USA). Plasma concentrations of the terminal SC5b-9 complement complex (TCC) were quantified by ELISA as previously described [21,22] using a specific monoclonal antibody (aE11) reacting with a neoepitope expressed in C9 only when activated and incorporated into TCC as capture antibody, and an anti-C6 antibody to detect the complex.
Statistics
All results were analysed using nonparametric statistics. The Mann–Whitney U-test (two-tailed) was used to determine differences between two groups of individuals. Frequencies were compared using the Chi-square test and, in case of categories with a natural order, the χ2 test for trends. Paired data were analysed using the Wilcoxon signed rank test. Coefficients of correlation were calculated by the Spearman's rank test. Data are given as medians and 25th to 75th percentiles (interquartile range) if not otherwise stated. P-values are two-sided and considered significant when < 0·05.
Results
Levels of the individual complement components, functional capacity of the complement pathways and complement activation products in CVID patients were compared to healthy controls and examined for possible relation to clinical features within the CVID group such as respiratory tract infections, bronchiectasis, splenomegaly and granulomatous disease.
The classical and alternative complement pathways
CVID patients had significantly raised serum levels of C1q, C4, C3 and factor B compared with healthy controls (Table 2). This increase in several complement components in CVID was accompanied by enhanced functional capacity of both the classical and alternative pathway (Table 3) as well as raised plasma levels of C3a, C4a and C5a (Fig. 1). Although there was no increase in TCC (data not shown), these findings suggest some degree of complement activation in CVID with release of biologically potent mediators (i.e. C5a).
Table 2. Serum concentrations of individual complement components and hsCRP among CVID patients (n = 71) and healthy controls (n = 30).
| Complement component | CVID patients | Controls | P-value |
|---|---|---|---|
| C1q (mg/l) | 154 (132–182) | 134 (118–142) | 0·003 |
| MBL (ng/ml) | 1219 (406–5023) | 1042 (304–1697) | 0·224 |
| C4 (g/l) | 0·26 (0·19–0·38) | 0·21 (0·16–0·24) | 0·010 |
| C3 (g/l) | 1·18 (1·00–1·44) | 1·06 (0·89–1·28) | 0·036 |
| Factor B (mg/l) | 410 (300–450) | 329 (283–362) | < 0·001 |
| hsCRP (mg/l) | 3·1 (1·6–7·3) | 1·2 (0·5–2·3) | < 0·001 |
Data are given as median and 25th to 75th percentiles. C3 and C4 were analysed in 53 patients, factor B and C1q in 59 patients and CRP in 50 patients. MBL, Mannose-binding lectin; hsCRP, high sensitivity C-reactive protein.
Table 3. Functional capacity and prevalence of functional deficiency in all three complement pathways among CVID patients (n = 68) and healthy controls (n = 30).
| Levels of functional capacity (%) | Prevalence of functional deficiency (%) | |||||
|---|---|---|---|---|---|---|
| Patients | Controls | P-value | Patients | Controls | P-value | |
| Complement pathway | ||||||
| ″Classical pathway | 120 (96–135) | 85 (79–97) | < 0·001 | 0 | 0 | – |
| ″Lectin pathway | 47 (0·4–124) | 60 (13–96) | 0·703 | 23·3 | 32·4 | 0·367 |
| ″Alternative pathway | 99 (82–116) | 75 (45–111) | 0·005 | 0 | 0 | – |
Classical pathway deficiency defined as < 40% capacity; lectin and alternative pathway deficiency defined as < 10% capacity. Capacity in the alternative pathway was analysed in 64 patients. Data for functional capacity are given as medians and 25th to 75th percentiles.
Fig. 1.
Serum concentrations of (a) C3a, (b) C4a and (c) C5a in CVID patients (n = 60) and controls (n = 30). Horizontal bars represent median values.
These signs of complement activation in vivo were particularly pronounced in CVID patients with autoimmune manifestations or other clinical features of immunological hyperactivity such as splenomegaly or granulomatous disease. Thus, patients with splenomegaly had raised levels of factor B (440 (368–450) mg/l versus 370 (280–423) mg/l, P = 0·01), patients with granulomatous disease had raised levels of factor B (450 (429–450) mg/l versus 382 (288–441) mg/l, P = 0·008), C1q (188 (156–212) mg/l versus 148 (130–176) mg/l, P = 0·025), C3 (1·31 (1.19–1.68) g/l versus 1·17 (0.99–1·38) g/l, P = 0·036) and capacity in the classical complement pathway (135 (128–144)%versus 115 (92–131)%, P = 0·009) and patients with idiopathic thrombocytopenia had raised levels of C3a (190 (158–220) mg/ml versus 145 (122–169) mg/ml, P = 0·009) compared to patients without these manifestations.
In contrast to these signs of enhanced complement activation in vivo, no evidence of complement deficiency in alternative and classical pathways was found in CVID (Table 3).
MBL and the lectin pathway
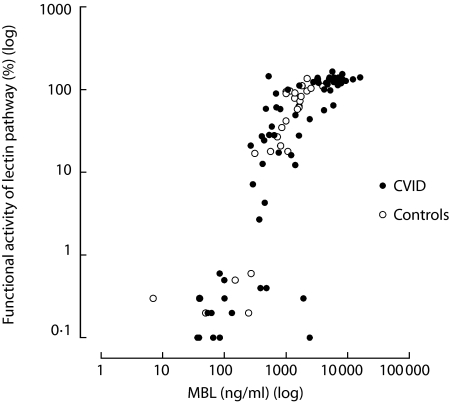
There was a close correlation between serum levels of MBL and the functional capacity of the lectin pathway for both patients (r = 0·841, P < 0·001) and controls (r = 0·907, P < 0·001) (Fig. 2). This correlation was present also when subjects with a lectin pathway deficiency was excluded (r = 0·722, P < 0·001 and r = 0·813, P < 0·001, patients and controls, respectively). The functional capacity of the lectin pathway and the serum concentrations of MBL (Fig. 3), as well as the prevalence of lectin pathway deficiency (Table 3), were not significantly different between CVID patients and controls. However, a significant proportion of both patients and controls had a lectin pathway deficiency, mainly reflecting mutations in the MBL gene affecting MBL synthesis [23]. When subjects with lectin pathway deficiency were excluded, in order to obtain a more reliable impression of MBL synthesis and capacity of the lectin pathway, we found both higher serum concentrations of MBL and higher functional capacity of the lectin pathway among patients than controls (Fig. 3). In fact half of these CVID patients had serum levels of MBL above the maximum range of the control group (4000 ng/ml).
Fig. 2.
Correlation between serum concentrations of MBL and functional activity of the lectin pathway in patients (•, r = 0·841, P < 0·001) and controls (○, r = 0·907, P < 0·001). Both parameters are shown on log scale.
Fig. 3.
Serum concentrations of MBL and functional activity of the lectin pathway in all CVID patients and controls (a, b) and after excluding patients and controls with lectin pathway deficiency (c, d). Serum concentrations of MBL are shown on log scale. Horizontal bars represent median values.
MBL levels and lectin pathway activity in relation to clinical manifestations
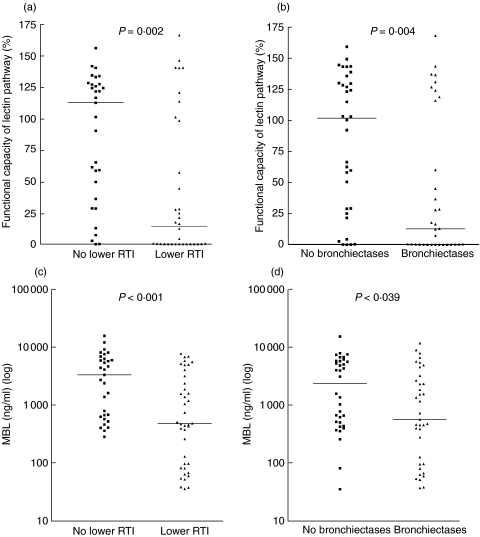
Based on our own data as well as previous studies [24–26] we considered a priori a level of less than 500 ng/ml to be low while a level above the range of the control group (i.e. 4000 ng/ml) was arbitrarily considered to be high. When all the CVID patients were divided into three groups according these predefined criteria (< 500 ng/ml, n = 26; 500–4000 ng/ml, n = 21; > 4000 ng/ml, n = 24), we found a strong association between levels of MBL and occurrence of bronchiectasis and lower RTI with increasing frequencies of those pulmonary complications along with decreasing MBL levels (Fig. 4). Moreover, when isolating patients with very low MBL levels (< 100 ng/ml, n = 10), this trend was even stronger (P = 0·008 and P < 0·001, bronchiectasis and increased frequency of lower RTI, respectively). In line with this, we found significantly lower serum concentrations of MBL and functional capacity of the lectin pathway in patients with lower RTI and bronchiectasis compared with patients without these manifestations (Fig. 5).
Fig. 4.
Frequency of lower respiratory tract infection (RTI) (a) and bronchiectasis (b) in CVID patients according to low (< 500 ng/ml), intermediate (500–4000 ng/ml) or high (> 4000 ng/ml) serum level of MBL.
Fig. 5.
Functional capacity of the lectin pathway and serum concentration of MBL in CVID patients with and without increased frequency of lower respiratory tract infections (RTI) (a, c, respectively) and bronchiectasis (b, d, respectively). Serum concentrations of MBL are shown on log scale. Horizontal bars represent median values.
In contrast, MBL levels and functional capacity of the lectin pathway were not associated with any of the other clinical features in the CVID group including increased frequency of upper RTI and age at debut (data not shown).
CRP levels in CVID
CVID patients had significantly raised serum levels of hsCRP compared with healthy controls further underscoring that inflammation is a characteristic of this group of immunodeficiency (Table 2). Within the CVID group, hsCRP levels correlated significantly to levels of C1q (r = 0·40, P = 0·06), C4 (r = 0·45, P = 0·001), C3 (r = 0·36, P = 0·012) and factor B (r = 0·59, P < 0·001). In contrast there was no correlation between serum levels of hsCRP and MBL within these patients.
Longitudinal testing
There was no significant longitudinal change in levels of complement components or functional capacity of complement pathways among the 26 CVID patients who were followed during the longitudinal testing (median observation time 18·5 months, range 9–29 months). This longitudinal pattern was regardless of clinical manifestations such as bronchiectasis, splenomegaly or autoimmunity and regardless of MBL level at baseline (data not shown).
Discussion
In the present study we show that CVID patients are characterized by some degree of complement activation, which is particularly associated with signs of immunological hyperactivity and autoimmunity. Moreover, although the prevalence of MBL deficiency in CVID was not different from healthy controls, low MBL levels as well as low capacity of complement activation through the lectin pathway, were significantly associated with increased frequency of lower RTI and bronchiectasis within the CVID group.
The number of controls in this study was relatively modest (n = 30). However, the distribution of most of the analysed parameters in the control group was relatively narrow and consistent with historical data from normal populations in our laboratories (Mollnes TE. Unpublished data). Furthermore, most of the observed differences between patients and controls regarding serum concentrations of complement components and split products were highly significant with P-values < 0·01. Although the variation in MBL levels and the functional activity in the lectin pathway within the control group was larger than for the other complement parameters, the values are consistent with observations in larger cohorts using the same laboratory kits as referred to by the manufacturer (MBL) and as published by Seelen et al. [23] (functional activity of the lectin pathway). We therefore consider the number and composition of our control group to be adequate.
There are a few reports, mostly case studies, on complement deficiencies in CVID and the possible contribution of defects in the classical and alternative pathways has been uncertain [14,15]. However, in this first systematic study of complement and CVID we found no significant deficiencies of the classical or alternative pathways in the CVID group compared to healthy controls. On the contrary, the CVID patients had increased functional capacity in both pathways compared to controls, with increased levels of complement split products (i.e. C3a, C4a and C5a). This complement activation, and in particular the release of inflammatory mediators such as C5a, could potentially contribute to inflammatory and autoimmune manifestations characterizing CVID. Indeed, we found an association between high levels of factor B and splenomegaly, between high levels of factor B, C1q and C3 and granulomatous disease and between high levels of C3a and idiopathic thrombocytopenia. Regardless of the possible clinical implications, our findings suggest that enhanced complement activation should be added to the list of features of immunological and inflammatory hyperactivity in CVID.
The present study did not show increased prevalence of low MBL levels or lectin pathway deficiency in CVID patients compared to controls. This is consistent with the finding of Mullighan et al. [13] in a study of 160 CVID patients in the UK. However, individuals with lectin pathway deficiency have a putative abnormal MBL synthesis [23] and this may mask relevant differences in functional capacity of the lectin pathway and serum concentration of MBL between patients and controls with a normal synthesis of the protein [27]. Thus, excluding subjects with lectin pathway deficiency, focusing on patients and controls with a normal MBL synthesis, functional capacity of the lectin pathway and MBL levels were significantly higher among CVID patients than controls. MBL has been considered a weak acute phase reactant, but although CRP levels were also raised among CVID patients, they did not correlate to MBL levels, suggesting at least partly different mechanisms of regulation. Moreover, the patients in the present study had no obvious acute infection at the time of sampling and the stable MBL levels found during longitudinal testing makes an elevation caused by intercurrent acute inflammation improbable. Elevated levels of MBL have also been reported in studies of chronic diseases such as cystic fibrosis [28], HIV infection [29] and rheumatic heart disease [30]. Little is known of factors regulating MBL beyond polymorphisms in the promoter regions and mutations in the MBL gene and the reason for elevated levels of MBL and the possible clinical correlates in these chronic diseases remain largely unexplained.
A major finding in the present study was a strong inverse correlation between MBL levels as well as functional capacity of the lectin pathway and the frequency of lower RTI and bronchiectasis in the CVID group. Although MBL deficiency has been associated both with respiratory infections in children [7,8,31] and a poor prognosis in cystic fibrosis [32,33] no relation to bronchiectasis or lower RTI in otherwise healthy adults has been found [9]. Turner [34] has proposed that pathology arising from MBL deficiency may require one or more coexisting immune deficits and our findings in CVID seem to support this. In the previously mentioned study by Andersen et al. [12], there was an association between structural mutations of the mbl2 gene and increased frequency of severe respiratory tract infections in CVID. In the present study we extend these findings in several ways. While most studies, including that by Andersen et al. [12], have focused on MBL deficiency as defined by the presence of mutations in the MBL gene, we show a more continuous association between serum concentrations of MBL and lower RTI and bronchiectasis. In fact, patients with MBL levels below 500 ng/ml had increased frequency of lower RTI and bronchiectasis, while patients with MBL levels above 4000 ng/ml had reduced frequency of these respiratory complications. Thus, it seems that while low MBL levels may predispose to lower RTI and bronchiectasis in CVID, high MBL levels may protect against these complications in CVID. MBL can bind to a variety of bacteria and other microbes as well as their potentially toxic components [35], neutralizing them and/or opsonizing them by activating complement through the lectin pathway. It is tempting to hypothesize that such mechanisms also could be operating in CVID within the pulmonary microenvironment, giving some protection against the development of respiratory tract complications including bronchiectasis.
There is a close correlation between the different MBL alleles and serum concentrations of MBL, but there is also a wide range of serum concentrations within each allele and a significant overlap of serum concentrations between these alleles [4–6]. A mapping of MBL alleles could potentially help to identify MBL deficient subjects. However, genetic studies will not discriminate between normal MBL allele individuals with intermediate and high serum levels of MBL. The present study suggests that such discrimination may be clinically relevant as the various effects of MBL may be dose-dependent. Our findings indicating a protective effect of particularly high MBL levels may also suggest a therapeutic potential for MBL infusions.
The present study shows no increased frequency of complement deficiencies in CVID. On the contrary there are signs of increased complement activation in these patients. There is an inverse correlation between serum levels of MBL and the occurrence of bronchiectasis and lower RTI in CVID, suggesting that the MBL system may protect against these respiratory tract complications in this immunodeficiency. The pathogenesis of bronchiectasis is incompletely understood, but probably depends both on the occurrence of lower RTI as well as other host factors. In view of the present results, suggesting the importance of the MBL system in CVID, future studies are warranted to clarify if MBL is of importance also in other groups of patients with bronchiectasis.
Acknowledgments
This work was supported by The Research Council of Norway, The Medinnova Foundation, The Research Council of Rikshospitalet, The Norwegian Foundation for Health and Rehabilitation and The Norwegian Plasmafractionation Research Foundation. We thank Merethe Sanna Borgen, Bodil Lunden, Anne Pharo, Azita Rashidi and Gunni Ulvund for excellent technical assistance.
References
- 1.Rosen FS, Eibl MM, Roifman CM, et al. Primary immunodeficiency diseases. Report of an IUIS Scientific Committee. Int Union Immunol Societies Clin Exp Immunol. 1999;118:1–28. doi: 10.1046/j.1365-2249.1999.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 3.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 4.Madsen HO, Garred P, Thiel S, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013–20. [PubMed] [Google Scholar]
- 5.Steffensen R, Thiel S, Varming K, Jersild C, Jensenius JC. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J Immunol Meth. 2000;241:33–42. doi: 10.1016/s0022-1759(00)00198-8. [DOI] [PubMed] [Google Scholar]
- 6.Garred P, Larsen F, Madsen HO, Koch C. Mannose-binding lectin deficiency – revisited. Mol Immunol. 2003;40:73–84. doi: 10.1016/s0161-5890(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 7.Summerfield JA, Sumiya M, Levin M, Turner MW. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. Brit Med J. 1997;314:1229–32. doi: 10.1136/bmj.314.7089.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cedzynski M, Szemraj J, Swierzko AS, et al. Mannan-binding lectin insufficiency in children with recurrent infections of the respiratory system. Clin Exp Immunol. 2004;136:304–11. doi: 10.1111/j.1365-2249.2004.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl M, Tybjaerg-Hansen A, Schnohr P, Nordestgaard BG. A Population-based Study of Morbidity and Mortality in Mannose-binding Lectin Deficiency. J Exp Med. 2004;199:1391–9. doi: 10.1084/jem.20040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner MW. The role of mannose-binding lectin in health and disease. Mol Immunol. 2003;40:423–9. doi: 10.1016/s0161-5890(03)00155-x. [DOI] [PubMed] [Google Scholar]
- 11.Ohlenschlaeger T, Garred P, Madsen HO, Jacobsen S. Mannose-binding lectin variant alleles and the risk of arterial thrombosis in systemic lupus erythematosus. N Engl J Med. 2004;351:260–7. doi: 10.1056/NEJMoa033122. [DOI] [PubMed] [Google Scholar]
- 12.Andersen P, Permin H, Andersen V, et al. Deficiency of somatic hypermutation of the antibody light chain is associated with increased frequency of severe respiratory tract infection in common variable immunodeficiency. Blood. 2005;105:511–7. doi: 10.1182/blood-2003-12-4359. [DOI] [PubMed] [Google Scholar]
- 13.Mullighan CG, Marshall SE, Welsh KI. Mannose binding lectin polymorphisms are associated with early age of disease onset and autoimmunity in common variable immunodeficiency. Scand J Immunol. 2000;51:111–22. doi: 10.1046/j.1365-3083.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- 14.Soto ME, Cordera F, Reyes PA. Congenital C2 (type I) deficiency associated with common variable immunodeficiency. Ann Intern Med. 2000;132:597. doi: 10.7326/0003-4819-132-7-200004040-00030. [DOI] [PubMed] [Google Scholar]
- 15.Sanal O, Yel L, Tezcan I, Ersoy F, Berkel AI. Homozygous C2 deficiency: association with defective alternative pathway function and immunoglobulin deficiency. Int Arch Allergy Immunol. 1996;110:195–8. doi: 10.1159/000237287. [DOI] [PubMed] [Google Scholar]
- 16.Aukrust P, Froland SS, Muller F. Raised serum neopterin levels in patients with primary hypogammaglobulinaemia; correlation to other immunological parameters and to clinical and histological features. Clin Exp Immunol. 1992;89:211–6. doi: 10.1111/j.1365-2249.1992.tb06934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munro NC, Cooke JC, Currie DC, Strickland B, Cole PJ. Comparison of thin section computed tomography with bronchography for identifying bronchiectatic segments in patients with chronic sputum production. Thorax. 1990;45:135–9. doi: 10.1136/thx.45.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsang KW, Tipoe GL. Bronchiectasis: not an orphan disease in the East. Int J Tuberc Lung Dis. 2004;8:691–702. [PubMed] [Google Scholar]
- 19.Aukrust P, Aandahl EM, Skalhegg BS, et al. Increased activation of protein kinase A type I contributes to the T cell deficiency in common variable immunodeficiency. J Immunol. 1999;162:1178–85. [PubMed] [Google Scholar]
- 20.Wu TL, Tsao KC, Chang CP, Li CN, Sun CF, Wu JT. Development of ELISA on microplate for serum C-reactive protein and establishment of age-dependent normal reference range. Clin Chim Acta. 2002;322:163–8. doi: 10.1016/s0009-8981(02)00172-9. [DOI] [PubMed] [Google Scholar]
- 21.Mollnes TE, Lea T, Froland SS, Harboe M. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand J Immunol. 1985;22:197–202. doi: 10.1111/j.1365-3083.1985.tb01871.x. [DOI] [PubMed] [Google Scholar]
- 22.Mollnes TE, Redl H, Hogasen K, et al. Complement activation in septic baboons detected by neoepitope-specific assays for C3b/iC3b/C3c, C5a and the terminal C5b-9 complement complex (TCC) Clin Exp Immunol. 1993;91:295–300. doi: 10.1111/j.1365-2249.1993.tb05898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seelen MA, Roos A, Wieslander J, et al. Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J Immunol Meth. 2005;296:187–98. doi: 10.1016/j.jim.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Kruse C, Rosgaard A, Steffensen R, Varming K, Jensenius JC, Christiansen OB. Low serum level of mannan-binding lectin is a determinant for pregnancy outcome in women with recurrent spontaneous abortion. Am J Obstet Gynecol. 2002;187:1313–20. doi: 10.1067/mob.2002.126846. [DOI] [PubMed] [Google Scholar]
- 25.Peterslund NA, Koch C, Jensenius JC, Thiel S. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637–8. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 26.Neth O, Hann I, Turner MW, Klein NJ. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: a prospective study. Lancet. 2001;358:614–8. doi: 10.1016/S0140-6736(01)05776-2. [DOI] [PubMed] [Google Scholar]
- 27.Dean MM, Minchinton RM, Heatley S, Eisen DP. Mannose Binding Lectin Acute Phase Activity in Patients with Severe Infection. J Clin Immunol. 2005;25:346–52. doi: 10.1007/s10875-005-4702-1. [DOI] [PubMed] [Google Scholar]
- 28.Carlsson M, Sjoholm AG, Eriksson L, et al. Deficiency of the mannan-binding lectin pathway of complement and poor outcome in cystic fibrosis: bacterial colonization may be decisive for a relationship. Clin Exp Immunol. 2005;139:306–13. doi: 10.1111/j.1365-2249.2004.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heggelund L, Mollnes TE, Ueland T, Christophersen B, Aukrust P, Froland SS. Mannose-binding lectin in HIV infection: relation to disease progression and highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;32:354–61. doi: 10.1097/00126334-200304010-00002. [DOI] [PubMed] [Google Scholar]
- 30.Schafranski MD, Stier A, Nisihara R, Messias-Reason IJ. Significantly increased levels of mannose-binding lectin (MBL) in rheumatic heart disease: a beneficial role for MBL deficiency. Clin Exp Immunol. 2004;138:521–5. doi: 10.1111/j.1365-2249.2004.02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch A, Melbye M, Sorensen P, et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. J Am Med Assoc. 2001;285:1316–21. doi: 10.1001/jama.285.10.1316. [DOI] [PubMed] [Google Scholar]
- 32.Garred P, Pressler T, Madsen HO, et al. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Invest. 1999;104:431–7. doi: 10.1172/JCI6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabolde M, Guilloud-Bataille M, Feingold J, Besmond C. Association of variant alleles of mannose binding lectin with severity of pulmonary disease in cystic fibrosis: cohort study. Brit Med J. 1999;319:1166–7. doi: 10.1136/bmj.319.7218.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–40. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 35.Jack DL, Turner MW. Anti-microbial activities of mannose-binding lectin. Biochem Soc Trans. 2003;31:753–7. doi: 10.1042/bst0310753. [DOI] [PubMed] [Google Scholar]