Abstract
The human 60 kDa and microbial 65 kDa heat shock proteins (HSP) have been implicated in the pathogenesis of chronic periodontitis (P) and coronary heart disease (CHD). We have studied four male non-smoking cohorts of 81 subjects, matched for age. Group (a) consisted of a healthy group with minimal gingivitis (n = 18), group (b) were patients with P (n = 23), group (c) patients with CHD and minimal gingivitis (n = 20) and group (d) patients with CHD and P (n = 20). T cells separated from peripheral blood were found to be primed to both microbial HSP65 and human HSP60 but significant CD4, human leucocyte antigen (HLA) class II-restricted proliferative responses were found only with the human HSP60 in patients with P (P < 0·001) and CHD without (P < 0·001) or with (P < 0·00001) periodontitis. Dose-dependent inhibition of T cell proliferative responses was carried out to determine the receptors involved in recognition of HSP60 and HSP65. Monoclonal antibodies to CD14 showed inhibition of T cell proliferation stimulated by both HSP60 and HSP65, consistent with the role of CD14 as a receptor for these HSPs in P and CHD. The toll-like receptor 2 (TLR-) and TLR-4 were then studied and these showed that TLR-4 was recognized by microbial HSP65, whereas TLR-2 was recognised by human HSP60 in both P and CHD. However, a dissociation was found in the HSP60 and TLR4 interaction, as TLR4 appeared to have been recognized by HSP60 in P but not in CHD. The results suggest an autoimmune or cross-reactive CD4+ class II-restricted T cell response to the human HSP60 in P and CHD. Further studies are required to determine if there is a common epitope within HSP60 that stimulates T cell proliferation in P and CHD.
Keywords: coronary heart disease, heat shock protein, peptides, periodontitis
Introduction
Chronic periodontitis (P) is an inflammatory disease characterized by connective tissue destruction and bone resorption. The aetiology of P is associated with a number of bacteria, autoimmunity or microbial cross-reactivity [1]. Although cross-sectional studies suggest an association between P and Porphyromonas gingivalis and Tannerella forsythensis, there are no longitudinal studies showing that P. gingivalis leads necessarily to P. In addition, more recent studies indicate that there are more potential periodontal Gram-positive and Gram-negative pathogens, as well as a significant number of unculturable species [2]. These findings support the hypothesis that the aetiology of P is polymicrobial. Heat shock proteins (HSP) are a group of highly conserved proteins found in eukaryotic and prokaryotic cells, including Gram-positive and Gram-negative microorganisms [3]. The high degree of homology between microbial and human HSP 60 [4] has led to the concept that molecular mimicry between the microbial and self HSP may be involved in the pathogenesis of some autoimmune diseases [5,6] and oral ulceration [7–9]. GroEL-like proteins belonging to the HSP60 family are found in two -major periodontopathic organisms: P. gingivalis [10,11] and Actinobacillus actinomycetemcomitans [12]. HSP60 has also been demonstrated in periodontal tissues, using anti-human HSP60 antibody which is cross-reactive with bacterial GroEL HSP65 [13]. More recently, a significant increase in serum antibodies and T cell proliferative responses to HSP60 has been detected in patients with P [14,15].
Epidemiological reports suggest that periodontitis may be associated with coronary artery disease [16,17]. Although this association has recently been questioned [18], tooth loss as a result of periodontitis is associated with peripheral arterial disease and coronary artery plaques [19]. In humans, both chlamydial and human HSP60 has been found in atheroma lesions and elevated anti-mycobacterial HSP antibodies have been detected in serum [20,21]. Thus, HSP may provide a link between these two diverse diseases, as antibodies to HSP65 have been implicated in the pathogenesis of atherosclerosis [20,22–24] and periodontal disease [25]. There is a wide range of pathogens implicated in atherosclerosis and anti-mycobacterial HSP antibodies can be detected in the sera of patients. Furthermore, in normocholesterolaemic rabbits, atherosclerotic lesions can be induced by immunization with the mycobacterial HSP65 [26,27]. Given the wide range of putative periodontopathogens and the role of mycobacterial HSP in atherosclerosis, we chose to investigate immune responses to human HSP and the mycobacterial HSP, which shows a high degree of homology with both Gram-positive and Gram-negative bacteria and the human [3,4].
The objectives of this investigation were to determine whether lymphocytes from patients with P and coronary heart disease (CHD) are primed to the microbial 65 kDa and/or human 60 kDa HSP, and to study the involvement of CD4 and CD8 T cells, the CD14 receptors and toll-like receptor 2 (TLR2) and TLR4.
Subjects and methods
Patients and controls
All subjects were male and non-smokers. Patients with P (n = 23) without any autoimmune or other systemic disease manifestations were selected from the out-patient clinic of the Department of Periodontology at GKT Dental Institute, London, UK (Table 1). Patients with CHD (n = 40) were selected from the Coronary Care Unit at St Thomas's Hospital. Healthy controls consisted of 18 male subjects broadly matched for age. Ethical committee approval and patient consent was obtained to withdraw 40 ml venous blood from all subjects. The control subjects had minimal gingival inflammation and bleeding on probing (percentage of bleeding sites, mean ± s.d., 1·8 ± 0·8), compared with the diseased groups, with bleeding sites in P (48·9 ± 11·7), coronary heart disease with gingivitis (CHD-G; 1·9 ± 0·7) and CHD-P (45·9 ± 8·7), and had probing depths of 2·2 ± 0·8. The CHD-G group also showed minimal gingival inflammation (1·9 ± 0·7), and probing depths of 2·2 ± 1·1. All CHD-G patients had CHD as determined by greater than 60% diameter stenosis in at least two major epicardial coronary arteries, and conversely only eight of the healthy control subjects were confirmed to be free of CHD based on angiography, the remaining members of the group denying any chest pain symptoms.
Table 1. Number, age, dental data and angiographic status of control subjects, patients with periodontitis and coronary heart disease without and with periodontitis.
| Controls | Periodontitis | Coronary heart disease and gingivitis | Coronary heart disease and periodontitis | |
|---|---|---|---|---|
| Number | n = 18 | n = 23 | n = 20 | n = 20 |
| Age | 44·6 ± 10·4 | 47 ± 9·5 | 55·6 ± 8·5 | 54·8 ± 7·3 |
| number of teeth | 26 ± 5·2 | 25·5 ± 5 | 24 ± 2·9 | 23·8 ± 3·3 |
| Mean percentage of siteswith probing depths of | ||||
| ″≥ 4 mm | 2·0 ± 0·86 | 10·6 ± 20·6 | 2·0 ± 0·86 | 14·6 ± 23·8 |
| ″≥ 5 mm | 0 | 26·0 ± 25·0 | 0 | 28·6 ± 21·3 |
| ″≥ 7 mm | 0 | 3·2 ± 1·8 | 0 | 2·1 ± 2·6 |
| Mean probing depth | 2·2 ± 0·8 | 5·1 ± 1·3 | 2·2 ± 1·1 | 5·8 ± 2·0 |
| Mean percentage of sites with bleeding on probing | 1·8 ± 0·8 | 48·9 ± 11·7 | 1·9 ± 0·7 | 45·9 ± 8·7 |
| Angiogram | 8 confirmed | 9 confirmed | 20/20 | 20/20 |
| negative | negative | positive | positive | |
The chronic periodontitis (P) group had probing depths of 5·1 ± 1·3, compared to 5·8 ± 2·0 in CHD-P (coronary heart disease with P). Although patients with CHD with or without periodontitis were older (mean age 54·8 ± 7·3, and 55·6 ± 8·5, respectively) than the healthy controls (44·6 ± 10·4) and P (47 ± 9·5), this was not statistically significant (P > 0·05).
HSP
Recombinant HSP65 derived from Mycobacterium bovis was prepared at the National Institute of Public Health and Environmental Protection, Bilthoven, the Netherlands and used at a predetermined optimal concentration of 10 µg/ml. Human HSP60 was purchased from Stressgen (Victoria, Canada). The two HSPs were detoxified using Detoxi-gel columns (Pierce, Oxford, UK) and the endotoxin level was determined by Limulus Amoebocyte Lysate assay (Sigma-Aldrich, Poole, Dorset, UK). The concentration of endotoxin was < 0·007 U/µg or 7 pg endotoxin/µg for both HSPs.
Separation of cells
Peripheral blood mononuclear cells (PBMC) were separated from blood by density gradient centrifugation and cultured as described previously [28]. Briefly, 105 cells were cultured in RPMI with or without antigens, including ovalbumin (Sigma, Poole, UK) as an unrelated protein control, in quadruplicate in 96-well round-bottomed plates for 6 days. Enriched monocytes were obtained by incubating the cells on plastic dishes (Falcon Labware, Oxnard, CA, USA) in RPMI-1640 and 10% autologous serum for 1 h at 37°C in 5% CO2. Adherent cells were recovered by washing the Petri dishes with ice-cold Hanks's buffered salt solution (HBSS, × 10 balanced salts) (Sigma). The proportion of CD14+ monocytes in the adherent cell population was determined by flow cytometry using monoclonal antibody (mAb) to CD14 and showed 69–76% of CD14+ cells (ATCC, Rockville, MD, USA). Plastic adherent cells were removed and then reconstituted at 10% with T cell fractions as a source of antigen-presenting cells. The non-adherent cells were separated into T cells and B cells by rosetting with sheep red blood cells (SRBC; Becton Dickinson; Cowley, Oxford, UK) treated with amino-ethylisothiouronium bromide (AET) (Sigma). The rosetted T cells were separated further by panning with a predetermined optimum concentration of monoclonal anti-CD4 culture supernatant fluid [American Type Culture Collection (ATCC), Rockville, MD, USA, ref. LRL8002] in HBSS and 10% fetal calf serum (FCS) for 45 min or overnight at 4°C. After washing, 15–20 × 106 cells in 4 ml HBSS, containing 10% FCS were added to Petri dishes (Falcon Plates, Becton Dickinson) and coated with affinity purified anti-mouse IgG antibody (Tago Inc., Burlingame, CA, USA), at 5 mg/ml in 0·05 M Tris HCL, pH 9·5) for 70 min at 4°C. The adherent cells consisted of a CD4-enriched subset and the non-adherent cells of CD8-enriched subset of cells. The purity of CD4 and CD8 enriched populations was determined by flow cytometry. This showed > 95% CD4 T cells (96·6–98·3%), with 3·2–5·2% CD8 T cells and > 85% CD8 T cells (85–89%, with 4·7–5·1% contaminating CD4 cells). The enriched T cell subsets were reconstituted with 10% monocytes (plastic adherent cells) and stimulated with or without antigen for 6 days under standard conditions. In the final 6 h of culture the cells were pulsed with [3H]-thymidine (0·5 µCi or 18·5 mBq per well; Amersham International, Amersham, UK). The results were assessed by calculating the stimulation index (SI), which is the ratio of antigen-stimulated to antigen-unstimulated cultures [28].
Monoclonal antibodies
Hybridomas W6/32, Genox 3·53, OKT3, OKT4 and OKT8 were obtained from ATCC (Rockville, MD, USA); isotype control immunoglobulins for IgG1a was anti-P. gingivalis (MAb PG3B3; obtained from Dr P. Shepherd and Mr J. Cridland) or anti-rhesus MHC class II (GM11, obtained from Dr Jonker, National Institute of Public Health, Bilthoven, the Netherlands). mAb recognizing microbial and human HSP were obtained from Stressgen (Canada). Culture supernatant fluids obtained using the hybridomas were concentrated, purified and then freeze-dried.
Inhibition experiments
Inhibition of proliferation of PBMC stimulated by human HSP60 or microbial HSP65 was carried out with mAb to CD14, TLR-2 and TLR-4 (eBioscience, Middlesex, UK). The specificity of the HSP-induced lymphoproliferation was also determined using mAb to human HSP60 and microbial HSP65. The mAb were added to the cultures at increasing concentrations of 6·25, 12·5 and 25 µg/ml. Isotype control immunoglobulins for IgG1a were anti-P. gingivalis (mAb PG3B3) or anti-rhesus MHC class II (GM11). The cultures were then incubated in 5% CO2, at 37°C for 6 days, pulsed and harvested as described for the proliferation assays [28].
Flow cytometry
The proportion of CD3, CD4 and CD8 cells was determined by indirect flow cytometry. The cells were incubated for 30 min at 4°C with mAb OKT3, OKT4 and OKT8 obtained from ATCC, washed twice with phosphate buffered saline containing 1% FCS (Sigma) and then incubated with goat anti-mouse IgG labelled with fluorescein isothiocyanate for 30 min at 4°C. After two washes, the cells were fixed in 1% formaldehyde. Controls included cells incubated with a single layer of second antibody alone and cells alone without first or second layer. mAb recognizing leucocyte common antigen (LCA) was used as a positive control. mAb used in the inhibition assays were also tested by flow cytometry. A Coulter Epics profile II (Coulter Electronics Ltd, Luton, UK) was used to analyse the stained cells.
Results
Proliferative responses of PBMCs stimulated with the human HSP60 and mycobacterial HSP65
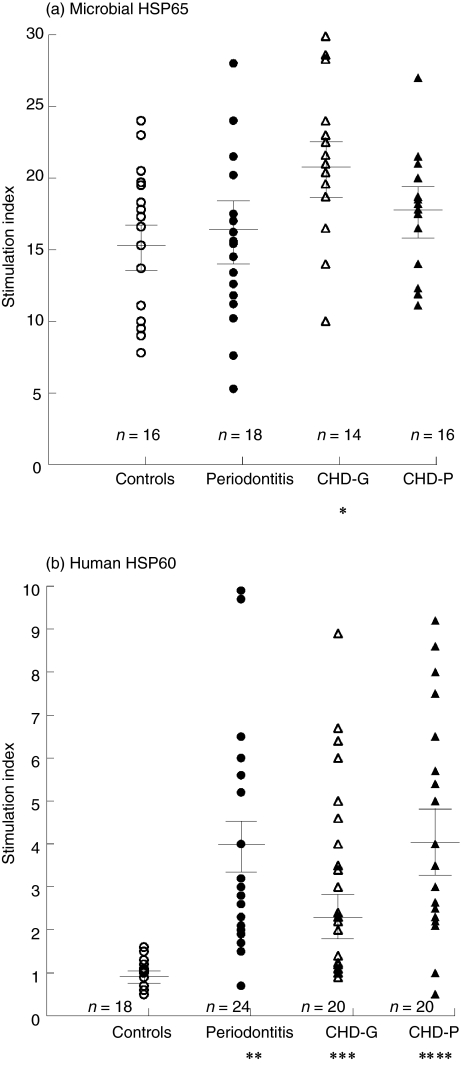
The microbial HSP65 stimulated PBMC in all groups to yield a mean (± s.e.m.) SI of 15·3 ± (1·30) in controls, 16·28 ± (1·58) in patients with P, 21·94 ± (1·73) in CHD-G and 18·69 ± (1·46) in CHD-P (Fig. 1). Analysis of variance (anova) showed a significant difference within these four groups, d.f. = 3, F = 3·75, P = 0·015 and using the unpaired t-test revealed significant increases in SI only when the CHD-G group was compared with the controls (P = 0·008).
Fig. 1.
Lymphoproliferative responses of peripheral blood mononuclear cells (PBMC) stimulated by microbial heat shock protein (HSP)65 (a) and human HSP60 (b) in healthy controls, periodontitis, coronary heart disease (CHD)-G and CHD-P. *P < 0·01; **P < 0·001; ***P < 0·0001; ****P < 0·00001.
The lymphoproliferative responses stimulated by the human HSP60 were lower than those obtained with the microbial HSP65 (Fig. 1). However, the anova test showed more significant differences ( d.f. = 3, F = 7·169, P = 0·0002) within the four groups. The SI was < 2 in all controls (mean SI ± s.e.m., 1·0 ± 0·6) compared with P (3·9 ± 0·6, P = 0·00025), with CHD-G (2·4 ± 0·5, P = 0·0001) and with CHD-P (4·2 ± 0·6, P = 0·00001). There was no statistical difference in SI between patients who had P and were confirmed angiographically negative (n = 9), compared with those with P who did not undergo angiography (n = 11) (P = 0·849). These results suggest that autoreactive T cells recognizing human HSP60 are present in patients with P and those with CHD irrespective of the presence of P (Fig. 1).
An unrelated protein control, ovalbumin, was also used to stimulate PBMC and failed to induce significant lymphoproliferation in any of the four groups (control subjects: SI, 1·1 ± 0·06; P, 1·1 ± 0·05; CHD-G, 1·1 ± 0·06 and CHD-P, 1·1 ± 0·06).
Excluding any activity due to contamination of HSP with lipopolysaccharide (LPS)
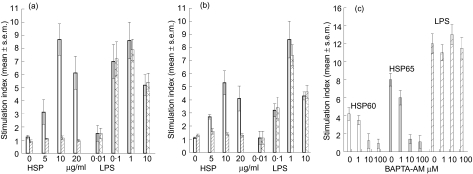
In order to exclude the possibility that any residual LPS might have contributed to the lymphoproliferative responses to HSP, we treated the HSPs with proteinase K and the calcium chelator (BAPTA-AM). Treatment with proteinase K (Fig. 2a, b) or BAPTA-AM (Fig. 2c) abrogated the lymphoproliferative responses stimulated by either microbial HSP65 or human HSP60 but had no effect on LPS (Escherichia coli LPS, L2654; Sigma)-stimulated T cell proliferation, making it unlikely that LPS contamination was responsible for the T cell proliferative responses.
Fig. 2.
T cell proliferative responses to microbial heat shock protein (HSP)65 (a), human HSP60 (b) before (open bars) and after treatment (hatched bars) with proteinase K or (c) BAPTA-AM and compared with lipopolysaccharide (LPS) before (grey bars) and after (hatched bars) treatment with proteinase K.
Proliferative responses of T cells, enriched CD4+ and CD8+ T cells stimulated with the HSPs
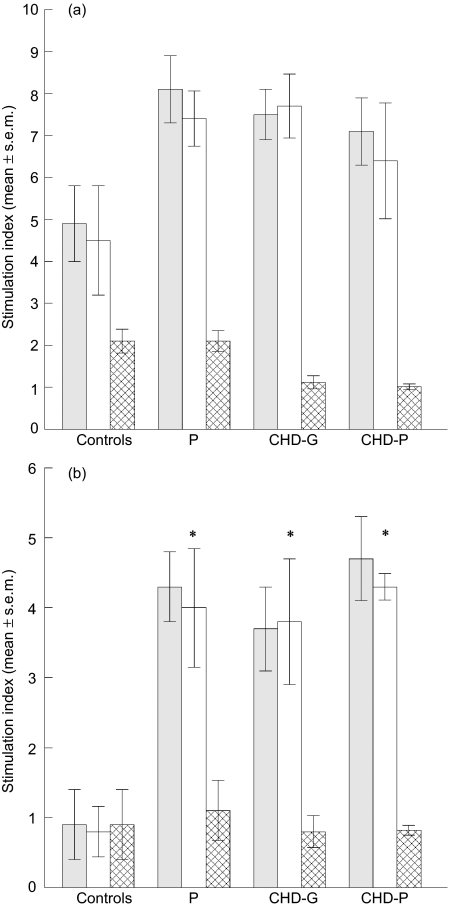
T cells from all subjects responded to the microbial HSP65 (anovaF = 0·061, P = 0·979) (Fig. 3a). However, human HSP60 elicited statistically significant T cell proliferation in all groups compared with the controls (anovaF = 19·643, P = 0·00001) (Fig. 3a).
Fig. 3.
Mean (± s.e.m.) stimulation indices of T cells (grey bars), enriched CD4 (open bars) and CD8 (cross-hatched bars) T cells stimulated with microbial heat shock protein (HSP)65 (a), human HSP60 (b), cells in healthy controls (n = 6), chronic periodontitis (P, n = 6) and coronary heart disease with gingivitis [coronary heart disease (CHD)-G, n = 6] or coronary heart disease with periodontitis (CHD-P, n = 6); *P < 0·001.
Enriched CD4+ and CD8+ T cells from the four groups were reconstituted with 10% enriched monocytes and stimulated with the two HSPs. The HSP65 stimulated only CD4+ T cells from the four groups of subjects (Fig. 3a), and there was no significant difference between the groups (F = 1·831, P = 0·174). In contrast, the human HSP60 stimulated significant CD4+ T cells proliferation, consistent with the findings using unseparated PBMC and T cells (Fig. 3b) (F = 19·94706, P = 0·00001). Further analysis of the individual groups of patients with the control group showed significant increases in T cell proliferation with the CD4+ T cells; P (P < 0·001), CHD-G (P < 0·001) and CHD-P (P < 0·001). There was, however, no significant difference between CHD-G and CHD-P (P = 0·691), or P and CHD-P (P = 0·915).
Inhibition assays
Inhibition of HSP induced proliferation by mAb to HLA classes I and II to study HLA restriction
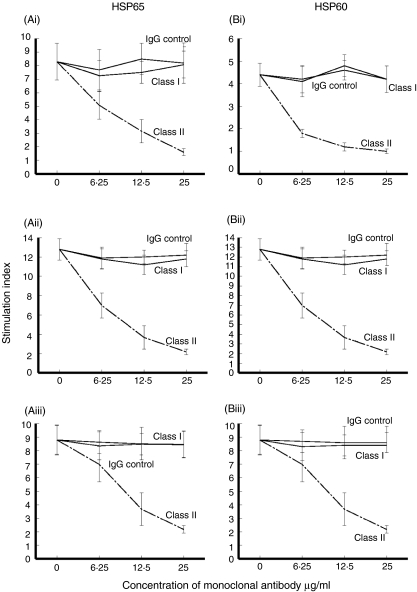
HLA restriction of the T cell proliferative responses was studied by dose-dependent inhibition with mAb to MHC classes I and II and the corresponding isotype controls in the three groups of patients (Fig. 4). In all groups, the T cell proliferative responses stimulated by HSP65 (Fig. 4a) or HSP60 (Fig. 4b) was inhibited to SI < 2·5 in a dose-dependent manner with mAb to MHC class II (n = 5), but not with mAb to MHC class I or the corresponding isotype controls (Fig. 4).
Fig. 4.
Inhibition of T cell proliferation with monoclonal antibody (mAb) to human leucocyte antigen (HLA) class I, HLA class II or isotype controls of peripheral blood mononuclear cells (PBMC) stimulated with microbial heat shock protein (HSP)65 (a), human HSP60 (b) in five patients each with periodontitis (i), coronary heart disease (CHD)-G (ii) and CHD-P (iii).
Investigation of HSP receptors by using mAb to CD14, TLR-2 and TLR-4
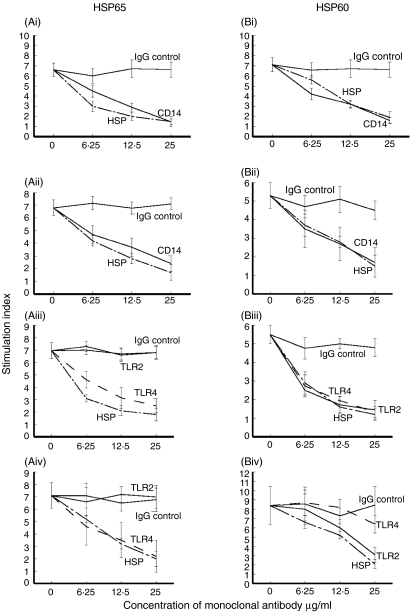
mAb recognizing CD14 inhibited T cell proliferative responses stimulated by microbial HSP65 or human HSP60 in all groups of patients, confirming that CD14 is a receptor for both HSPs. HSP65-induced proliferation was inhibited by anti-TLR4 but not by anti-TLR2 antibodies in periodontitis (n = 7; Fig. 5Ai) and CHD group (n = 7; Fig. 5Aii) with periodontitis (n = 4) or without periodontitis (n = 3). However, whereas HSP60 stimulated T cell proliferation showed inhibition with both mAb to TLR2 and TLR4 in periodontitis, only mAb to TLR-2 inhibited lymphoproliferation in patients with CHD (Fig. 5).
Fig. 5.
Inhibition T cell proliferation with monoclonal antibody (mAb) to CD14 of peripheral blood mononuclear cells (PBMC) stimulated with microbial heat shock protein (HSP)65 (a), human HSP60 (b), in patients each with periodontitis (i), coronary heart disease (CHD)-G (ii) and using monoclonal antibody (mAb) recognizing Toll-like receptor (TLR)2, TLR4 or isotype controls in periodontitis (iii) and CHD (iv); mean ± s.e.m. of seven patients in each group. mAb to HSP65 and HSP60 were also added to the cultures to confirm the antigen-specificity of this lymphoproliferation.
In order to confirm the antigen specificity of these responses, mAb to HSP65 and HSP60 were added to the HSP65- and HSP60-stimulated cultures, respectively. Lymphoproliferation was inhibited in a dose-dependent manner (Fig. 5).
Discussion
Specific T cell proliferation was stimulated with the human HSP60 in patients with P, and those without or with CHD. Some patients with CHD had myocardial infarction 2–5 years previously, and comparison of these with patients without previous myocardial infarction showed no differences in T cell proliferative responses. This is important as myocardial damage may release HSP into the surrounding tissues and induce immune responses [29].
The results of the T cell proliferative studies support enhanced humoral immune responses to HSP reported in P [14] and CHD [22,30]. However, reduced T cell responses to human and microbial HSP60/65 were reported in patients with periodontitis [31]. This is in contrast to the findings by Yamazaki et al. [15], that a significant increase is found in human HSP60-specific T cell responses in peripheral blood as well as cells eluted from periodontal lesions. The T cells, however, did not respond to P. gingivalis HSP. P. gingivalis is just one of many potential periodontopathogens which may be involved in the pathogenesis of P. Recent studies suggest that there are at least twice as many unculturable bacteria, whose roles in P are yet to be determined [2]. Mycobacterial HSP has been shown not only to induce and modulate atherosclerosis in animal models but also has a high degree of homology between Gram-positive and Gram-negative bacteria [3, 26, 27] For these reasons we sought to investigate mycobacterial HSP, which has been demonstrated previously to be a common factor in atherosclerosis and P. Yamazaki et al. have used HSP from P. gingivalis and found no skewing of the cytokine responses to Th1 or Th2, whereas the human HSP did elicit high levels of interferon-gamma [15].
None the less, the results are consistent with a HSP60/65 cross-reactive pathogenesis between human and microbial HSP. Any cross-reactive epitope remains to be mapped. It is noteworthy that cross-reactivity between HSP65 and human oral mucosa was demonstrated [32]. The T cell proliferative responses to microbial HSP65 in all four groups were rather high (mean SI > 15), so it is unlikely that an increase in proliferation from 15·3 ± 1·30 in controls to 21·94 ± (1·73) in CHD-G is of pathogenic significance, although this difference was statistically significant.
The T cell subset was then determined with mAb to CD4 and CD8, which showed clearly that only the CD4 subset of T cells responds to stimulation with both the microbial 65 kDa and human HSP60. Inhibition studies with monoclonal antibodies to HLA classes I and II antigens showed that anti-HLA class II antibodies inhibited HSP-induced T cell proliferation in a dose-dependent way in all responding groups, and is consistent with class II restriction of the HSP65/HSP60 responses [15,33]. Altogether these results confirmed that CD4+ HLA class II-restricted T cells respond to HSP60 and HSP65.
It should to be emphasized that in this investigation we excluded smokers, as smoking is a well-known risk factor in periodontitis as well as in CHD [34]. We have also excluded non-Caucasian subjects. The possibility that contamination of the HSP preparation with LPS may affect the immune response was excluded by means of polymixin and the detoxified HSP showed < 0·007 U LPS/pg of HSP. In the proliferation assays, the lowest concentration of LPS used was 0·01 µg/ml (that is more than 100× greater than the LPS contaminating the detoxified HSPs), and yet this concentration failed to induce significant T cell proliferation. Treatment of the HSPs with proteinase K showed that T cell proliferation was abrogated, unlike similar treatment of LPS. The calcium chelating agent (BAPTA-AM) was then used, as HSP responses are calcium-dependent, and this resulted in inhibition of the HSP-stimulated response but not that stimulated by LPS [35,36].
The CD14, TLR2 and TLR4 receptors were then explored as these have been implicated in interactions with HSPs. Antibodies to CD14 inhibited both human HSP60 and mycobacterial HSP65-stimulated cultures, confirming that CD14 is a receptor for the HSP, as has been reported previously [37]. CD14 is also a high-affinity receptor for bacterial LPS sharing signalling pathways with HSP [38–40]. Further inhibition studies showed that T cells stimulated with microbial HSP65 are inhibited with anti-TLR4 in patients with P and CHD with or without P. This is consistent with other reports that TLR4 is a receptor for HSP65 [41–43]. However, treatment with anti-TLR4 of the human HSP60-stimulated T cells showed a dose-dependent inhibition in patients with P but not those with CHD. This discrepancy is difficult, at present, to account for, as TLR4 has been reported to mediate human HSP60 signalling [43]. Treatment with anti-TLR-2 of the human HSP60-stimulated cultures showed dose-dependent inhibition of T cell proliferation in both P and CHD, suggesting that TLR-2 is another receptor for human HSP60. Thus, the discrepancy in inhibition with anti-TLR-4 raises the possibility that there is a difference in TLR function in periodontitis and CHD and this point requires further studies.
There is evidence that both TLR-2 and TLR-4 might be involved in the recognition of human and microbial HSP. Thus, whereas a HSP-unresponsive human fibroblast cell line transfected with TLR-4 alone was not sufficient to confer HSP responsiveness, TLR-4 with accessory molecule MD-2 conferred responsiveness to both human and chlamydial HSP60 [44]. TLR-2 and TLR-4 are influenced by other TLR which are able to form heterocomplexes [45]. TLR-4 has been detected in macrophages and endothelial cells of atherosclerotic plaques [41, 42, 46]. Indeed, TLR-4 may function as a receptor in both infection and arterial injury to induce inflammation in atherosclerosis, as TLR4 can also bind LPS and fibronectin [42]. Our results suggest that human HSP60 is recognized by TLR-2 and TLR-4 in P and only TLR-2 in CHD. It is possible that TLR-2 forms heterocomplexes with TLR-4 or other TLRs so as to enhance HSP recognition.
As both P and CHD exhibit significant increases in CD4+ HLA-restricted T cell responses to human HSP60, the possibility needs to be explored that they may share a common immune response to HSP60, elicited either by a cross-reacting response to homologous epitopes within microbial HSP65 [4] or through the release of constitutive HSP60 from inflammatory cells as a result of tissue damage [15,47]. The important question of whether P may contribute to the pathogenesis of CHD cannot be excluded in view of the circulating CD4+ T cells primed to HSP60 in P and CHD. Further studies are required to determine the stimulatory epitopes within HSP60, as has been demonstrated in recurrent aphthous stomatitis [7] and Behçet's disease [28].
Acknowledgments
This project was supported by a British Society of Periodontology Fellowship.
References
- 1.Listgarten MA, Loomer PM. Microbial identification in the management of periodontal diseases. A systematic review. Ann Periodontol. 2003;8:182–92. doi: 10.1902/annals.2003.8.1.182. [DOI] [PubMed] [Google Scholar]
- 2.Kumar P, Griffin A, Barton J, Paster B, Moeschberger M, Leys E. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;82(5):338–44. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- 3.Thole JE, Hindersson P, de Bruyn J, et al. Antigenic relatedness of a strongly immunogenic 65 kDA mycobacterial protein antigen with a similarly sized ubiquitous bacterial common antigen. Microb Pathogen. 1988;4:71–83. doi: 10.1016/0882-4010(88)90049-6. [DOI] [PubMed] [Google Scholar]
- 4.Jindal S, Dudani AK, Singh B, Harley CB, Gupta RS. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989;9:2279–83. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feige U, van Eden W. Infection, autoimmunity and autoimmune disease. EXS. 1996;77:359–73. doi: 10.1007/978-3-0348-9088-5_24. [DOI] [PubMed] [Google Scholar]
- 6.van Eden W, Koets A, van Kooten P, Prakken B, van der Zee R. Immunopotentiating heat shock proteins: negotiators between innate danger and control of autoimmunity. Vaccine. 2003;21:897–901. doi: 10.1016/s0264-410x(02)00538-8. [DOI] [PubMed] [Google Scholar]
- 7.Hasan A, Childerstone A, Pervin K, et al. Recognition of a unique peptide epitope of the mycobacterial and human heat shock protein 65–60 antigen by T cells of patients with recurrent oral ulcers. Clin Exp Immunol. 1995;99:392–7. doi: 10.1111/j.1365-2249.1995.tb05563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasan A, Fortune F, Wilson A, et al. Role of gamma delta T cells in pathogenesis and diagnosis of Behçet's disease. Lancet. 1996;347:789–94. doi: 10.1016/s0140-6736(96)90868-5. [DOI] [PubMed] [Google Scholar]
- 9.Hasan A, Shinnick T, Mizushima Y, van der Zee R, Lehner T. Defining a T-cell epitope within HSP 65 in recurrent aphthous stomatitis. Clin Exp Immunol. 2002;128:318–25. doi: 10.1046/j.1365-2249.2002.01757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotokezaka H, Hayashida H, Ohara N, Nomaguchi H, Kobayashi K, Yamada T. Cloning and sequencing of the GroESL homologue from Porphyromonas gingivalis. Biochim Biophys Acta. 1994;1219:175–8. doi: 10.1016/0167-4781(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 11.Maeda H, Miyamoto M, Hongyo H, Nagai A, Kurihara H, Murayama Y. Heat shock protein 60 (GroEL) from Porphyromonas gingivalis: molecular cloning and sequence analysis of its gene and purification of the recombinant protein. FEMS Microbiol Lett. 1994;119:129–35. doi: 10.1111/j.1574-6968.1994.tb06879.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakano Y, Inai Y, Yamashita Y, et al. Molecular and immunological characterization of a 64-kDa protein of Actinobacillus actinomycetemcomitans. Oral Microbiol Immunol. 1995;10:151–9. doi: 10.1111/j.1399-302x.1995.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 13.Ando T, Kato T, Ishihara K, Ogiuchi H, Okuda K. Heat shock proteins in the human periodontal disease process. Microbiol Immunol. 1995;39:321–7. doi: 10.1111/j.1348-0421.1995.tb02208.x. [DOI] [PubMed] [Google Scholar]
- 14.Tabeta K, Yamazaki K, Hotokezaka H, Yoshie H, Hara K. Elevated humoral immune response to heat shock protein 60 (hsp60) family in periodontitis patients. Clin Exp Immunol. 2000;120:285–93. doi: 10.1046/j.1365-2249.2000.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamazaki K, Ohsawa Y, Tabeta K, et al. Accumulation of human heat shock protein 60-reactive T cells in the gingival tissues of periodontitis patients. Infect Immun. 2002;70:2492–501. doi: 10.1128/IAI.70.5.2492-2501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. BMJ. 1993;306:688–91. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67(Suppl. 10):1123–37. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 18.Hujoel P, Drangsholt M, Spiekerman C, Deroeun T. Examining the link between coronary heart disease and the elimination of chronic dental infections. J Am Dent Assoc. 2001;132:883–9. doi: 10.14219/jada.archive.2001.0300. [DOI] [PubMed] [Google Scholar]
- 19.Desvarieux M, Demmer R, Rundek T, et al. Relationship between periodontal disease, tooth loss and carotid artery plaques. Stroke. 2003;34:2120–5. doi: 10.1161/01.STR.0000085086.50957.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Q, Willeit J, Marosi M, et al. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993;341:255–9. doi: 10.1016/0140-6736(93)92613-x. [DOI] [PubMed] [Google Scholar]
- 21.Xu Q, Schett G, Seitz CS, Hu Y, Gupta RS, Wick G. Surface staining and cytotoxic activity of heat-shock protein 60 antibody in stressed aortic endothelial cells. Circ Res. 1994;75:1078–85. doi: 10.1161/01.res.75.6.1078. [DOI] [PubMed] [Google Scholar]
- 22.Wick G, Kleindienst R, Schett G, Amberger A, Xu Q. Role of heat shock protein 65/60 in the pathogenesis of atherosclerosis. Int Arch Allergy Immunol. 1995;107:130–1. doi: 10.1159/000236952. [DOI] [PubMed] [Google Scholar]
- 23.Hoppichler F, Lechleitner M, Traweger C, et al. Changes of serum antibodies to heat-shock protein 65 in coronary heart disease and acute myocardial infarction. Atherosclerosis. 1996;126:333–8. doi: 10.1016/0021-9150(96)05931-x. [DOI] [PubMed] [Google Scholar]
- 24.Schett G, Xu Q, Amberger A, et al. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest. 1995;96:2569–77. doi: 10.1172/JCI118320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schett G, Metzler B, Kleindienst R, et al. Salivary anti-HSP65 antibodies as a diagnostic marker for gingivitis and a possible link to atherosclerosis. Int Arch Allergy Immunol. 1997;114:246–50. doi: 10.1159/000237675. [DOI] [PubMed] [Google Scholar]
- 26.Xu Q, Kleindienst R, Schett G, et al. Regression of arteriosclerotic lesions induced by immunization with heat shock protein 65-containing material in normocholesterolemic, but not hypercholesterolemic, rabbits. Atherosclerosis. 1996;123:145–55. doi: 10.1016/0021-9150(96)05800-5. [DOI] [PubMed] [Google Scholar]
- 27.Metzler B, Mayr M, Dietrich H, et al. Inhibition of arteriosclerosis by T-cell depletion in normocholesterolemic rabbits immunized with heat shock protein 65. Arter Thromb Vasc Biol. 1999;19:1905–11. doi: 10.1161/01.atv.19.8.1905. [DOI] [PubMed] [Google Scholar]
- 28.Pervin K, Childerstone A, Shinnick T, et al. T cell epitope expression of mycobacterial and homologous human 65-kilodalton heat shock protein peptides in short term cell lines from patients with Behçet's disease. J Immunol. 1993;151:2273–82. [PubMed] [Google Scholar]
- 29.Schett G, Metzler B, Kleindienst R, et al. Myocardial injury leads to a release of heat shock protein (hsp) 60 and a suppression of the anti-hsp65 immune response. Cardiovasc Res. 1999;42:685–95. doi: 10.1016/s0008-6363(99)00012-7. [DOI] [PubMed] [Google Scholar]
- 30.Metzler B, Schett G, Kleindienst R, et al. Epitope specificity of anti-heat shock protein 65/60 serum antibodies in atherosclerosis. Arter Thromb Vasc Biol. 1997;17:536–41. doi: 10.1161/01.atv.17.3.536. [DOI] [PubMed] [Google Scholar]
- 31.Petit MD, Wassenaar A, van der Velden U, van Eden W, Loos BG. Depressed responsiveness of peripheral blood mononuclear cells to heat-shock proteins in periodontitis patients. J Dent Res. 1999;78:1393–400. doi: 10.1177/00220345990780080401. [DOI] [PubMed] [Google Scholar]
- 32.Lehner T, Lavery E, Smith R, van der Zee R, Mizushima Y, Shinnick T. Association between the 65-kilodalton heat shock protein, Streptococcus sanguis, and the corresponding antibodies in Behçet's syndrome. Infect Immun. 1991;59:1434–41. doi: 10.1128/iai.59.4.1434-1441.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ottenhoff TH, Haanen JB, Geluk A, et al. Regulation of mycobacterial heat-shock protein-reactive T cells by HLA class II molecules: lessons from leprosy. Immunol Rev. 1991;121:171–91. doi: 10.1111/j.1600-065x.1991.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 34.Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontal disease and coronary heart disease risk. JAMA. 2000;284:1406–10. doi: 10.1001/jama.284.11.1406. [DOI] [PubMed] [Google Scholar]
- 35.MacAry P, Javid B, Andres Floto R, et al. HSP70 peptide binding mutants separate antigen delivery from dendritic cell stimulation. Immunity. 2004;20:95–106. doi: 10.1016/s1074-7613(03)00357-1. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Kelly CG, Singh M, et al. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J Immunol. 2002;169:2422–9. doi: 10.4049/jimmunol.169.5.2422. [DOI] [PubMed] [Google Scholar]
- 37.Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–17. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- 38.Underhill DM, Ozinsky A, Hajjar AM, et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–15. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 39.Aliprantis AO, Yang RB, Mark MR, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 40.Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301–4. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- 41.Vink A, Schoneveld AH, van der Meer JJ, et al. In vivo evidence for a role of toll-like receptor 4 in the development of intimal lesions. Circulation. 2002;106:1985–90. doi: 10.1161/01.cir.0000032146.75113.ee. [DOI] [PubMed] [Google Scholar]
- 42.Xu XH, Shah PK, Faure E, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–8. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 43.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 44.Vabulas RM, Ahmad-Nejad P, da Costa C, et al. Endocytosed HSP60s use Toll-like receptor 2 (TLR2) and TLR4 to activate the Toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–9. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 45.Ozinsky A, Underhill DM, Fontenot JD, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc Nat Acad Sci USA. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of Toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–61. [PubMed] [Google Scholar]
- 47.Kol A, Sukhova GK, Lichtman AH, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation. 1998;98:300–7. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]