Abstract
The recent development of T cell receptor phage display opens up the possibility of engineering human T cell receptors with antibody-like binding properties for cell-surface peptide antigens. In this review we briefly discuss recent developments in molecular targeting of peptide antigens. We then discuss potential clinical applications of engineered high-affinity T cell receptors in autoimmunity and cancer.
Keywords: antibody-like, antigen targeting, high-affinity, phage display, TCR
Antibodies: the first class of engineered antigen targeting proteins
T cell receptors (TCRs) and antibodies are the only rearranged primary antigen receptor molecules of the adaptive immune system. A huge diversity of different TCRs and antibodies (> 1014) are generated by somatic rearrangement within T and B cells, respectively, which then undergo selection to delete self-reactive molecules. Since Kohler and Milstein produced the first monoclonal antibodies (mAbs) from mouse hybridomas in 1975 [1], antibodies have been developed extensively as diagnostic and therapeutic targeting agents. As well as being expressed on the surface of B cells, antibodies are naturally produced as soluble molecules and often have high affinities for their targets, making them ideal for many diagnostic uses. However, their therapeutic development was hampered initially by the murine origin of conventional mAbs which leads to an immune response when they are administered to humans: the human anti-mouse antibody (HAMA) response [2]. The production of mAbs from mouse hybridomas also limits the number of antibody variants which can be screened for the desired specificity and affinity. These problems were later overcome by various molecular engineering techniques, most prominently the expression of mAbs on the surface of bacteriophage (phage) [3] fused to a phage coat-protein (so-called ‘phage display’ [4]). This enabled manipulation of mAb characteristics by allowing artificial introduction of random variation in the mAb gene(s) to produce a ‘library’ followed by selection for affinity, specificity, and sometimes stability as well. Phage display was also used to generate phage libraries containing naive human antibody genes [5], allowing the selection of fully human mAbs specific for a selected target. This provides a potential starting point for the development human therapeutic targeting agents.
T cell receptors and their peptide antigens
Whereas antibodies target largely intact foreign bodies, often proteins, the natural ligands for TCRs are small peptide antigens presented on the surface of host cells by major histocompatibility complex (MHC) proteins [in humans also called human leucocyte antigens (HLA)] [6,7]. Eight to 11 amino acid peptides derived from the cytosolic protein pool are processed and presented by class I MHC proteins on the surface of all nucleated human cells where they are recognized by CD8+ T cells. Also, 12–20 amino acid peptides derived from the extracellular environment are processed by specialized antigen-presenting cells, such as dendritic cells and macrophages, and presented by class II MHC where they are recognized by CD4+ T cells. Class I MHC-presented peptide antigens are of particular interest for targeting applications because they are derived from the total pool of expressed proteins within a given cell. Thus, intracellular protein targets which are inaccessible to mAbs are presented on the cell-surface in the form of peptide-MHC class I protein complexes (see Fig. 1) where they can potentially be targeted by reagents with the requisite peptide-MHC specificity.
Fig. 1.
Schematic diagram showing the presentation of intracellular antigens as processed peptides by cell-surface major histocompatibility complex (MHC) molecules where they can be recognized by T cell receptors (TCRs). TAP: transporter associated with antigen processing; ERAAP: endoplasmic reticulum aminopeptidase associated with antigen processing.
TCRs are expressed naturally on the surface of T cells as α/β heterodimeric integral membrane proteins, each subunit comprising a short intracellular segment, a single transmembrane α-helix and two globular extracellular Ig-superfamily domains. The α/β heterodimer is stabilized by an extracellular, membrane proximal, inter-chain disulphide bond. TCRs therefore have four extracellular globular domains, the membrane proximal (C-terminal), two of which are constant, and the N-terminal, two of which are variable. The variable domains are encoded by variable gene segments which are rearranged with junctional and constant gene segments (and diversity gene segments in the case of β-chains) to produce the massive TCR diversity observed in the mature immune system.
In the immune system, TCRs are exclusively expressed on the surface of T cells and do not exist naturally as soluble molecules. The repertoire of T cells is controlled tightly by processes of negative and positive selection in the thymus; as a consequence, naturally occurring TCRs have low affinities, typically in the range 1–100 µ M [7]. Increasing the affinity of TCRs would require their display in a recombinant form suitable for in vitro evolution, but the production of recombinant TCRs was limited initially by their relatively poor stability. Therefore, initial efforts to generate targeting agents for peptide–MHC antigens were focused upon monoclonal antibodies.
Peptide–MHC specific monoclonal antibodies
Although monoclonal antibodies are often raised readily against intact proteins, it has proved more difficult to generate peptide–MHC specific antibodies which recognize the peptide component rather than the MHC component. Only a few ‘peptide-specific’ mAbs have been raised using the conventional mouse immunization approach [8,9]. Such ‘peptide-specific’ antibodies appear to be very rare in the mature immune system, implying that they are cross-reactive with self-MHC. The major obstacle in the generation of ‘peptide-specific’ mAbs is that antibodies have evolved such that the natural repertoire does not respond to pMHC in a peptide-specific manner, because this would result in an undesirable autoimmune antibody response against host antigen-presenting cells during infections. This lack of response must be at a fundamental structural level (rather than the level of selection) as antigenic peptides are not present during antibody negative selection. Indeed, a recent X-ray structure of a peptide-specific antibody indicates that a different binding mode is adopted compared to TCRs [10]. If this is a general phenomenon, it may explain some of the difficulties in making highly peptide-specific monoclonal antibodies.
Greater progress has been achieved in generating ‘peptide-specific’ mAbs using naive phage display libraries [11–13]. This shows that antibodies can be engineered to recognize peptide–MHCs, although there is evidence that they may bind in a structurally distinct manner to TCRs [10]. The affinities obtained with peptide-specific mAbs are typically ∼5–60 n M [14–17], although detailed and rigorous binding studies, e.g. surface plasmon resonance studies, have not generally been reported. For at least some peptide-specific mAbs, their affinity is achieved by fast on-rates [18], whereas high specificity is generally generated by non-covalent bond formation to the ligand, resulting in slow off-rates. Also, these mAbs are expressed as recombinant ‘single-chain’ constructs with a potentially immunogenic flexible linker connecting the C-terminus of one chain with the N-terminus of the other. To date, in vivo targeting experiments have only been performed using mAbs directed against conventional cell-surface antigens [19] rather than peptide–MHCs.
High-affinity TCRs: a new class of antigen targeting proteins
Unlike antibodies, TCRs are not naturally expressed as soluble proteins, and their extracellular domains are not stable in the absence of their natural inter-chain disulphide bond. A number of potential solutions to this problem have been tried before, including ‘single-chain’ TCRs [20–22] and fusions to stabilizing jun–fos leucine zippers [23]. We designed an alternative soluble TCR construct with the aim of producing highly stable TCR molecules with the minimum of sequence change from the wild-type in order to retain antigen specificity while avoiding host anti-TCR immune responses. A non-native disulphide bond, predicted by molecular modelling of a known TCR crystal structure [24], was engineered into the interface between the TCR constant domains, and the resulting TCR protein refolded correctly and was highly stable [25]. Because of their stability and globular structure, soluble TCRs made in this way have the additional advantage of being relatively easy to crystallize, enabling much more routine TCR X–ray structure solution [25,26] (and Jakobsen et al., unpublished data).
Biacore™ surface plasmon resonance analysis of soluble TCR binding to immobilized pMHC indicates that wild-type TCR affinities are in the range ∼1–100 µ M and have half-lives of ∼1–10 s [7]. This range of half-lives it too low to allow soluble TCRs to localize on target cells. In contrast, antibodies specific for conventional targets have affinities ranging up to pM [27] and fM [28], with most diagnostic and therapeutic antibodies being in the low nM range with binding half-lives of minutes to hours [29,30].
A well-established technology using tetrameric peptide–MHCs (MHC tetramers) allows the specific labelling of T cells [31] through an avidity effect made possible by the high density of TCR on the surface of T cells. Similarly, multimerization of TCRs has been used to generate higher avidity molecules (TCR tetramers) which can label antigen-presenting cells pulsed with peptide in vitro [32,33] and can specifically inhibit T cell activation [33]. However, this approach is limited by the low level of antigenic peptide, specific for a given TCR, naturally presented by cell surface MHC molecules: typically ≤ 1000 molecules per cell [34]. The low surface density of specific ligand reduces the number of multivalent binding sites for TCR tetramers, effectively negating the multimeric avidity effect. Furthermore, the low sensitivity of flow cytometry means that a cell must be labelled with > 1000 fluorochromes in order to be detected. It has therefore only been possible to use TCR tetramers to detect naturally processed and presented peptide antigens in cases where specific peptide antigen is expressed artificially at very high levels, although much lower levels may be detected indirectly [33].
Display of TCRs on yeast cells has been used previously to select stabilized variants of the single-chain alloreactive mouse 2C TCR [35] and to increase its affinity by a reported ∼100-fold to 9 n M [36], but similar engineering of other TCRs has not yet been reported. The high-affinity single-chain 2C TCR confers high peptide sensitivity and CD8 independence to transfected T cells [37], but shows significant cross-reactivity to self-peptide antigens [38]. Phage display of a single-chain mouse TCR has also been reported [39], but high-affinity TCR generation was not achieved. These technologies have therefore not had the same impact as monoclonal antibodies or mAb library display and, in order to solve this problem, a more effective way of engineering TCRs was clearly required.
TCR affinity enhancement by phage display
The unique stability of the novel disulphide-linked TCR [25] led us to attempt to display it on the surface of phage. We displayed several TCRs successfully as fusions to the phage geneIII coat protein and engineered TCRs with nanomolar to picomolar affinities for their cognate peptide–MHCs [40]. In particular, we engineered a TCR specific for the NY-ESO-1157−165 tumour-associated peptide antigen (SLLMWITQC) presented in the context of HLA-A*0201, to an affinity of 26 p M. Selected mutations were predominantly in the complementarity determining regions (CDRs) which contact the peptide-MHC in wild-type TCRs, and their effect was primarily to lengthen the half-life of binding of the TCR from approximately 7 s to > 15 h. High-affinity TCRs also bound antigen presenting cell-surface MHC at very low levels of peptide and, significantly, showed no cross-reactivity to endogenous non-specific peptides [40].
Further studies with the phage engineered A6 high-affinity TCR [40] specific for the HLA-A*0201-presented tax11−19 peptide (LLFGYPVYV) showed no evidence of any significant increase in affinity for the known peptides [41] to which its wild-type parent is cross-reactive [33]. This indicates that specificity of high-affinity TCRs can be increased by phage display-directed evolution, implying that many of the selected mutations interact specifically with the antigenic peptide rather than with the MHC. This high specificity is likely to be crucial to the success of TCRs as clinical tools because of the high levels of background endogenous peptide presentation on most antigen-presenting cells.
Applications of high-affinity TCRs: autoimmunity
High-affinity TCRs have been shown to be effective at competitively inhibiting specific T cell responses [33], implying that they could be used to mask specific antigens in the treatment of autoimmunity (Fig. 2). However, relatively few autoimmune peptide antigens have been identified unambiguously to date, and many of those which have been proposed do not have a defined role in disease aetiology or progression. Furthermore, ‘epitope spread’ is thought to occur in many autoimmune diseases [42], potentially reducing the efficacy of inhibiting the T cell response to a single antigen. However, the identification of autoimmune antigens is currently the subject of much research effort, and some of the causative antigens are becoming apparent for diseases such as type I diabetes [43] although much further investigation remains to be conducted. Also, it is possible that epitope spread may work in reverse, i.e. inhibition of one or very few T cell responses may restore the balance of the immune system and provide effective regulation of the autoimmune response. This is clearly a subject which requires further research for which peptide-specific high-affinity TCRs are ideally suited.
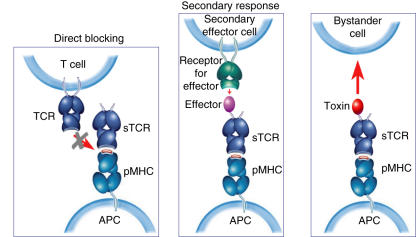
Fig. 2.
Diagram showing the main potential modes of action of high-affinity T cell receptors (TCRs) and their conjugates. High-affinity TCRs can directly block specific T cell activation by masking peptide–major histocompatibility complex (MHC); high-affinity TCR–effector conjugates can elicit secondary responses, either activating or inhibitory; high-affinity TCR–toxin conjugates can localize cytotoxic effects.
A more versatile, and potentially more effective, approach is to use high-affinity TCRs as targeting agents for immunomodulatory molecules (Fig. 2) which can modify disease-related immune responses. The localized presence of cytokines such as interleukin (IL)-10 [44] and IL−13 [45] can have significant qualitative effects on immune responses, e.g. by inducing regulatory T cell activity or by shifting the balance between Th1 and Th2 responses. High-affinity TCR targeting to tissue-specific antigens, that need not even be involved in disease, could enable the effects of such cytokines to be localized at the disease locus, where they will gain selective exposure to the relevant immune cells. This represents a novel concept for immune modulation that is now accessible due to the ability of TCRs to target the much broader range of intracellular antigens which are presented as processed peptides by MHC molecules on the cell surface.
Applications of high-affinity TCRs: cancer
There are many ‘tumour-associated antigens’ (TAAs), some of which are expressed on the tumour cell surface and can be targeted by monoclonal antibodies [46,47]. Many more are expressed only within the tumour cell, but all TAAs can be processed to produce ‘tumour-associated peptide antigens’ (TAPAs) presented by MHC/HLA molecules on the tumour cell surface [48]. The MHC locus is highly polymorphic, but some human class I HLAs are relatively common in certain ethnic populations, e.g. HLA-A*0201 in Caucasians and HLA-A*2402 in Asians. This polymorphism means that complete coverage of all patients is unlikely to be feasible with a TCR-mediated approach, but up to 85% coverage of the Caucasian population can be achieved by targeting just three HLA class I alleles.
A further potential problem with targeting peptide–MHCs in cancer is that many tumours are reported to down-regulate their class I HLAs in order to escape immune surveillance [49]. However, this is by no means a universal phenomenon and many tumour cells express normal levels of MHC/HLA. Furthermore, natural killer (NK) T cell surveillance is likely to ensure that at least a very low level of MHC/HLA expression is maintained on tumour cells in vivo, if not for tumour cell lines in vitro. MHC/HLA presented TAPAs can be recognized by T cells, although tumours often induce a state of immune tolerance which blunts the effectiveness of the anti-tumour immune response [50]. Attempts to boost the host T cell response to tumours using various vaccination strategies have had limited success − despite often inducing a specific T cell response, clinical tumour regression has very rarely been observed [51]. Preliminary studies show that tumour cell lines can be targeted specifically by high-affinity TCRs (Jakobsen et al., unpublished data), providing the basis for a variety of possible therapeutic approaches to cancer using high-affinity TCRs (Fig. 2).
Adoptive immunotherapy provides a way of redirecting the host immune response towards a tumour, by either selectively expanding tumour-specific host T cells or by transfecting host T cells in vitro with a tumour-specific TCR, and then reintroducing them into the autologous host [52–54]. In the latter case, it is clearly critical to use a TCR which confers high avidity, specificity and activity to the transfected host T cells, and engineered high-affinity TCRs are therefore likely to improve the efficacy of such approaches. Furthermore, the expression of high-affinity tumour-specific TCRs on both CD8+ and CD4+ T cells is likely to lead to activation of both subsets in response to tumour antigen, because the requirement for CD8 or CD4 co-receptor signalling is bypassed by high strength TCR signalling [55].
Also, like antibodies, TCRs can be engineered to deliver radionuclides, toxins or immunomodulatory molecules. Targeted radionuclide tumour treatment requires large-scale targeting of the radionuclide by the targeting molecule. Given the reported low levels of specific peptide antigen on tumour cells of ≤ 1000 molecules per cell [34], high-affinity TCRs are unlikely to be effective targeting molecules for such an approach. This relatively low level of peptide antigen target also requires that any targeted toxin be highly potent. Furthermore, there is no evidence to date that high-affinity TCRs are internalized upon binding to tumour cells (Jakobsen et al., unpublished data), so any targeted toxin must either work on the tumour cell-surface or must carry an internalization signal. However, there are reports of wild-type single-chain TCRs fused to, e.g. exotoxin A protein, specifically killing cells presenting p53 peptide antigens [56], and high-affinity engineering may improve the efficacy of such approaches.
Fusion of wild-type single-chain TCRs to IL-2 have been reported to reduce lung metastases in an HLA-A*0201 transgenic mouse tumour model [57], and immune effector function may also be provided by fusion to IgG1 H [58]. High-affinity engineering is likely to improve the efficacy of conjugates such as these. Other potential fusion partners include superantigens [59], antibodies, e.g. against CD3 [60,61], antigenic HLA molecules [19,62] and also IL−12 and tumour necrosis factor (TNF), which have shown efficacy in a mouse tumour model when fused to a tumour targeting antibody [63,64]. Work is in progress to elucidate the optimal conditions for high-affinity TCR targeting of tumours and which conjugate gives the best clinical response; in this respect the large body of data generated for antibody targeting of tumours is likely to be extremely valuable in guiding future research.
Conclusions
High-affinity TCRs derived by phage display directed evolution represent exciting novel reagents with similar binding properties to high-affinity monoclonal antibodies to their cognate peptide–MHC ligands. Although their potential clinical applications need to take into account the polymorphic nature of the peptide presenting MHC/HLA and the relatively low level of specific peptide antigen presentation, the majority of expressed antigens generate processed peptide fragments displayed in the form of cell-surface peptide–MHCs allowing targeting of a broader range of, particularly intracellular, disease or tissue markers. The potential for high-affinity soluble TCRs is extremely broad, particularly for targeting so-called universal cancer antigens which are intracellular and for delivering immune modulatory molecules to specific tissues affected by immune dysregulation.
Acknowledgments
We would like to thank A. K. Sewell for critical reading of the manuscript and for many helpful discussions and T. Miles for preparing the figures.
References
- 1.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 2.Tjandra JJ, Ramadi L, McKenzie IF. Development of human anti-murine antibody (HAMA) response in patients. Immunol Cell Biol. 1990;68:367–76. doi: 10.1038/icb.1990.50. [DOI] [PubMed] [Google Scholar]
- 3.McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552–4. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 4.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–7. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 5.Clackson T, Hoogenboom HR, Griffiths AD, Winter G. Making antibody fragments using phage display libraries. Nature. 1991;352:624–8. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- 6.Davis MM, Boniface JJ, Reich Z, et al. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–44. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 7.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–84. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 8.Messaoudi I, LeMaoult J, Nikolic-Zugic J. The mode of ligand recognition by two peptide : MHC class I-specific monoclonal antibodies. J Immunol. 1999;163:3286–94. [PubMed] [Google Scholar]
- 9.Uchanska-Ziegler B, Nossner E, Schenk A, Ziegler A, Schendel DJ. Soluble T cell receptor-like properties of an HLA-B35-specific monoclonal antibody (TU165) Eur J Immunol. 1993;23:734–8. doi: 10.1002/eji.1830230325. [DOI] [PubMed] [Google Scholar]
- 10.Hulsmeyer M, Chames P, Hillig RC, et al. An MHC : peptide-restricted antibody and TCR molecules recognize their target by distinct binding modes: crystal structure of HLA-A1: MAGE-A1 in complex with Fab-Hyb3. J Biol Chem. 2005;280:2972–80. doi: 10.1074/jbc.M411323200. [DOI] [PubMed] [Google Scholar]
- 11.Andersen PS, Stryhn A, Hansen BE, Fugger L, Engberg J, Buus S. A recombinant antibody with the antigen-specific, major histocompatibility complex-restricted specificity of T cells. Proc Natl Acad Sci USA. 1996;93:1820–4. doi: 10.1073/pnas.93.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen CJ, Denkberg G, Segal D, Reiter Y. Generation of recombinant immunotoxins for specific targeting of tumor-related peptides presented by MHC molecules. Meth Mol Biol. 2003;207:269–82. doi: 10.1385/1-59259-334-8:269. [DOI] [PubMed] [Google Scholar]
- 13.Chames P, Hufton SE, Coulie PG, Uchanska-Ziegler B, Hoogenboom HR. Direct selection of a human antibody fragment directed against the tumor T-cell epitope HLA-A1-MAGE-A1 from a nonimmunized phage-Fab library. Proc Natl Acad Sci USA. 2000;97:7969–74. doi: 10.1073/pnas.97.14.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen CJ, Sarig O, Yamano Y, Tomaru U, Jacobson S, Reiter Y. Direct phenotypic analysis of human MHC class I antigen presentation: visualization, quantitation, and in situ detection of human viral epitopes using peptide-specific, MHC-restricted human recombinant antibodies. J Immunol. 2003;170:4349–61. doi: 10.4049/jimmunol.170.8.4349. [DOI] [PubMed] [Google Scholar]
- 15.Lev A, Denkberg G, Cohen CJ, et al. Isolation and characterization of human recombinant antibodies endowed with the antigen-specific, major histocompatibility complex-restricted specificity of T cells directed toward the widely expressed tumor T-cell epitopes of the telomerase catalytic subunit. Cancer Res. 2002;62:3184–94. [PubMed] [Google Scholar]
- 16.Denkberg G, Cohen CJ, Lev A, Chames P, Hoogenboom HR, Reiter Y. Direct visualization of distinct T cell epitopes derived from a melanoma tumor-associated antigen by using human recombinant antibodies with MHC- restricted T cell receptor-like specificity. Proc Natl Acad Sci USA. 2002;99:9421–6. doi: 10.1073/pnas.132285699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Held G, Matsuo M, Epel M, et al. Dissecting cytotoxic T cell responses towards the NY-ESO-1 protein by peptide/MHC-specific antibody fragments. Eur J Immunol. 2004;34:2919–29. doi: 10.1002/eji.200425297. [DOI] [PubMed] [Google Scholar]
- 18.Mareeva T, Lebedeva T, Anikeeva N, Manser T, Sykulev Y. Antibody specific for the peptide-MHC complex: is it TCR-like? J Biol Chem. 2004;279:44243–9. doi: 10.1074/jbc.M407021200. [DOI] [PubMed] [Google Scholar]
- 19.Lev A, Noy R, Oved K, et al. Tumor-specific Ab-mediated targeting of MHC-peptide complexes induces regression of human tumor xenografts in vivo. Proc Natl Acad Sci USA. 2004;101:9051–6. doi: 10.1073/pnas.0403222101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schodin BA, Schlueter CJ, Kranz DM. Binding properties and solubility of single-chain T cell receptors expressed in E. coli. Mol Immunol. 1996;33:819–29. doi: 10.1016/0161-5890(96)00038-7. [DOI] [PubMed] [Google Scholar]
- 21.Chung S, Wucherpfennig KW, Friedman SM, Hafler DA, Strominger JL. Functional three-domain single-chain T-cell receptors. Proc Natl Acad Sci USA. 1994;91:12654–8. doi: 10.1073/pnas.91.26.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plaksin D, Polakova K, McPhie P, Margulies DH. A three-domain T cell receptor is biologically active and specifically stains cell surface MHC/peptide complexes. J Immunol. 1997;158:2218–27. [PubMed] [Google Scholar]
- 23.Willcox BE, Gao GF, Wyer JR, et al. Production of soluble alphabeta T-cell receptor heterodimers suitable for biophysical analysis of ligand binding. Protein Sci. 1999;8:2418–23. doi: 10.1110/ps.8.11.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding YH, Smith KJ, Garboczi DN, Utz U, Biddison WE, Wiley DC. Two human T cell receptors bind in a similar diagonal mode to the HLA-A2/Tax peptide complex using different TCR amino acids. Immunity. 1998;8:403–11. doi: 10.1016/s1074-7613(00)80546-4. [DOI] [PubMed] [Google Scholar]
- 25.Boulter JM, Glick M, Todorov PT, et al. Stable, soluble T-cell receptor molecules for crystallization and therapeutics. Protein Eng. 2003;16:707–11. doi: 10.1093/protein/gzg087. [DOI] [PubMed] [Google Scholar]
- 26.Chen JL, Stewart-Jones G, Bossi G, et al. Structural and kinetic basis for heightened immunogenicity of T cell vaccines. J Exp Med. 2005;201:1243–55. doi: 10.1084/jem.20042323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanes J, Schaffitzel C, Knappik A, Pluckthun A. Picomolar affinity antibodies from a fully synthetic naive library selected and evolved by ribosome display. Nat Biotechnol. 2000;18:1287–92. doi: 10.1038/82407. [DOI] [PubMed] [Google Scholar]
- 28.Boder ET, Midelfort KS, Wittrup KD. Directed evolution of antibody fragments with monovalent femtomolar antigen-binding affinity. Proc Natl Acad Sci USA. 2000;97:10701–5. doi: 10.1073/pnas.170297297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerstner RB, Carter P, Lowman HB. Sequence plasticity in the antigen-binding site of a therapeutic anti-HER2 antibody. J Mol Biol. 2002;321:851–62. doi: 10.1016/s0022-2836(02)00677-0. [DOI] [PubMed] [Google Scholar]
- 30.Einhauer A, Jungbauer A. Affinity of the monoclonal antibody M1 directed against the FLAG peptide. J Chromatogr A. 2001;921:25–30. doi: 10.1016/s0021-9673(01)00831-7. [DOI] [PubMed] [Google Scholar]
- 31.Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. [PubMed] [Google Scholar]
- 32.Subbramanian RA, Moriya C, Martin KL, et al. Engineered T-cell receptor tetramers bind MHC-peptide complexes with high affinity. Nat Biotechnol. 2004;22:1429–34. doi: 10.1038/nbt1024. [DOI] [PubMed] [Google Scholar]
- 33.Laugel B, Boulter JM, Lissin N, et al. Design of soluble recombinant T cell receptors for antigen targeting and T cell inhibition. J Biol Chem. 2005;280:1882–92. doi: 10.1074/jbc.M409427200. [DOI] [PubMed] [Google Scholar]
- 34.Schirle M, Keilholz W, Weber B, et al. Identification of tumor-associated MHC class I ligands by a novel T cell-independent approach. Eur J Immunol. 2000;30:2216–25. doi: 10.1002/1521-4141(2000)30:8<2216::AID-IMMU2216>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Shusta EV, Holler PD, Kieke MC, Kranz DM, Wittrup KD. Directed evolution of a stable scaffold for T-cell receptor engineering. Nat Biotechnol. 2000;18:754–9. doi: 10.1038/77325. [DOI] [PubMed] [Google Scholar]
- 36.Holler PD, Holman PO, Shusta EV, O'Herrin S, Wittrup KD, Kranz DM. In vitro evolution of a T cell receptor with high affinity for peptide/MHC. Proc Natl Acad Sci USA. 2000;97:5387–92. doi: 10.1073/pnas.080078297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–64. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 38.Holler PD, Chlewicki LK, Kranz DM. TCRs with high affinity for foreign pMHC show self-reactivity. Nat Immunol. 2003;4:55–62. doi: 10.1038/ni863. [DOI] [PubMed] [Google Scholar]
- 39.Weidanz JA, Card KF, Edwards A, Perlstein E, Wong HC. Display of functional alphabeta single-chain T-cell receptor molecules on the surface of bacteriophage. J Immunol Meth. 1998;221:59–76. doi: 10.1016/s0022-1759(98)00153-7. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Moysey R, Molloy PE, et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23:349–54. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 41.Hausmann S, Biddison WE, Smith KJ, et al. Peptide recognition by two HLA-A2/Tax11-19-specific T cell clones in relationship to their MHC/peptide/TCR crystal structures. J Immunol. 1999;162:5389–97. [PubMed] [Google Scholar]
- 42.Tuohy VK, Yu M, Yin L, et al. The epitope spreading cascade during progression of experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol Rev. 1998;164:93–100. doi: 10.1111/j.1600-065x.1998.tb01211.x. [DOI] [PubMed] [Google Scholar]
- 43.Kent SC, Chen Y, Bregoli L, et al. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435:224–8. doi: 10.1038/nature03625. [DOI] [PubMed] [Google Scholar]
- 44.Pauza ME, Neal H, Hagenbaugh A, Cheroutre H, Lo D. T-cell production of an inducible interleukin-10 transgene provides limited protection from autoimmune diabetes. Diabetes. 1999;48:1948–53. doi: 10.2337/diabetes.48.10.1948. [DOI] [PubMed] [Google Scholar]
- 45.Zaccone P, Phillips J, Conget I, et al. Interleukin-13 prevents autoimmune diabetes in NOD mice. Diabetes. 1999;48:1522–8. doi: 10.2337/diabetes.48.8.1522. [DOI] [PubMed] [Google Scholar]
- 46.Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–9. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook RC, Connors JM, Gascoyne RD, Fradet G, Levy RD. Treatment of post-transplant lymphoproliferative disease with rituximab monoclonal antibody after lung transplantation. Lancet. 1999;354:1698–9. doi: 10.1016/S0140-6736(99)02058-9. [DOI] [PubMed] [Google Scholar]
- 48.Renkvist N, Castelli C, Robbins PF, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2001;50:3–15. doi: 10.1007/s002620000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meissner M, Reichert TE, Kunkel M, et al. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clin Cancer Res. 2005;11:2552–60. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 50.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riddell SR. Finding a place for tumor-specific T cells in targeted cancer therapy. J Exp Med. 2004;200:1533–7. doi: 10.1084/jem.20042004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris EC, Bendle GM, Stauss HJ. Prospects for immunotherapy of malignant disease. Clin Exp Immunol. 2003;131:1–7. doi: 10.1046/j.1365-2249.2003.02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–23. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holler PD, Lim AR, Cho BK, Rund LA, Kranz DM. CD8(-) T cell transfectants that express a high affinity T cell receptor exhibit enhanced peptide-dependent activation. J Exp Med. 2001;194:1043–52. doi: 10.1084/jem.194.8.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Epel M, Ellenhorn JD, Diamond DJ, Reiter Y. A functional recombinant single-chain T cell receptor fragment capable of selectively targeting antigen-presenting cells. Cancer Immunol Immunother. 2002;51:565–73. doi: 10.1007/s00262-002-0312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Card KF, Price-Schiavi SA, Liu B, et al. A soluble single-chain T-cell receptor IL-2 fusion protein retains MHC-restricted peptide specificity and IL-2 bioactivity. Cancer Immunol Immunother. 2004;53:345–57. doi: 10.1007/s00262-003-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mosquera LA, Card KF, Price-Schiavi SA, et al. In vitro and in vivo characterization of a novel antibody-like single-chain TCR human IgG1 fusion protein. J Immunol. 2005;174:4381–8. doi: 10.4049/jimmunol.174.7.4381. [DOI] [PubMed] [Google Scholar]
- 59.Erlandsson E, Andersson K, Cavallin A, et al. Identification of the antigenic epitopes in staphylococcal enterotoxins A and E and design of a superantigen for human cancer therapy. J Mol Biol. 2003;333:893–905. doi: 10.1016/j.jmb.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 60.Davol PA, Smith JA, Kouttab N, Elfenbein GJ, Lum LG. Anti-CD3 × anti-HER2 bispecific antibody effectively redirects armed T cells to inhibit tumor development and growth in hormone-refractory prostate cancer-bearing severe combined immunodeficient beige mice. Clin Prostate Cancer. 2004;3:112–21. doi: 10.3816/cgc.2004.n.021. [DOI] [PubMed] [Google Scholar]
- 61.Gao Y, Xiong D, Yang M, et al. Efficient inhibition of multidrug-resistant human tumors with a recombinant bispecific anti-P-glycoprotein × anti-CD3 diabody. Leukemia. 2004;18:513–20. doi: 10.1038/sj.leu.2403267. [DOI] [PubMed] [Google Scholar]
- 62.Savage P, Gao L, Vento K, et al. Use of B cell-bound HLA-A2 class I monomers to generate high-avidity, allo-restricted CTLs against the leukemia-associated protein Wilms tumor antigen. Blood. 2004;103:4613–5. doi: 10.1182/blood-2003-11-3903. [DOI] [PubMed] [Google Scholar]
- 63.Halin C, Gafner V, Villani ME, et al. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor alpha. Cancer Res. 2003;63:3202–10. [PubMed] [Google Scholar]
- 64.Dela Cruz JS, Huang TH, Penichet ML, Morrison SL. Antibody-cytokine fusion proteins: innovative weapons in the war against cancer. Clin Exp Med. 2004;4:57–64. doi: 10.1007/s10238-004-0039-y. [DOI] [PubMed] [Google Scholar]